Abstract
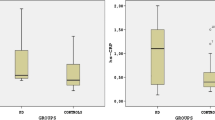
This study explored the potential relationship between levels of high-sensitivity C-reactive protein (hs-CRP) in plasma and freezing of gait (FOG) in Parkinson's disease (PD) in China. A total of 72 healthy subjects, 62 PD patients with FOG, and 83 PD patients without FOG from our center were enrolled in this prospective study. Patients with FOG showed significantly higher hs-CRP levels than controls, but patients without FOG did not. Binary logistic regression analysis identified levels of hs-CRP in plasma to be an independent risk factor for FOG among the patients in our cohort (OR 6.371, 95% CI 2.589–15.678, p < 0.001). In fact, a cut-off level of 0.935 mg/L distinguished patients with or without FOG [area under the ROC curve (AUC) = 0.908, sensitivity 87.1%, specificity 89.2%]. Our study suggests that high levels of hs-CRP in plasma are associated with the occurrence of FOG in PD. The pooled data combined with a previous study carried out in Spain also indicate a positive association between plasma hs-CRP levels and FOG in PD. However, more research is still needed to verify the plasma hs-CRP as a potential biomarker of FOG.



Similar content being viewed by others
References
Zhang ZX, Roman GC, Hong Z et al (2005) Parkinson’s disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet 365:595–597. https://doi.org/10.1016/S0140-6736(05)17909-4
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Adams B, Nunes JM, Page MJ et al (2019) Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci 11:210. https://doi.org/10.3389/fnagi.2019.00210
Dorszewska J, Kowalska M, Prendecki M et al (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16:1383–1391. https://doi.org/10.4103/1673-5374.300980
Wei Z, Li X, Liu Q et al (2018) Oxidative stress in Parkinson’s disease: a systematic review and meta-analysis. Front Mol Neurosci 11:236–236. https://doi.org/10.3389/fnmol.2018.00236
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. https://doi.org/10.1056/NEJM199902113400607
Luan YY, Yao YM (2018) The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol 9:1302. https://doi.org/10.3389/fimmu.2018.01302
Song I-U, Kim J-S, Chung S-W et al (2009) Is there an association between the level of high-sensitivity C-reactive protein and idiopathic Parkinson’s disease? A comparison of Parkinson’s disease patients, disease controls and healthy individuals. Eur Neurol 62:99–104. https://doi.org/10.1159/000222780
Qiu X, Xiao Y, Wu J et al (2019) C-Reactive protein and risk of Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 10:384. https://doi.org/10.3389/fneur.2019.00384
Verghese J, Holtzer R, Lipton RB et al (2012) High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 41:541–545. https://doi.org/10.1093/ageing/afs038
Kositsawat J, Barry LC, Kuchel GA (2013) C-reactive protein, vitamin D deficiency, and slow gait speed. J Am Geriatr Soc 61:1574–1579. https://doi.org/10.1111/jgs.12403
Sousa ACPA, Zunzunegui M-V, Li A et al (2016) Association between C-reactive protein and physical performance in older populations: results from the International Mobility in Aging Study (IMIAS). Age Ageing 45:274–280. https://doi.org/10.1093/ageing/afv202
Nutt JG, Bloem BR, Giladi N et al (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet 10:734–744. https://doi.org/10.1016/S1474-4422(11)70143-0
Kader M, Ullen S, Iwarsson S et al (2017) Factors contributing to perceived walking difficulties in people with Parkinson’s disease. J Parkinsons Dis 7:397–407. https://doi.org/10.3233/JPD-161034
Santos-Garcia D, De Deus FT, Suarez Castro E et al (2019) High ultrasensitive serum C-reactive protein may be related to freezing of gait in Parkinson’s disease patients. J Neural Transm (Vienna) 126:1599–1608. https://doi.org/10.1007/s00702-019-02096-8
Goetz CG (2010) Movement disorder society-unified Parkinson’s disease rating scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease. Rev Neurol (Paris) 166:1–4. https://doi.org/10.1016/j.neurol.2009.09.001
Hoehn MM (1983) Parkinsonism treated with levodopa: progression and mortality. J Neural Transm Suppl 19:253
Dubois B, Burn D, Goetz C et al (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324. https://doi.org/10.1002/mds.21844
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. https://doi.org/10.1136/jnnp.23.1.56
Amboni M, Stocchi F, Abbruzzese G et al (2015) Prevalence and associated features of self-reported freezing of gait in Parkinson disease: the DEEP FOG study. Parkinsonism Relat Disord 21:644–649. https://doi.org/10.1016/j.parkreldis.2015.03.028
Onyou H (2013) Role of oxidative stress in Parkinson’s Disease. Exp Neurobiol 22:11–17. https://doi.org/10.5607/en.2013.22.1.11
Blesa J, Trigo-Damas I, Quiroga-Varela A et al (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91–91. https://doi.org/10.3389/fnana.2015.00091
Rocha N, De Miranda A, Teixeira A (2015) Insights into neuroinflammation in Parkinson’s disease: from biomarkers to anti-inflammatory based therapies. Biomed Res Int 2015:628192–628192. https://doi.org/10.1155/2015/628192
Scalzo P, Kümmer A, Cardoso F et al (2010) Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci Lett 468:56–58. https://doi.org/10.1016/j.neulet.2009.10.062
Emberson JR, Whincup PH, Morris RW et al (2004) Extent of regression dilution for established and novel coronary risk factors: results from the British Regional Heart Study. Eur J Cardiovasc Prev Rehabil 11:125–134. https://doi.org/10.1097/01.hjr.0000114967.39211.e5
Kuhlmann CR, Librizzi L, Closhen D et al (2009) Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke 40:1458–1466. https://doi.org/10.1161/STROKEAHA.108.535930
Closhen D, Bender B, Luhmann HJ et al (2010) CRP-induced levels of oxidative stress are higher in brain than aortic endothelial cells. Cytokine 50:117–120. https://doi.org/10.1016/j.cyto.2010.02.011
Li YN, Qin XJ, Kuang F et al (2008) Alterations of Fc gamma receptor I and Toll-like receptor 4 mediate the antiinflammatory actions of microglia and astrocytes after adrenaline-induced blood–brain barrier opening in rats. J Neurosci Res 86:3556–3565
Juma WM, Lira A, Marzuk A et al (2011) C-reactive protein expression in a rodent model of chronic cerebral hypoperfusion. Brain Res 1414:85–93. https://doi.org/10.1016/j.brainres.2011.07.047
Moghaddam HS, Valitabar Z, Ashraf-Ganjouei A et al (2018) Cerebrospinal fluid C-reactive protein in Parkinson’s disease: associations with motor and non-motor symptoms. NeuroMol Med 20:376–385. https://doi.org/10.1007/s12017-018-8499-5
Jin H, Gu H, Mao C et al (2020) Association of inflammatory factors and aging in Parkinson’s disease. Neurosci Lett 736:135259–135259. https://doi.org/10.1016/j.neulet.2020.135259
Baran A, Bulut M, Kaya MC et al (2019) High-sensitivity C-reactive protein and high mobility group box-1 levels in Parkinson’s disease. Neurol Sci 40:167–173. https://doi.org/10.1007/s10072-018-3611-z
Sawada H, Oeda T, Umemura A et al (2015) Baseline C-reactive protein levels and life prognosis in Parkinson disease. PLoS ONE 10:e0134118. https://doi.org/10.1371/journal.pone.0134118
Ou R, Cao B, Wei Q et al (2017) Serum uric acid levels and freezing of gait in Parkinson’s disease. Neurol Sci 38:955–960. https://doi.org/10.1007/s10072-017-2871-3
Beavers DP, Kritchevsky SB, Gill TM et al (2021) Elevated IL-6 and CRP levels are associated with incident self-reported major mobility disability: a pooled analysis of older adults with slow gait speed. J Gerontol A Biol Sci Med Sci 76:2293–2299. https://doi.org/10.1093/gerona/glab093
Renner SW, Qiao Y, Gmelin T et al (2021) Association of fatigue, inflammation, and physical activity on gait speed: the Long Life Family Study. Aging Clin Exp Res. https://doi.org/10.1007/s40520-021-01923-x
Umemura A, Oeda T, Yamamoto K et al (2015) Baseline plasma C-reactive protein concentrations and motor prognosis in Parkinson disease. PLoS ONE 10:e0136722. https://doi.org/10.1371/journal.pone.0136722
Gan J, Liu W, Cao X et al (2021) Prevalence and clinical features of FOG in Chinese PD patients, a multicenter and cross-sectional clinical study. Front Neurol 12:568841. https://doi.org/10.3389/fneur.2021.568841
Martens KE, Hall JM, Gilat M et al (2016) Anxiety is associated with freezing of gait and attentional set-shifting in Parkinson’s disease: a new perspective for early intervention. Gait Posture 49:431–436. https://doi.org/10.1016/j.gaitpost.2016.07.182
Lafer B, Renshaw PF, Sachs GS (1997) Major depression and the basal ganglia. Psychiatr Clin N Am 20:885–896. https://doi.org/10.1016/s0193-953x(05)70350-6
Martens KAE, Lewis SJ (2017) Pathology of behavior in PD: what is known and what is not? J Neurol Sci 374:9–16. https://doi.org/10.1016/j.jns.2016.12.062
Lewis SJ, Barker RA (2009) A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 15:333–338. https://doi.org/10.1016/j.parkreldis.2008.08.006
Giladi N, Hausdorff JM (2006) The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J Neurol Sci 248:173–176. https://doi.org/10.1016/j.jns.2006.05.015
Lieberman A (2006) Are freezing of gait (FOG) and panic related? J Neurol Sci 248:219–222. https://doi.org/10.1016/j.jns.2006.05.023
Herman T, Shema-Shiratzky S, Arie L et al (2019) Depressive symptoms may increase the risk of the future development of freezing of gait in patients with Parkinson’s disease: findings from a 5-year prospective study. Parkinsonism Relat Disord 60:98–104. https://doi.org/10.1016/j.parkreldis.2018.09.013
Nonnekes J, Snijders AH, Nutt JG et al (2015) Freezing of gait: a practical approach to management. Lancet Neurol 14:768–778. https://doi.org/10.1016/S1474-4422(15)00041-1
Choi S-M, Jung H-J, Yoon G-J et al (2019) Factors associated with freezing of gait in patients with Parkinson’s disease. Neurol Sci 40:293–298. https://doi.org/10.1007/s10072-018-3625-6
Moore O, Peretz C, Giladi N (2007) Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord 22:2192–2195. https://doi.org/10.1002/mds.21659
Macht M, Kaussner Y, Möller JC et al (2007) Predictors of freezing in Parkinson’s disease: a survey of 6,620 patients. Mov Disord 22:953–956. https://doi.org/10.1002/mds.21458
Acknowledgements
We thank the patients with Parkinson's disease and the healthy control group for their participation in our study.
Funding
This research was supported by Yunnan Province Clinical Research Center for Neurological Disease(202002AA100204), National Natural Science Foundation of China [grant numbers: 81960242], Applied Basic Research Foundation of Yunnan Province[grant numbers: 202101AY070001-115], Yunnan Province Clinical Research Center for Geriatric Disease [grant number: 202102AA310069].
Author information
Authors and Affiliations
Contributions
JL contributed to the acquisition of the data, statistical analysis, and interpretation of the data, and drafted the manuscript. XY contributed to the study concept and design, acquisition of the data, statistical analysis, and critical revision of the manuscript for important intellectual content. WY, CZ, YZ, MG, BL and HR contributed to the acquisition of the data and clinical assessment.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report. The study does not present any potential conflicts.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all patients and their families for their anonymized clinical data to be published for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Yin, W., Zhou, C. et al. Association between levels of high-sensitivity C-reactive protein in plasma and freezing of gait in Parkinson's disease. Aging Clin Exp Res 34, 1865–1872 (2022). https://doi.org/10.1007/s40520-022-02134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02134-8