Abstract
Previous observational studies have investigated the relationship between obesity and the biliary tract and pancreas. The causality, however, is still to be confirmed. This study was designed to explore the causality between obesity which included body mass index(BMI), circumference (WC), hip circumference (HC) and waist-to-hip ratio (WHR), and pancreatobiliary diseases with a Two-Sample Mendelian Randomization(MR) analysis. single-nucleotide polymorphisms used in our study were derived from genome-wide association studies (GWAS). The inverse variance weighted was the dominated method to evaluate the causality. The heterogeneity was validated by Cochran's Q test. The pleiotropy was validated by MR-Egger regression and MR-PRESSO. The stability and reliability of the results were illustrated by the ‘leave-one-out’sensitivity analysis. The MR results explored positive causal effects of BMI (OR: 1.021; 95% CI: from 1.016 to 1.027; P = 4.25 × 10−15) and WC (OR: 1.021; 95% CI: from 1.015 to 1.028; P = 1.65 × 10−10) on pancreatobiliary diseases. However, no causality existed between HC, WHR and pancreatobiliary diseases. This study reminded that general obesity and abdominal obesity required weight loss to prevent pancreatic biliary disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, defined as a pathological condition that impairs health due to abnormal or excessive accumulation of lipids in adipose tissue, have been a global health crisis. According to a research, it is estimated that there are 603.7 million adults and 107.7 million children globally being obese [1]. Diagnosed at a body mass index (BMI) ≥ 30 kg/m2, obesity is regarded as a kind of chronic inflammation and is associated with the occurrence and development of many diseases [2]. A study indicated that BMI could increase risk of gallbladder and pancreatic cancer with a positive dose–response relationship [3]. In addition, another study found that non-biliary cancers were more associated with BMI than biliary cancers [4]. Previous studies maybe have reached a consensus that risk factors for pancreatic and biliary diseases included smoking, type 2 diabetes mellitus (T2DM), obesity and alcohol consumption and so on [5, 6]. However, it's worth noting that most of these literatures were based on observational data, which can only indicate the association and can not determine causal association between risk factors and outcomes due to the existence of confounding factors. Generally, the most common parameter to determine obesity is BMI. However, it might be inaccurate to assess obesity by BMI level alone because of the presence of abdominal obesity. It has been reported that it might be more effective to combine BMI with anthropometric characteristics such as waist circumference (WC), hip circumference (HC), and waist-to-hip ratio (WHR) when assessing obesity [7]. A few studies combined BMI and body type characteristics to assess obesity levels which were unable to establish a causal relationship between obesity and pancreatobiliary diseases.
Mendelian randomization (MR) analysis is an emerging epidemiological method employing genetic variation as an instrumental variables (IVs) [8, 9]. This method is similar to traditional randomized controlled trials(RCT), but can minimize the confounding factors of RCT [10], because the variables are distributed randomly and equally between the population, and that the genotypes are existence before the disease and independent of external factor after birth [11]. Therefore, it is widely used to explore causal relationships. In this study, single-nucleotide polymorphisms (SNPs) were employed as the IVs to conduct MR analysis to explore causal relationships between obesity (BMI, WC, HC, and WHR) and pancreatobiliary diseases.
Materials and methods
Study design
MR which can be carried out is based on the theory that because different genotypes determine different intermediate phenotypes which act as an individual's exposure, the association between the genotype and the disease can represent the effect of the exposure on the disease [12]. As shown in Fig. 1, SNPs worked as IVs to indirectly explore the relationship between exposure and outcome. However, in order to properly and reasonably deduce causal relationship between exposure and outcome, three important assumption must be met [13]:
-
1.
The SNPs should be strongly associated with exposure.
-
2.
The SNPS selected should have little to do with confounding factors.
-
3.
The SNPs should influence results only through exposure but not the direct correlation.
The flowchart of the study is shown in Fig. 2. The SNPs sassociated with BMI, WC, HC and WHR were employed to research the causality between obesity and pancreatobiliary diseases.
Data sources
The data in our study were obtained from the genome-wide association studies (GWAS) datasets. Genetic variants for BMI were obtained from the GIANT Consortium based on GWAS with 339,224 participants and 2,555,511 SNPs [14]. The genetic variants for WC, HC and WHR, respectively, brought 232,101 (2,565,408 SNPs), 225,487 (2,542,663 SNPs) and 224,459 (2,562,516 SNPs) participants from GIANT Consortium into the study [15]. Genetic predictors of disorders of gallbladder, biliary tract and pancreas were obtained from UK Biobank, which enrolled 361,194 individuals (13,586,589 SNPs) and consisted of 13,922 cases and 347,272 controls. The ethical approval in our study was not required, because of available data in public GWAS data set (https://gwas.mrcieu.ac.uk).
Instrumental variable
In our study, the following criteria were applied to select the IVs: (1) The SNPs associated with each genus at the locus-wide significance threshold (P ≤ 1.0 × 10–8) were extracted as potential IVs. the F-statistics were calculated with the following equation to assess the strength of each IV. To reduce the bias caused by weak IVs, weak IVs with F < 10 were excluded [16]. (2) The linkage disequilibrium (LD) was detected to guarantee that the SNPs were independent (r2 < 0.001,window size = 10,000 kb) and removed if containing LD. (3) The SNPs involved in the confounding factors were removed at Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/).
where R2 the proportion of variance in the exposure explained by the genetic variants, N sample size, EAF the effect allele frequency, beta the estimated genetic effect on physical activity, SE (beta) the standard error of the genetic effect
Statistical analysis
The inverse variance weighted (IVW), weighted median, MR-Egger, Simple mode and Weighted mode methods were applied to evaluate the causal associations between BMI, WC, HC, WHR, and pancreatobiliary diseases. IVW was the dominant analysis, which analyzed each Wald ratio and provided the most accurate estimate and was sensitivity to pleiotropy [17]. The heterogeneity was validated by Cochran's Q test. The MR-Egger regression methods and the MR-PRESSO were applied to conduct sensitivity analyses to examine whether the potential violation of the second and third assumptions of MR was existence[18]. MR-PRESSO global test and MR-PRESSO outlier test was used to examine the pleiotropy and remove the pleiotropy of IVs, respectively. There was no directional pleiotropy with the Egger-intercept of the linear regression close to 0 and horizontal pleiotropy of the IVs with P-value of MR-PRESSO global test > 0.05, then the exclusivity assumption could be considered to be valid[19]. The stability and reliability of the results were illustrated by the ‘leave-one-out’sensitivity analysis. All statistical analyses can be conducted by the ‘Two-Sample MR’package and MR-PRESSO packages in R (version 4.2.2) software. Statistically significant difference was considered at P < 0.05.
Results
In our study, the characteristics of obesity were selected as risk factors for pancreatobiliary diseases for MR Analysis. Then, conversely, MR Analysis was performed with pancreaticobiliary disease as a risk factor and obesity characteristics as an outcome. According to the described selection criteria, there were 67 BMI-related SNPs with F ranging between 29.9 and 239.9, 34 WC-related SNPs with F ranging between 29.3 and 128.4, 66 HC-related SNPs with F ranging between 30.9 and 188.1, and 27 WHR-related SNPs with F ranging between 29.2 and 169.8 selected for MR analysis for pancreaticobiliary disease outcome. Details of the relevant SNPS for MR analysis were Presented in Tables S1–S4.
Mendelian randomization analyses
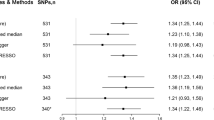
Results presented in Table 1, which were reported as odds ratios (OR), made the casual relationship between obesity and pancreaticobiliary disease clear. As shown in Figs. 3 and 4, the IVW method indicated that BMI was causal risk factor of pancreaticobiliary disease (OR: 1.021; 95% CI: from 1.016 to 1.027; P = 4.25 × 10−15) with no heterogeneity (Q = 76.14, P = 0.1845) and WC was causal risk factor of pancreaticobiliary disease (OR: 1.021;95% CI: from 1.015 to 1.028; P = 1.65 × 10−10) with no heterogeneity (Q = 31.24, P = 0.5548). The results of HC, WHR and pancreaticobiliary diseases were listed in Table 1. However, the MR analysis indicated no causal relationship was existence between HC and WHC on pancreaticobiliary diseases as shown in the IVW result respectively (OR: 0.999; 95% CI: from 0.994 to 1.003; P = 0.585) and (OR: 1.009; 95% CI: from 1.001 to 1.018; P = 0.0206).
The MR results of BMI on pancreaticobiliary disease. Scatter plot about the causal effect of BMI on pancreaticobiliary disease. Forest plot for the overall causal effects of BMI on pancreaticobiliary disease. Leave-one-out analysis for the causal effect of BMI on pancreaticobiliary disease. Funnel plot of SNPs related to BMI and pancreaticobiliary disease
The MR results of WC on pancreaticobiliary disease. Scatter plot about the causal effect of WC on pancreaticobiliary disease. Forest plot for the overall causal effects of WC on pancreaticobiliary disease. Leave-one-out analysis for the causal effect of WC on pancreaticobiliary disease. Funnel plot of SNPs related to WC and pancreaticobiliary disease
Sensitivity analysis
The F-statistic for all IVs was >10, which meant that our estimate of the causal relationship between obesity and pancreaticobiliary disease was more accurate and precise. As shown in Table 1, no directional pleiotropy and horizontal pleiotropy was found for the analyze of BMI and WC. However, horizontal pleiotropy and heterogeneity were found by MR-PRESSO and Cochran's Q test when it came to HC and WHR with PMR-PRESSO = 0.0194, Q = 92.7, PQ test = 0.0204 and PMR- PRESSO = 0.019, Q = 48.2, PQ test = 0.0139, respectively. MR-PRESSO outlier test was carried out. Actually, several potentially pleiotropic SNPs were excluded. Specifically speaking, rs1053593 and rs4239437 were excluded from the analysis of HC; rs12549058, rs17451107 and rs2287019 were excluded from the analysis of WHR. As shown in Table 1, Similar results were observed with no pleiotropy and heterogeneity when these SNPs were excluded from the analysis. Scatter-plot revealed there was no causal relationship between WHR and pancreaticobiliary disease, although IVW indicated OR: 1.009, 95% CI: from 1.001 to 1.018, P = 0.0206.
Discussion
The purpose of our study was to investigate the relationship between obesity and pancreaticobiliary disease by two-sample Mendelian randomization analysis. Although BMI played an important role in assessing obesity, it was difficult to distinguish between abdominal and peripheral fat [20]. Therefore, BMI and body type characteristics (WC, HC, and WHR) was applied to assess the causal effect of obesity on pancreaticobiliary disease in our study, which made it clear that BMI and WC, rather than HC and WHR, had causal effects on pancreaticobiliary disease.
Pancreatic diseases are a large category of diseases occurring in the pancreas. Patients with pancreatic disease and obesity are common in clinical practice. Previous studies have shown that WC was associated with the development of acute pancreatitis [21, 22], meaning that for every 10 cm increase in waist circumference, the probability of acute pancreatitis increased by 40% [22]. Navina et al. found that the increased risk of acute pancreatitis due to increased abdominal circumference may be related to increased retroperitoneal, peripancreatic, or intrapancreatic volume [23]. There are a few literatures on chronic pancreatitis and obesity. A study focused on impact of pediatric acute recurrent and chronic pancreatitis showed that obese or overweight children were diagnosed with chronic pancreatitis at an older age, which meant obesity might be a long-term proinflammatory state that eventually progressed to a chronic inflammatory state [24]. Obesity is regarded as a kind of chronic inflammation and can increase the levels of TNFα, IL-1β, IL-6, and IL-18 within adipose tissue and systemically, such as through inflammasome activation in macrophages [25]. An animal study showed that obesity caused deficiency of PGC-1α in the pancreas, which significantly enhanced NF-kB-mediated up-regulation of IL-6 in pancreas, resulting in serious inflammatory responses [26].
Biliary tract disease is a general term of gallbladder cancer, ampulla cancer, cholelithiasis, cholecystitis and so on and may be related with body fatness through oxidative stress, increased inflammation and so on [4]. A meta-analysis of BMI, WC, WHR, and gallbladder disease risk showed a summary relative risk of 1.46 for every 10 cm increase in WC and 1.44 for every 0.1 unit increase in WHR. Even within the "normal" BMI range, the risk almost twofold increase [27]. According to a European cohort, obesity, especially abdominal obesity, was associated with the risk of gallbladder cancer [28]. Interesting, obesity was thought to be associated with cholangiocarcinoma in Western countries, but had no effect on its development in Asia population [29].
Although obesity was shown in our study to be associated with pancreatobiliary diseases. However, there were still some limitations. Firstly, this study was primarily based on European population, whether similar results would be obtained in other population was unknown. Secondly, because of the different etiopathogenesis and heterogeneous of the pancreatobiliary diseases, and unclear criteria which had been specifically taken into account from the genome-wide association studies (GWAS) datasets obtained from the GIANT Consortium and from UK Biobank, it was hard to conduct subgroup analysis in pancreatobiliary diseases, making our results seemed to be excessive. Nevertheless, our results give a new insight, especially regarding which types of obesity requires weight loss to prevent pancreatic biliary disease”.
Conclusion
This MR study revealed that body shape traits were associated with pancreatobiliary diseases and health benefits of losing weight within reasonable target to prevent pancreatic biliary disease still remained attractive.
Data Availability
Data supporting the findings of this study are available within the paper and its supplementary information files.
References
Collaborators GBDO et al (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27
Council of the Obesity Society (2008) Obesity as a disease: the obesity society council resolution. Obesity (Silver Spring) 16(6):1151
Lauby-Secretan B et al (2016) Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med 375(8):794–798
Park M et al (2014) Body mass index and biliary tract disease: a systematic review and meta-analysis of prospective studies. Prev Med 65:13–22
Wang GJ et al (2009) Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol 15(12):1427–1430
Jackson SS et al (2020) Challenges in elucidating cholangiocarcinoma etiology. Hepatobiliary Surg Nutr 9(4):537–539
Jayedi A et al (2020) Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 370:m3324
Birney E (2022) Mendelian randomization. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a041302
Sekula P et al (2016) Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27(11):3253–3265
Lawlor DA et al (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8):1133–1163
Lyu L et al (2022) Causal relationships of general and abdominal adiposity on osteoarthritis: a two-sample mendelian randomization study. J Clin Med 12(1):320
Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23(R1):R89-98
Carter AR et al (2021) Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 36(5):465–478
Locke AE et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538):197–206
Shungin D et al (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518(7538):187–196
Papadimitriou N et al (2020) Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun 11(1):597
Burgess S et al (2017) Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology 28(1):30–42
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525
Verbanck M et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698
Nishida C, Ko GT, Kumanyika S (2010) Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr 64(1):2–5
Sawalhi S et al (2014) Does the presence of obesity and/or metabolic syndrome affect the course of acute pancreatitis? A prospective study. Pancreas 43(4):565–570
Sadr-Azodi O et al (2013) Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol 108(1):133–139
Navina S et al (2011) Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 3(107):107ra110
Uc A et al (2018) Impact of obesity on pediatric acute recurrent and chronic pancreatitis. Pancreas 47(8):967–973
Sakai NS, Taylor SA, Chouhan MD (2018) Obesity, metabolic disease and the pancreas-Quantitative imaging of pancreatic fat. Br J Radiol 91(1089):20180267
Perez S et al (2019) Obesity causes PGC-1alpha deficiency in the pancreas leading to marked IL-6 upregulation via NF-kappaB in acute pancreatitis. J Pathol 247(1):48–59
Aune D, Norat T, Vatten LJ (2015) Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol 30(9):1009–1019
Schlesinger S et al (2013) Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer 132(3):645–657
Osataphan S, Mahankasuwan T, Saengboonmee C (2021) Obesity and cholangiocarcinoma: a review of epidemiological and molecular associations. J Hepatobiliary Pancreat Sci 28(12):1047–1059
Acknowledgements
This work was made possible by publicly available statistics from GWAS. We thank the contributors of the original GWAS datasets.
Funding
This work was supported by the grants from the project of Chengdu Municipal Health Commission (No. 2020106 and 2022236), and the project of Sichuan Hospital Management and Development Research Center (No. SCYG2022-03).
Author information
Authors and Affiliations
Contributions
Jian Zou and Dan Huang provided the overall design of the study. Dan Huang wrote the main manuscript text and Jian Zou supervised the progress in the implementation. Yu Liu and Wenjun Gong performed the main analysis of this study and summarised and collated the results into the table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical and informed consent
Mendelian randomization analysis is conducted using summary data obtained from GWAS. The collection of this data adhered to the principles of written informed consent and received ethics approval. It is important to note that no ethical permit is necessary for the secondary analysis of summary data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, D., Liu, Y., Gong, W. et al. Causal relationships between obesity and pancreatobiliary diseases: a two-sample Mendelian randomization study. Eat Weight Disord 28, 63 (2023). https://doi.org/10.1007/s40519-023-01592-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-023-01592-x