Abstract
Background
Vegetarian diets and aerobic exercise are increasingly accepted as a common way to improve lifestyle. Several studies have shown that vegetarian diets combined with aerobic exercise interventions have a significant effect on preventing and reducing the risk of metabolic diseases.
Methods
A search of the PubMed, EBSCO, Embase, CENTRAL, and Web of Science databases was conducted for comparative studies of pre- and post-vegetarian diet adoption combined with aerobic exercise interventions on glycemic control and body composition. Qualitative reviews and meta-analyses of fixed and random effects were conducted to pool available data. The results were validated by sensitivity analysis.
Results
A total of 27 studies were selected for meta-analysis. Combining the studies included in the meta-analysis showed a mean difference for homeostasis model assessment of insulin resistance of − 0.75 (− 1.08 to − 0.42), fasting plasma glucose of − 0.27(− 0.30 to − 0.23), waist circumference of − 1.10 (− 5.06 to 2.86) and body mass index of − 0.70 (− 1.38 to − 0.01).
Conclusion
In summary, our findings suggest that participants who adopted a vegetarian diet combined with aerobic exercise intervention had significantly lower fasting plasma glucose and insulin levels and improved body composition compared to preintervention participants.
Level of evidence
Level I, systematic review and meta-analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lifestyle and health-related behaviors, mainly including diet and physical activity, are strongly associated with global morbidity and mortality for many chronic diseases [1, 2]. Poor diet and lack of exercise are common high-risk lifestyle behaviors among people of all ages worldwide [3], which have led to increased rates of obesity, a major chronic disease that affects the overall health of humans. As the increase in the number of people with body mass index (BMI) in the overweight and obese range is closely related to the occurrence and development of chronic diseases, such as diabetes, metabolic syndrome, cardiovascular disease (CVD) and cancer, the prevention and treatment of obesity has become a global issue [4]. Growing evidence supports the role of diet, exercise, stress management, and smoking in the pathogenesis of metabolic diseases [5,6,7].
Lifestyle changes can alter the state of the body and prevent chronic diseases such as hypertension, diabetes, and CVD. What’s more, with more people being aware of the importance of environmental issues and compassion for animals and the potential health benefits of a vegetarian diet (VD), there has been a clear trend towards vegetarianism over the past few years [8]. VD [9, 10] is defined as a diet that excludes all animal products, including dairy products, eggs and honey. VD includes lacto-ovo-vegetarian diet (LOV), plant-based diet (PBD) and vegan diet, as well as some other dietary patterns. Compared to omnivorous diets, VD is rich in many vitamins, phytochemicals, and antioxidants, but lower in cholesterol, total fat, saturated fatty acids and serum vitamin B12 concentrations, especially omega-3 polyunsaturated fatty acids. This property is associated with lower blood pressure and BMI [11]. The impact of physical activity on human health and wellbeing has been demonstrated and is associated with a reduced risk of death [12]. Exercise recommendations include participation in regular moderate-intensity aerobic exercise for at least 150 min per week or more than 300 min per week to achieve long-term weight control and moderate-intensity weight lifting twice per week with 10 to 15 repetitions of resistance exercise [13].
The Pritikin Institute has conducted studies [14, 15] demonstrating improved glycemic control with a PBD combined with exercise as part of type 2 diabetes treatment. David and colleagues [15] demonstrated the potential benefits of a VD for glycemic control and insulin resistance (IR). Several meta-analyses [16,17,18,19] also confirmed the role of exercise in IR, glycemic control, and body composition. To our knowledge, there is no systematic review and meta-analysis on the effects of combined VD and aerobic exercise interventions on glycemic control, IR, and body composition. Therefore, we conducted a systematic literature search to summarize and synthesize the available data and performed a meta-analysis of published clinical trials to determine whether the combination of VD and exercise has additional benefits on glycemic control, IR, and body composition compared with VD or aerobic exercise alone.
Materials
Protocol and registration
This study is based on the PRISMA Statement Guidelines [20]. The protocol reviewed was preregistered with PROSPERO: CRD42022318778.
Literature search strategy
In this meta-analysis, we considered only studies published in English. These studies were obtained by searching five electronic databases, namely: EBSCO, PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science. The search was finished on September 4, 2022. The search strategy to identify all studies examining vegetarian diet combined with exercise interventions was (“plant-based diet”, “vegan diet”, “lacto-ovo-vegetarian diet” or “vegetarian diet”, etc.) and (“aerobic exercise”, “exercise”, or “exercise performance”). We searched “All field” in PubMed and for “subject terms” + “entry words”, “subject terms” in EBSCO, Embase, and Web of Science, and searched unrestrictedly in CENTRAL. In addition, we checked the reference lists of all primary studies and any relevant review articles for additional references. The search strategy of five databases is shown in Table S1 (Supplementary material 1 Table 1).
Study selection and eligibility criteria
Inclusion criteria
Studies were included if they met the following criteria: (1) Randomized controlled trials (RCTs), nonrandomized controlled trials (Non-RCTs), and cohort studies; (2) Written in English and translation of non-English articles into English; (3) The intervention was a combination of both vegetarian diet and exercise; (4) The outcome measures included one of the following: body composition, fasting plasma glucose (FPG), insulin, or homeostasis model assessment of insulin resistance(HOMA-IR); and (5) Availability of summary statistics for mean and standard deviation (SD), change in mean and SD, quartiles, or 95% confidence interval (CI) before and after the intervention for outcome measures.
Exclusion criteria
Studies were excluded if they met the following criteria: (1) Reviews, animal studies, comments, letters, books, conferences published in abstract form only; (2) No comparison based on pre- and postintervention. (3) Articles published in languages other than English. (4) Studies published later than the time of the literature search.
Data extraction
The three authors (YL, JM-Y, HY) independently extracted data from the selected studies into Microsoft Excel spreadsheets; any disagreements were resolved at a consensus meeting. Table 1 presents a summary of the extracted data, which provides information on (1) Authors, year, and country of publication; (2) Study design; (3) Characteristics of the study population (i.e., physical health status, age, sample size, number of men and women); (4) Type of vegetarian intervention, and type of exercise intervention; and (5) Duration of intervention. One author (YL) extracted the data, and two authors (JM-Y, HY) checked and verified the extracted data.
Quality assessment
Revman 5.4 software and the Cochrane Risk of Bias Assessment Tool [21] (RBT) were used to produce the literature risk of bias and quality for selected randomized controlled trials (RCTs). The risk of bias for cohort studies was assessed using the Newcastle Ottawa Observational Study Scale [21] (NOS). Nonrandomized controlled trials (Non-RCTs) were assessed using the methodological index for nonrandomized studies (MINORS) [22]. The assessment was first performed independently by two authors (YL, JM-Y), and when there was a lack of consensus, it was resolved by discussion and agreement with the third review author (HY).
Statistical analysis
Meta-analysis was performed using Stata SE 15.0. Effect sizes were estimated using weighted mean differences (WMDs), standardized mean differences (SMDs), and 95% CIs. We used forest plots and I2 to assess heterogeneity between studies, where 0–40%, 30–60%, 50–90%, and 75–100% indicated possible irrelevance, moderate heterogeneity, substantial heterogeneity, and greater heterogeneity [23]. The fixed-effects model was used to assess studies with less heterogeneity, while the random-effects model was used to assess studies with greater heterogeneity. If the heterogeneity of the meta-results was high (p < 0.1 or/and I2 > 50%), subgroup and sensitivity analyses were produced to elucidate the sources of heterogeneity and to examine whether the risk of bias would alter the combined results. When more than seven articles were included, publication bias in the meta-analysis was detected by making funnel plots, Egger’s regression test [24], and Begg’s rank correlation test [25].
Results
Study selection
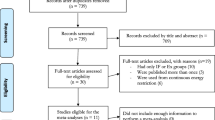
Data for eligible publications were searched until September 4, 2022. The literature search resulted in 3109 articles with 1161, 129, 731, 853, and 235 studies on PubMed, EBSCO, Embase, Web of Science, and the Cochrane Library, respectively. Exclusion of duplicates resulted in 1143 articles, of which the abstracts were screened for eligibility. A total of 1966 studies were retrieved from the full-text screening, and 27 studies were considered eligible for systematic evaluation and meta-analysis after screening by full-text reading. The process is shown in Fig. 1.
Excluded studies
Fifty-four studies were excluded after screening the full text. The most common reason was that the outcome indicator after the intervention was not body composition, FPG, insulin, or HOMA-IR; some articles were excluded because they did not fit the combined VD and aerobic exercise intervention, and others were excluded because they had more than just VD and exercise interventions but also added supplements, specific medications, etc. Seven articles were excluded because the full text was not available, and one article was excluded because it did not provide the data we needed and we were not able to rectify the situation even after contacting the original author numerous times.
Included studies
Details of the included study are shown in Table 1. The effect of a VD combined with an aerobic exercise intervention on people was systematically evaluated from the perspective of glucose metabolism and weight control by comparing postintervention measures with preintervention measures in the same subjects. Variables used as proxies for carbohydrate metabolism included FPG and IR; body weight (BW), BMI, waist circumference (WC), and hip circumference (HC) for body composition measures.
Study characteristics
A total of 27 studies with a total of 9053 subjects were included in this paper, and in general, more female subjects than male subjects were included. Fourteen interventional studies and thirteen observational studies were included. The intervention studies were subdivided into 9 RCTs and 5 non-RCTs, and the observational studies included cohort studies. The sample sizes ranged from 12 to 5046 individuals, and the study durations ranged from 6 days to 24 months.
Three studies included only female subjects, three studies included only male subjects, 13 studies included healthy subjects, and 14 studies included patients with metabolic abnormalities such as overweight or obese BMI, coronary artery disease (CAD), diabetes, or hypertension. All studies were published between 1990 and 2022, and approximately two-thirds of the included studies were published after 2010. All of them were published in India, Mexico, Germany, Australia, Italy, Korea, Canada, Israel, the Czech Republic, Poland and the United States, with nearly half of the studies published in the United States.
Results from quality assessments
The risk of bias graph in RevMan lists the risk of bias for the nine RCTs [26,27,28,29,30,31,32,33,34]. The results indicate that the included RCTs were of high quality. The details are shown in Fig. 2. The risk of bias for the five included non-RCT studies [35,36,37,38,39] is presented in Table S2. According to the MINORS entry, one studies [37] showed a high risk of bias, with overall scores of 11, because many details of the study protocol were not reported in the publication. The other four studies had an overall low to moderate risk of bias, with total scores of 14, 15, 16, and 16, respectively. Points were deducted from total scores due to a lack of prospective data collection details, statistical analysis methods, recruitment of participants, blinding of results, and problems with using participants as their own controls and parameters being estimated at two different time points. The quality of the literature was assessed for the thirteen cohort studies, indicating that the quality of the literature was high quality and the overall risk was low, details of which can be found in Table S3.
Effect of intervention on glycemic control
Seven studies [26, 34, 40,41,42,43,44] reported FPG data for 5862 subjects, and five studies [30, 38, 39, 45, 46]reported glucose data for 878 subjects. Pooled results under a fixed effects model showed that individuals adhering to the VD and aerobic exercise interventions had significantly lower FPG (SMD: − 0.27; 95% CI: − 0.30 to − 0.23; p < 0.001; I2: 41%) and a random effects model showed nonsignificant changes in glucose (SMD: − 0.22; 95% CI − 0.32 to − 0.13; p < 0.001; I2: 32.7%). There was significant heterogeneity in both groups (Figs. 3 and 4).
Forest figure of FPG. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Forest figure of glucose. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Effect of intervention on IR
Three studies [26, 30, 34] reported HOMA-IR data for 166 subjects, and six studies [26, 27, 30, 34, 38, 40] reported insulin data for 419 subjects. The pooled results under a fixed-effects model showed that HOMA-IR scores were significantly lower in individuals who adhered to a VD combined with exercise intervention (WMD: − 0.75; 95% CI − 1.08 to − 0.42; p < 0.001; I2:0%). Insulin data for study individuals were then used in a random-effects model, and the pooled results showed significantly lower insulin levels (WMD: − 2.03; 95% CI − 3.40 to − 0.67; p = 0.003; I2:40.5%) (Figs. 5 and 6).
Forest Figure of HOMA-IR. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Forest Figure of insulin. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Effect of intervention on body composition
Twenty studies [26,27,28, 30,31,32,33, 35,36,37,38,39, 44,45,46,47,48,49,50,51] reported data on BW in 3152 subjects. Eighteen studies [26, 28,29,30, 33, 37,38,39,40,41,42,43,44,45,46,47, 51, 52] reported data on BMI in 7574 subjects. Eight studies [26, 27, 29, 34, 38, 46, 51, 52] reported data on waist circumference (WC) in 624 subjects. Four studies [26, 46, 52, 53] reported data on the hip circumference (HC) in 177 subjects. Six studies [26, 30, 35, 47, 49, 51] reported data on body fat percentage (BF%) in 923 subjects.
Pooled results under a fixed-effects model indicated that individuals adhering to a VD combined with exercise intervention had a nonsignificant change in HC was significantly lower (WMD: − 2.55; 95% CI − 4.04 to − 1.05; p = 0.001; I2: 0%). No heterogeneity was found in HC of the integration of results under the fixed effects model.
For the study individuals, BW, BMI, BF% and WC were used in a random effects model, and the combined results showed a significant reduction in BW (SMD: − 0.24; 95% CI − 0.40 to − 0.09; p = 0.003; I2: 86.2%), a significant reduction in BMI (WMD: − 0.70; 95% CI − 1.38 to − 0.01; p = 0.046; I2:91.8%). BF% was significantly reduced (WMD: − 1.87; 95% CI − 3.50 to − 0.24; p = 0.025; I2: 85.0%), and WC was also significantly reduced (WMD: − 1.10; 95% CI − 5.06 to 2.86; p = 0.02; I2:94.7%). Substantial heterogeneity was present in all groups (Figs. 7, 8, 9 and 10).
Forest Figure of BW. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Forest Figure of BMI. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
To elucidate substantial heterogeneity in body composition, subgroup analyses were [31, 36]. Heterogeneity increased in the ≥ 1-month subgroup and decreased in the < 1-month subgroup, suggesting that the duration of intervention may be a source of heterogeneity. (Supplementary material 2: Figs. 1, 2, 3, and 4).
Forest Figure of BF%. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies
Forest Figure of WC. For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis
Sensitivity analysis
The results of the sensitivity analysis were stable, with no significant effect of any individual effect size on the overall effect size of all parameters (Supplementary material 2: Figs. 5, 6, and 7).
Publication bias
Begg’s test demonstrated no publication bias for FPG (p = 0.548), glucose (p = 1.000), insulin (p = 0.452), HOMA-IR (p = 1.000), BW (p = 0.347), BMI (p = 0.363), HC (p = 0.734), WC (p = 0.711), or BF% (p = 0.707). Egger’s test showed no significant publication bias for all indicators except weight. Visual inspection of the funnel plot yielded the same results (Supplementary material 2: Figs. 8, 9, and 10).
Discussion
Effect of the vegetarian diet combined with aerobic exercise
The results of our systematic review and meta-analysis showed that VD combined with aerobic exercise intervention had variable effects on glycemic control and IR in various populations compared to preintervention, mainly in terms of significant reductions in FPG, HOMA-IR and insulin levels, and nonsignificant reductions in glucose values. The effects of a VD on glycemic management, IR, and body composition have been confirmed by several clinical trials and narrative reviews. The addition of aerobic exercise training has been shown to further enhance the improving effects of a VD, it can reduce diabetes and its complications caused by chronic glycotoxicity caused by persistent hyperglycemia [27, 54].
The combination of VD and exercise interventions reduced body composition levels, including laboratory measures (weight, body fat, BF%) and anthropometric measures (HC and WC), and the reduction in anthropometric measures was generally greater than that in laboratory measures. In subgroup analyses, the effects of intervention duration ≥ 1 month and < 1 month were statistically significant, and the effects were more pronounced for intervention duration < 1 month. We observed a significant reduction in WC and HC in all subjects compared to the preintervention baseline values. This was mainly associated with a reduction in the level of adipose tissue, which confirms the effect of vegetarianism and exercise [55], with an improvement in metabolic profile consistent with a reduction in body weight, fat mass, and WC. Previous studies have shown that lower upper body fat positioning is associated with a lower incidence of glucose tolerance and IR [56, 57]. The reduction in WC in the absence of changes in weight and body composition is clinically meaningful because WC is inversely associated with the risk of chronic disease [58, 59].
A study [30] showed that in healthy subjects, BW, BMI, FPG, and HOMA-IR levels were significantly lower with a VD combined with exercise intervention compared to a control group on a regular diet and no exercise. In two RCTs of obese individuals, WC, FPG, and HOMA-IR levels were significantly lower in the VD group with the same level of exercise, and the decrease in WC was greater than that in the control group [34]. The results of another RCT [26] showed a greater decrease in FPG levels after the intervention than in the control group. In another study [60] investigating the effects of diet and exercise (alone or in combination) on weight and body composition in obese menopausal women, there was a significant weight loss effect in the diet and moderate-intensity aerobic exercise groups, but the greatest change was observed in the group where the two interventions were used in combination. Together, these studies demonstrate that the combined effect of a VD and exercise is more effective than exercise alone.
Lee [30] suggested that short-term lifestyle programs, including diet and exercise, can improve the physical health and physical fitness of healthy adults, which could explain the heterogeneity that can be delineated by the duration of the intervention. Heterogeneity decreased in all subgroup analyses where the duration of intervention was less than one month. Interestingly, in the subgroup analysis, only the BF% increased and was greater than 50% of the heterogeneity for interventions < 1 month. The rest of the metrics had zero heterogeneity for intervention times < 1 month, while the heterogeneity was large for intervention times ≥ 1 month. In addition, some studies have suggested that vegetarians may not be able to exercise optimally at high intensity because they do not eat meat and therefore have lower creatine concentrations [61]. However, short-term vegetarian diets and aerobic exercise programs have been shown to improve skeletal muscle strength, insulin sensitivity, and blood lipids, as well as aid in weight loss and alter body composition. For example, a previous study reported that a 14-day diet and exercise intervention reduced BMI and insulin sensitivity [62].
The vast majority of the included studies indicated that subjects had lower BW and BMI after the intervention compared to preintervention, and the results were statistically significant. Only two studies [46, 63] demonstrated an increase rather than a decrease in BW after the intervention compared to the preintervention period. The only study of elevated BMI, with a six-day clinical trial led by Ahrens et al. [46], showed substantial improvements in lipid profiles and blood pressure associated with cardiovascular disease, although the changes in BW and BMI were minimal.
The beneficial effects of a VD with aerobic exercise training may be related to several possible mechanisms. Exercise training in individuals with an overweight or obese BMI, including aerobic and moderate intensity training, enhances mitochondrial function, and increases mitochondrial volume and protein turnover (due to impaired protein degradation and new functional protein synthesis), skeletal muscle changes in metabolic enzymes, the capillary to muscle fiber ratio, and insulin sensitivity. In turn, exercise training decreases catabolic mRNA expression, cardiac changes, and catheter artery changes, leading to improved cardiovascular health [64]. PBD may contain low levels of saturated fats (SFAs), mainly from oils such as palm oil and coconut oil [65]. The accumulation of free fatty acid (FFA) intermediates, ceramides, diacylglycerols, and SFAs, especially palmitates, can inhibit insulin signaling in myocytes at the cytosolic level [66]. In addition, excessive palmitate oxidation promotes mitochondrial dysfunction, which reduces ATP synthesis, thereby decreasing the bioavailability of ATP for insulin signaling and increasing oxidative stress [67, 68].
Strengths and limitations
The current meta-analysis has several strengths and limitations. The strengths are that this meta-analysis included a total of 9053 participants, regardless of gender or race and physical health status, with broad coverage, making the study results more reliable.
Some limitations remain in our study. RCTs are considered the gold standard for determining causality findings; However, there are fewer RCTs of vegetarian diets combined with aerobic exercise interventions published in the literature based on our inclusion criteria. Nearly one-third of the included literature was cross-sectional studies. Thus, the study is less informative and does not allow for causal inferences and is susceptible to limitations common to all observational studies, including recommendation bias, self-reported adherence measures, and low screening-to-enrollment rates[47].
Another limitation of this systematic review and meta-analysis is that the studies provide different definitions of different types of VD but do not discuss and analyze each type of VD in separate groups but rather combine data from PBD, low-fat PBD, LOV, and VD for a unified analysis. Since individual studies did not report data separately for men and women, our study did not use gender as a classification criterion but rather combined them for discussion. Therefore, it is uncertain whether the effects of VD combined with exercise interventions on glycemic control, IR, and body composition were the same in men and women. Only a limited number of studies included in the current analysis focused on the effects of vegetarian diets and exercise interventions on insulin and HOMA-IR. In addition, with less than one-third of the studies included in this systematic review and meta-analysis being classified RCTs, the level of evidence-based data is greatly reduced.
What is already known on this subject?
In recent years, a vegetarian and aerobic lifestyle has received increasing interest. Its benefits to the body are numerous, including glycemic control, body composition regulation and IR.
What does this study add?
While the effects of a single VD or single aerobic exercise on body composition have been described, this is the first meta-analysis of the effects of a vegetarian diet combined with aerobic exercise on body composition improvement, which demonstrates that the combined effect of the two is greater than the effect of a large mono-vegetarian diet or single exercise.
Conclusion
In conclusion, our study provides evidence that a VD combined with exercise intervention can be effective in glycemic control, IR, and improving body composition. We suggest that rather than focusing only on what to eat or how to exercise, a more comprehensive community-based lifestyle intervention program should be considered that focuses on promoting a VD and encouraging daily moderate-intensity physical activity. Just as multiple factors interact to affect the health of all individuals, comprehensive lifestyle interventions may improve the health status of the entire population. This has important public health implications for interventions to treat abnormal FPG and IR, to control obesity, and especially for the prevention and management of CVD and diabetes, among others.
Data availability
Published data used for the systematic review and meta-analysis are available from the authors.
References
Ford ES, Zhao G, Tsai J, Li C (2011) Low-risk lifestyle behaviors and all-cause mortality: findings from the national health and nutrition examination survey III mortality study. Am J Public Health 101(10):1922–1929
Ford ES, Croft JB, Posner SF, Goodman RA, Giles WH (2013) Co-occurrence of leading lifestyle-related chronic conditions among adults in the United States, 2002–2009. Prev Chronic Dis 10:E60
Allman-Farinelli MA (2015) Nutrition promotion to prevent obesity in young adults. Healthcare (Basel) 3(3):809–821
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7(5):e330–e341
Kornitzer M, De Backer G, Dramaix M, Kittel F, Thilly C, Graffar M, Vuylsteek K (1983) Belgian heart disease prevention project: incidence and mortality results. Lancet 1(8333):1066–1070
Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW (1983) Social stress and atherosclerosis in normocholesterolemic monkeys. Science 220(4598):733–735
Warner KE, Davis RM, Holbrook JH, Novotny TE, Ockene JK, Rigotti NAJCfCDP (1989) Smoking HPOo, health: reducing the health consequences of smoking: 25 years of progress: a report of the surgeon general: 1989 executive summary
Menzel J, Jabakhanji A, Biemann R, Mai K, Abraham K, Weikert C (2020) Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci Rep 10(1):21736
Orlich MJ, Singh PN, Sabaté J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE (2013) Vegetarian dietary patterns and mortality in Adventist health study 2. JAMA Intern Med 173(13):1230–1238
Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, Estruch R, Casas R (2020) Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients 12(10):2983
Li D (2011) Chemistry behind vegetarianism. J Agric Food Chem 59(3):777–784
Kujala UM, Kaprio J, Sarna S, Koskenvuo M (1998) Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA 279(6):440–444
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine position stand (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359
Barnard RJ, Massey MR, Cherny S, O’Brien LT, Pritikin N (1983) Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of NIDDM patients. Diabetes Care 6(3):268–273
Barnard RJ, Lattimore L, Holly RG, Cherny S, Pritikin N (1982) Response of non-insulin-dependent diabetic patients to an intensive program of diet and exercise. Diabetes Care 5(4):370–374
Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, Gundmi S, Jadhav R (2019) Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med 62(2):98–103
Nery C, Moraes SRAD, Novaes KA, Bezerra MA, Silveira PVDC, Lemos A (2017) Effectiveness of resistance exercise compared to aerobic exercise without insulin therapy in patients with type 2 diabetes mellitus: a meta-analysis. Braz J Phys Ther 21(6):400–415
Marson EC, Delevatti RS, Prado AKG, Netto N, Kruel LFM (2016) Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med 93:211–218
Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ (2001) Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 286(10):1218–1227
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716
Higgins J, Green SR (2011) Cochrane handbook for systematic review of interventions version 5.1.0
Irwig L, Macaskill P, Berry G, Glasziou P (1998) Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ 316(7129):470
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Bhardwaj S, Misra A, Gulati S, Anoop S, Kamal VK, Pandey RM (2017) A randomized controlled trial to evaluate the effects of high protein complete (lActo) VEgetaRian (PACER) diet in non-diabetic obese Asian Indians in North India. Heliyon 3(12):e00472
Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, Skoch A, Hajek M, Hill M, Kahle M et al (2011) Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 28(5):549–559
Kahleova H, Matoulek M, Bratova M, Malinska H, Kazdova L, Hill M, Pelikanova T (2013) Vegetarian diet-induced increase in linoleic acid in serum phospholipids is associated with improved insulin sensitivity in subjects with type 2 diabetes. Nutr Diabetes 3:e75
Klimis H, Thiagalingam A, McIntyre D, Marschner S, Von Huben A, Chow CK (2021) Text messages for primary prevention of cardiovascular disease: the TextMe2 randomized clinical trial. Am Heart J 242:33–44
Lee KS, Lee JK, Yeun YR (2017) Effects of a 10-day intensive health promotion program combining diet and physical activity on body composition, physical fitness, and blood factors of young adults: a randomized pilot study. Med Sci Monit 23:1759–1767
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL et al (1998) Intensive lifestyle changes for reversal of coronary heart disease. JAMA 280(23):2001–2007
Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL (1990) Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet 336(8708):129–133
Toobert DJ, Glasgow RE, Radcliffe JL (2000) Physiologic and related behavioral outcomes from the women’s lifestyle heart trial. Ann Behav Med 22(1):1–9
Tsaban G, Yaskolka Meir A, Rinott E, Zelicha H, Kaplan A, Shalev A, Katz A, Rudich A, Tirosh A, Shelef I et al (2020) The effect of green Mediterranean diet on cardiometabolic risk; a randomised controlled trial. Heart 107(13):1054–1061
Pischke CR, Weidner G, Elliott-Eller M, Scherwitz L, Merritt-Worden TA, Marlin R, Lipsenthal L, Finkel R, Saunders D, McCormac P et al (2006) Comparison of coronary risk factors and quality of life in coronary artery disease patients with versus without diabetes mellitus. Am J Cardiol 97(9):1267–1273
Dod HS, Bhardwaj R, Sajja V, Weidner G, Hobbs GR, Konat GW, Manivannan S, Gharib W, Warden BE, Nanda NC et al (2010) Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol 105(3):362–367
Cairo J, Williams L, Bray L, Goetzke K, Perez AC (2020) Evaluation of a mobile health intervention to improve wellness outcomes for breast cancer survivors. J Patient-Cent Res Rev 7(4):313–322
Koeder C, Kranz R-M, Anand C, Husain S, Alzughayyar D, Schoch N, Hahn A, Englert H (2022) Effect of a 1-year controlled lifestyle intervention on body weight and other risk markers (the healthy lifestyle community programme, cohort 2). Obes Facts 15(2):228–239
Slavícek J, Kittnar O, Fraser GE, Medová E, Konecná J, Zizka R, Dohnalová A, Novák V (2008) Lifestyle decreases risk factors for cardiovascular diseases. Cent Eur J Public Health 16(4):161–164
Chainani-Wu N, Weidner G, Purnell DM, Frenda S, Merritt-Worden T, Pischke C, Campo R, Kemp C, Kersh ES, Ornish D (2011) Changes in emerging cardiac biomarkers after an intensive lifestyle intervention. Am J Cardiol 108(4):498–507
Kent L, Morton D, Rankin P, Ward E, Grant R, Gobble J, Diehl H (2013) The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status - a cohort study. Nutr Metab 10:58
Kent LM, Grant RS, Watts G, Morton DP, Rankin PM, Ward EJ (2018) HDL subfraction changes with a low-fat, plant-based complete health improvement program (CHIP). Asia Pac J Clin Nutr 27(5):1002–1009
Morton D, Rankin P, Kent L, Sokolies R, Dysinger W, Gobble J, Diehl H (2014) The complete health improvement program (CHIP) and reduction of chronic disease risk factors in Canada. Can J Diet Pract Res 75(2):72–77
Suazo EMH, Chagoya LAM, Gutierrez LGF (2021) Improvement on biometrics in individuals undergoing a 10 and 21-day lifestyle intervention in a lifestyle medicine clinic in Mexico. J Lifestyle Med 11(2):66–73
Diehl HA (1998) Coronary risk reduction through intensive community-based lifestyle intervention: the coronary health improvement project (CHIP) experience. Am J Cardiol 82(10B):83T-87T
Ahrens AP, Culpepper T, Saldivar B, Anton S, Stoll S, Handberg EM, Xu K, Pepine C, Triplett EW, Aggarwal M (2021) A six-day, lifestyle-based immersion program mitigates cardiovascular risk factors and induces shifts in gut microbiota, specifically lachnospiraceae, ruminococcaceae, faecalibacterium prausnitzii: a pilot study. Nutrients 13(10):3459
Marshall DA, Walizer EM, Vernalis MN (2009) Achievement of heart health characteristics through participation in an intensive lifestyle change program (coronary artery disease reversal study). J Cardiopulm Rehabil Prev 29(2):84–94
Koertge J, Weidner G, Elliott-Eller M, Scherwitz L, Merritt-Worden TA, Marlin R, Lipsenthal L, Guarneri M, Finkel R, Saunders DE et al (2003) Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the multicenter lifestyle demonstration project. Am J Cardiol 91(11):1316–1322
Pischke CR, Weidner G, Elliott-Eller M, Ornish D (2007) Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the multicenter lifestyle demonstration project. Eur J Heart Fail 9(9):928–934
Null G, Feldman M (1996) Comprehensive lifestyle interventions in the community: a preliminary analysis. Age 19(3):91–100
Świątkiewicz I, Di Somma S, De Fazio L, Mazzilli V, Taub PR (2021) Effectiveness of intensive cardiac rehabilitation in high-risk patients with cardiovascular disease in real-world practice. Nutrients 13(11):3883
Telles S, Naveen VK, Balkrishna A, Kumar S (2010) Short term health impact of a yoga and diet change program on obesity. Med Sci Monit 16(1):CR35–CR40
Zamboni M, Armellini F, Turcato E, Robbi R, Micciolo R, Todesco T, Mandragona R, Angelini G, Bosello O (1996) Effect of altitude on body composition during mountaineering expeditions: interrelationships with changes in dietary habits. Ann Nutr Metab 40(6):315–324
Luo X, Wu J, Jing S, Yan LJ (2016) Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis 7(1):90–110
Tremblay A, Després J-P, Bouchard C (1987) Alteration in body fat and fat distribution with exercise. In: The Québec Meeting on "Fat distribution and metabolic risk factors during growth and later health outcomes"
Bouchard C, Bray GA, Hubbard VS (1990) Basic and clinical aspects of regional fat distribution. Am J Clin Nutr 52(5):946–950
Zamboni M, Armellini F, Turcato E, Todesco T, Bissoli L, Bergamo-Andreis IA, Bosello O (1993) Effect of weight loss on regional body fat distribution in premenopausal women. Am J Clin Nutr 58(1):29–34
Poirier P, Després J-P (2003) Waist circumference, visceral obesity, and cardiovascular risk. J Cardiopulm Rehabil 23(3):161–169
Perez-Martinez P, Alcala-Diaz JF, Delgado-Lista J, Garcia-Rios A, Gomez-Delgado F, Marin-Hinojosa C, Rodriguez-Cantalejo F, Delgado-Casado N, Perez-Caballero AI, Fuentes-Jimenez FJ et al (2014) Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur J Clin Invest 44(11):1053–1064
Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang C-Y, Blackburn GL, McTiernan A (2012) Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 20(8):1628–1638
Shomrat A, Weinstein Y, Katz A (2000) Effect of creatine feeding on maximal exercise performance in vegetarians. Eur J Appl Physiol 82(4):321–325
Izadpanah A, Barnard RJ, Almeda AJE, Baldwin GC, Bridges SA, Shellman ER, Burant CF, Roberts CK (2012) A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. Am J Physiol Endocrinol Metab 303(4):E542–E550
Wells AM, Haub MD, Fluckey J, Williams DK, Chernoff R, Campbell WW (2003) Comparisons of vegetarian and beef-containing diets on hematological indexes and iron stores during a period of resistive training in older men. J Am Diet Assoc 103(5):594–601
Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ (2019) Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag 15:89
Chen Z, Zuurmond MG, van der Schaft N, Nano J, Wijnhoven HAH, Ikram MA, Franco OH, Voortman T (2018) Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam study. Eur J Epidemiol 33(9):883–893
Nolan CJ, Larter CZ (2009) Lipotoxicity: why do saturated fatty acids cause and monounsaturates protect against it? J Gastroenterol Hepatol 24(5):703–706
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7(1):45–56
Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, Chang C-R, Tsai Y-S (2012) Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32(2):309–319
Funding
Mao-yuan Wang was supported by the fund for Less Developed Regions of the National Science Foundation of China (grant no. 82060420).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of this systematic review. JM-Y and HY performed the literature search, screening, data extraction, and quality check of the included articles. JH-Z and HY-X provided additional assistance to complete the quality check of the articles and compile the results. XT provided advice on the implementation of the meta-analysis model. YL wrote the paper. The remaining authors including YB-Z assisted in interpreting the data and editing the final manuscript. MY-W did the quality control.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Ethical approval
This study does not involve human or animal studies, and no ethical application is applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Long, Y., Ye, H., Yang, J. et al. Effects of a vegetarian diet combined with aerobic exercise on glycemic control, insulin resistance, and body composition: a systematic review and meta-analysis. Eat Weight Disord 28, 9 (2023). https://doi.org/10.1007/s40519-023-01536-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-023-01536-5