Abstract
Purpose
Eating disturbances are complex heritable conditions that can be influenced by both genetic and environmental factors but are poorly studied in early development. The aim of this research was to investigate the association of genetic polymorphisms within dopaminergic pathways with early feeding problems.
Methods
We analyzed the presence of VNTR polymorphisms of DRD4 (rs1805186) and DAT1 (rs28363170) in overeating (N = 45), undereating (N = 48) and control (N = 44) young children. We also assessed presence of externalizing, internalizing and dysregulation symptoms by the Child Behavior Checklist and quality of mother–child interactions during feeding by the Italian adaptation of the Scale for the Assessment of Feeding Interaction, respectively.
Results
Both polymorphisms were associated with children’s eating behavior, psychological symptoms and quality of interaction with their mothers, suggesting that: (a) the DRD4 4-repeat allele behaves as a protective factor, the 2-repeats and 7-repeats alleles as risk factors, for undereating behavior, the general quality of mother–child interaction and internalizing, externalizing and dysregulated symptoms; and (b) the DAT1 9-repeats allele behaves as a protective factor, the 10-repeats allele as a risk factor, for overeating behavior, the general quality of mother–child interaction, internalizing, externalizing and dysregulated symptoms. Finally, a gene x gene interaction is suggested between the DAT1 9-repeat or 10-repeat allele and the DRD4 4-repeat allele.
Conclusions
Our results suggest a role for DRD4 and DAT1 in an early susceptibility to eating disturbances.
Level of evidence III
Evidence obtained from well-designed case–control analytic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eating disturbances (EDs) in early childhood affect approximately 25% of children with typical development and 80% of children with developmental disorders [1, 2]. They may resolve spontaneously during development or produce more structured and severe problems for which late interventions would harm their outcome [3]. These difficulties are studied by focusing on both the child who, independently from food availability, feeds inadequately [undereating (UE)] or excessively [overeating (OE)] [4], and possible early-arising maladaptive aspects of the parent–child relationship with associated low-quality interactions during feeding and play [5, 6]. During feeding, a negative maternal affective state and interactions characterized by conflictual, non-collaborative, and non-empathetic communication, may contribute to children’s food refusal and to a negative emotional climate. Maternal intrusiveness or withdrawal in interactive exchanges with the child may depend on a lack of pleasure in reciprocal interactions [7], probably produced by an impairment (s) in the neurobiological circuits of reward [8]. Conversely, parents’ attention and sensitivity to the needs of the child, including the recognition of cues of hunger and satiety from breastfeeding and weaning, appear as key factors in the prevention of early EDs [9,10,11]. Observational procedures for the assessment of the quality of mother–child interactions during feeding such as the Scale for the Assessment of Feeding Interaction [12] have been used to evaluate at-risk patterns of caregiver–children’s interactions associated with children’s low ability to regulate affects and behavior, frequently correlated with EDs.
A genetic influence on EDs has been established as well [13, 14]. Maladaptive relational indicators may have a genetic basis, may emerge early during the first year of life and constitute a very significant risk factor for the appearance of children’s disordered eating [15]. Genetic studies, supported by the identification of neurotransmitters, hormones and peptides possibly regulating eating behavior and involved in the pathophysiology of EDs, have been investigated, among others, genes encoding factors responsible for dopamine (DA) dynamics, including receptors, membrane transporters and metabolizing enzymes [16,17,18], due to DA activity in subcortical and hypothalamic circuits controlling brain reward systems, appetite and satiety pathways [19]. Altered functioning of DA circuitry is involved in psychopathology, including abnormal feeding [20], and may be associated with the dysregulation of adaptive emotions and to internalized states of hypervigilance, withdrawal and inhibition, depression, anxiety and attachment insecurity [21].
Despite the large amount of information gathered on the genetics of major EDs in adults, data on young children are scarce or completely lacking. Indeed, studies on genetic variables associated with children’s EDs would be particularly interesting in Developmental Psychology, allowing the proposal of paradigm (s) intertwining psychological and biological variables.
In an initial attempt to close this gap, we have investigated the possible genetic and psychopathological correlates of EDs in young Italian children with diagnoses of overeating or undereating or with normal feeding behavior.
Genetic analysis was focused on variable number of tandem repeats (VNTR) polymorphisms of genes encoding for the dopamine D4 receptor (DRD4) and dopamine transporter (DAT1). Among DA receptors, DRD4 appears involved in EDs in a complex manner, being expressed in neurons of the prefrontal cortex and basal ganglia, including the striatum and nucleus accumbens, the limbic system and the thalamus [22,23,24]. DRD4 maps on chromosome 11p15.5 and encodes for a G protein-coupled receptor (GPCR) that inhibits activity of the enzyme adenylyl-cyclase down-regulating neuronal activity. A reduced expression of DRD4 is strongly associated with the risk for Anorexia Nervosa (AN) [25]. Interestingly, a 48 bp VNTR polymorphism (rs1805186) in exon 3 of this gene produces various changes in the length of the receptor protein: among known alleles, the 4-repeat-containing (4R) one produces more efficient receptor proteins and neuronal inhibition than other alleles, such as the 2-repeat (2R) and the 7-repeat (7R) ones, with behavioral consequences that include AN [26, 27] and binge eating disorder (BED) [28].
Together with the enzyme catechol-O-methyltransferase (COMT), the presynaptic transmembrane DA transporter (DAT) represents the neuronal factor responsible for DA deactivation after its synaptic release. DAT is expressed predominantly by neurons located in subcortical structures, including the limbic system and basal ganglia [29], and is, therefore, involved in the regulation of motivated behavior, including feeding. DAT is encoded by the DAT1 gene (SLC6A3) mapped on chromosome 5p15.3. DAT1 has been associated with bulimia nervosa (BN) [30], alcohol withdrawal and dependence [31, 32], and also shown to modify the risk for AN and modulate associated psychopathological features [27]. A 40 bp VNTR polymorphism (rs28363170) is present in the 3′-untranslated region (3′UTR), with most common alleles containing 9- (9R) or 10-repeats (10R) [33]. Although a clear effect of these alleles has not been assessed [34, 35], they appear to produce reduced [36] and increased DAT expression [37, 38], respectively.
Since DA availability in subcortical synapses depending on DAT1 activity may influence DRD4 responses, similarly to other studies carried out on inhibition/impulsivity [39] or in hyperactivity [40], we investigated the possible interaction between the two gene polymorphisms for UE and OE behaviors.
Genetic aspects were studied in association with behavioral features, which were investigated by the Italian adaptation of the Scale for the Assessment of Feeding Interaction (Scala di Valutazione delle Interazioni Alimentari, SVIA) [41] and the Child Behavior Checklist (CBCL) questionnaire [42]. While the SVIA assesses symptoms of feeding-related dysregulation, the CBCL analyzes the presence of externalizing, internalizing and dysregulation symptoms in affective, behavioral and cognitive areas.
Materials and methods
Sample
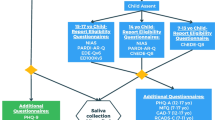
This study, consistent with the Declaration of Helsinki and authorized by the Sapienza Ethical Committee (n. 809/2020), involved 224 3-year-old children and their mothers recruited in Clinical Nutrition Services of pediatric Italian hospitals [N = 68 undereating and N = 76 overeating] and kindergartens [N = 80 normal controls]. Children were matched for age, gender and socio-demographic characteristics of their family. Undereating and Overeating diagnoses were made by pediatricians, based on DC: 0–5 classification criteria [43]. Inclusion criteria for children and mothers were no referred comorbidity with physical or mental disorders, no previous medical or psychological treatment. Eighty-seven duos were excluded due to children’s medical conditions (N = 10) and/or comorbidity (N = 10 with sensory processing disorder; N = 8 with sleep disorder; N = 14 with mood disorder; N = 9 with obsessive compulsive disorder), or mothers’ medical conditions (N = 24), as well as psychiatric diagnoses (N = 2 with bipolar disorder; N = 10 with anxiety disorder). The final overall sample was composed of 137 children-mother duos, divided according to children’s diagnoses in N = 48 UE; N = 45 OE; N = 44 controls. Mothers were all Caucasian and the biological parent of the children.
All mothers signed the written informed consent and received information about the aims, measures and procedures of the study in preliminary meetings.
Psychological/behavioral assessments
Mothers helped caregivers completing the Child Behavior Check List (CBCL) 1.5–5 [44], a questionnaire assessing children’s abilities and specific behavior/emotions according to eight subscales (anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior) and two global scales: internalizing problems (with anxious/depressed, withdrawn/depressed, and somatic complaints subscales) and externalizing problems (with rule-breaking behavior and aggressive behavior subscales). The dysregulated profile (DP) (aggregating anxious/depressed, aggressive behavior and attention problems subscales) was also assessed [42].
Specifically trained psychologists evaluated the quality of mother–child interaction during feeding by the Italian adaptation [41] of the Scale for the Assessment of Feeding Interaction [12] (SVIA). The SVIA can be applied to children 1-to-36 months of age and identifies normal and/or risky parent–child interactions after recording for at least 20 min during feeding, coding and evaluated according to 41 items. These are distributed among four subscales, measured on a Likert scale ranging from 0 (none) to 4 (a lot): (a) parent’s affective state (parents’ happiness or sadness/distress during feeding, higher scores referring to greater difficulties in showing positive affection and to more frequent signs of negative affection); (b) interactive conflict (conflictual, non-collaborative and non-empathetic interaction, higher scores referring to parents following their own emotions and intentions rather than children’s signals); (c) food refusal behavior (children’s refusal to open their mouths, being easily distracted and showing opposition or negativity); and (d) Dyad’s affective state (i.e., parents’ and children’s contribution to joy or sadness during feeding, higher scores referring to growing difficulties of caregivers in supporting children’s autonomous initiatives and/or distress and opposition). The inter-evaluator agreement for SVIA items for this sample is excellent (Pearson r = 0.9). Moreover, the instrument has good reliability in terms of internal consistency, Cronbach’s α = 0.94. The means of the four subscales were also used to derive a total one-dimensional score according to Fadda and Lucarelli (2017) [45], equal to the sum of the four scores [12]. Lucarelli et al. (2002) [41] indicated a cutoff of 54 (2 SDs from the M) to discriminate clinical scores.
Biological sampling for DNA isolation
Participants’ buccal cell samples were collected by trained personnel through buccal swabs and chilled on ice at 4 °C. DNA was extracted using the Buccal-Prep Plus DNA isolation kit (Isohelix, Cell Projects Ltd., U.K.) according to the manufacturer’s instructions.
Genotyping
DRD4 rs1805186 genotypes were obtained by PCR amplification using primers with the following sequences: 5′-GCGACTACGTGGTCTACTCG-3′ (DRD4 Forward), 5′-AGGACCCTCATGGCCTTG-3′ (DRD4 Reverse). Reactions were carried out in a final volume of 50 μl with 50 ng DNA, 1.5 mM MgCl2, 200 μM dNTPs, 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 0.25 μM primers, 5% DMSO and 1 U Taq DNA polymerase (Promega Corporation Madison WI, USA). An initial denaturation at 95 °C for 3 min was followed by 30 cycles at 94 °C for 45 s, 54 °C for 40 s and 72 °C for 40 s.
DAT1 rs28363170 genotypes were also obtained by PCR amplification using primers with the following sequences: 5′-TGTGGTGTAGGGAACGGCCTGAG-3′ (DAT1 Forward), 5′-CTTCCTGGAGGTCACGGCTCAAGG-3′ (DAT1-Reverse). Reactions were carried out in a final volume of 20 μl with 50 ng genomic DNA, 1.5 mM MgCl2, 200 μM dNTPs, 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 0.25 μM primers and 1 U Taq DNA polymerase. An initial denaturation at 95 °C for 3 min was followed by 30 cycles at 94 °C for 45 s, 57 °C for 30 s and 72 °C for 30 s.
For both polymorphisms, samples were resolved by 3% agarose gel electrophoresis after staining with Syber Safe (Invitrogen Corporation, Waltham MA, USA) and matching to the 100 bp Ladder DNA molecular weight standard (Promega Corporation Madison WI, USA). Genotyping procedures were carried out at least twice for each DNA sample.
Where not stated otherwise, chemicals were purchased from Sigma-Aldrich Co. (St. Louis MO, USA).
Statistical analysis
Genotype and allele frequencies of DRD4 and DAT1 polymorphisms in EU/OE and control subjects were compared by χ2 test.
The association of the 2R, 4R and 7R DRD4 genotypes and 9R and 10R DAT1 genotypes with UE and OE and their interaction were further investigated by χ2 test comparison with the control sample after grouping into three categories: +/+ (homozygous carriers), +/− (heterozygous carriers) or −/− (non-carriers).
The effect of specific genotypes was evaluated by calculation of the odds ratios (OR) with the 95% confidence interval (CI) using the Wilson method [46].
Finally, the scores of the three groups or whole sample on all measures were compared by analyses of variance (ANOVAs). All variables conformed to the assumptions underlying such analyses, including normality of distributions.
A power analysis was conducted according to Cohen’s (2013) [47] suggestions; α was set at 0.05 and a power of 0.861 was obtained with a large effect size of (f2 = 0.45). Sample sizes were calculated according to the cross sectional studies formula of Pourhoseingholi et al. (2013) [48].
All data were analyzed using IBM SPSS statistics, Version 25 (IBM, Armonk, NY, USA).
Results
Study sample
Control, UE and OE groups were balanced, overall features being: children’s gender: 69 males, 68 females; children’s and mothers’ ages: 34.8 ± 1.21 months and 33.6 ± 3.4 years, respectively; household income: ≥ 2500 euros, N = 120, ≥ 2000 and < 2500 euros, N = 17; maternal education, ≥ 13 school years N = 123, 12 school years, N = 14. Table 1 shows the sample characteristics stratified for sub-groups (Control, UE, and OE).
DRD4 and DAT1 allele frequencies in control, UE and OE groups
DRD4 and DAT1 allele frequencies in the three groups are reported in Table 2.
In the control group, 4R was the most frequent allele for the DRD4 polymorphism (81.8%). In the UE group, 2R, 4R and 7R alleles had similar frequencies of 26.0%, 30.2% and 28.1%, respectively. In the OE group, 4R and 2R alleles displayed frequencies of 62.2% and 18.9%, respectively. Differences in allele frequencies between UE and control subjects were observed for the 2R, 4R and 7R alleles (p < 0.01). OR evaluation suggests that presence of 2R and 7R alleles represents a risk factor for UE (p = 0.004 and p = 0.0006, respectively), while that of the 4R allele is a protective factor (p = 0.0001). A difference in allele frequency between OE and control subjects was observed only for the 4R allele (p < 0.01), also displaying a protective effect (p = 0.004). In the control group, 9R was the most represented allele for the DAT1 polymorphism (79.5%). In the UE group, 9R and 10R alleles displayed the highest frequencies (63.5% and 27.1%, respectively). In the OE group, the 9R allele was present in 27.8% of the subjects, while the 10R allele displayed the highest frequency (56.7%). Differences in allele frequencies between UE and control subjects were observed for both 9R and 10R alleles (p < 0.05 and p < 0.0001, respectively). OR evaluation suggests that while presence of the 9R allele represents a protective factor for UE (p = 0.018), that of the 10R allele is a risk factor (p = 0.001). A difference in allele frequency between OE and control subjects was also observed for 9R and 10R alleles (p < 0.00001), suggesting that the former represents a protective factor (p = 0.0001), the latter a risk factor (p = 0.0001).
Genotype frequencies of DRD4 alleles and DAT1 alleles in control, UE and OE groups
We investigated genotype distributions of most frequent alleles, namely 2R, 4R and 7R for DRD4, 9R and 10R for DAT1 in the three groups (Table 3).
For DRD4: (i) genotypes containing the 2R allele and 7R allele were more frequent in UE than control subjects (p < 0.01 and p < 0.00001, respectively); and (ii) genotypes containing the 4R allele were less frequent in UE/OE than control subjects (UE, p < 0.00001; OE, p < 0.01).
Regarding DAT1: (i) genotypes containing the 9R allele were less frequent in UE/OE than control subjects (UE, p < 0.05; OE, p < 0.00001); and (ii) genotypes containing the 10R allele were more frequent in UE/OE than control subjects (UE, p < 0.05; OE, p < 0.00001).
Dominant and recessive genotype effects of 4R DRD4, 9R and 10R DAT1 alleles in control, UE and OE groups
To dissect the effect of each allele, we analyzed genotype distributions according to dominant or recessive models in the three groups (Table 4).
While no additional information was found for 2R and 7R DRD4 alleles, the 4R DRD4 allele was associated with UE according to both the dominant (p < 0.0005) and the recessive model (p < 0.00001), suggesting a role as a protective factor (p = 0.0005 and p < 0.00001, respectively). The 4R allele was also associated with OE according to the recessive model (p < 0.001) as a putative protective factor (p = 0.001). The 9R DAT1 allele was associated with OE according to the dominant and the recessive model (p < 0.00001 for both) as a putative protective factor (p < 0.001 for both). The 10R DAT1 allele was associated with UE and OE according to both genetic models (see Table 3 for significance levels) as a putative risk factor.
Interaction of DRD4 and DAT1 genotypes in control, UE and OE groups
We finally investigated possible gene × gene interactions between DRD4 2R, 4R, 7R alleles and DAT1 9R, 10R alleles. Since receptor activity may depend on synaptic DA availability and, therefore, on DAT1 activity, we evaluated the distributions of DRD4 genotypes in the three groups, sorted according to the dominant and recessive models, in the presence or absence of DAT1 genotypes in the dominant/recessive models as well (Table 5).
No interaction was observed for 2R and 7R DRD4 alleles (not shown). On the contrary, presence of the DAT1 9R allele, in both dominant and recessive models, displayed interactions with recessive model-DRD4 4R allele on OE (p < 0.00001 for both). In the presence of the DRD4 4R allele, non-carriers (d −) and heterozygous carriers (r −) of the DAT1 9R allele were more frequent in OE subjects.
Presence of the 10R allele also displayed an interaction with dominant model-DRD4 4R allele on UE (p = 0.00002). Heterozygous carriers (r −) of the DAT1 10R allele were more frequent in control subjects carrying one or two copies of the DRD4 4R allele and more frequent in non-carrier UE subjects. Finally, presence of the DAT1 10R allele displayed interactions with recessive model-DRD4 4R allele on OE (p < 0.00001 for both dominant and recessive DAT1 10R models), being more frequent in OE subjects than controls.
Quality of mother–child interaction in control, UE and OE groups
Mother–child interactions during feeding were inspected by the SVIA and the CBCL scale (Table 6).
Differences between groups were assessed by one-way ANOVA. In contrast to control dyads, UE and OE dyads exceeded the clinical SVIA cutoff, thus being at risk for the quality of their interactions during feeding. Moreover, UE dyads presented more maladaptive SVIA scores than the other groups. As for CBCL internalizing, externalizing and dysregulation problems, UE and OE children showed significantly higher scores than control children, with UE children displaying the highest scores.
Quality of mother–child interaction relative to DRD4 and DAT1 genotypes in the whole sample
Specific DRD4 and DAT1 genotype effects on SVIA and CBCL scores, regardless of their involvement in UE and OE, were evaluated in the whole sample by one-way ANOVA (Table 7).
In line with observations on effects of various alleles on UE and OE groups, subjects carrying the DRD4 2R and 7R alleles displayed values that exceeded the clinical cutoffs of the SVIA and CBCL scores when compared to 4R homozygotes, who instead displayed scores below the clinical significance. As for DAT1 genotypes, 9R homozygotes showed the lowest scores for the SVIA and CBCL scores when compared to 9R heterozygotes and 10R allele-containing genotypes.
Discussion
The present results suggest that DRD4 and DAT1 genes: (i) have a role in abnormal feeding in young children; (ii) affect the general quality and specific aspects of mother–child interaction during feeding; (iii) influence each other, allowing to confirm a proposed functional mechanism that regulates DA dynamics underlying specific phenotypic effects. As such, they extend observations already reported for these genes on ADHD [40, 49] and impulsivity [39].
Effects of DRD4 alleles on UE and OE behavior
The DRD4 7R allele was associated with UE behavior as a putative risk factor, in line with the hypothesis that DA dysfunction is among neuronal mechanisms that increase susceptibility to AN in adolescents and adults [26, 27]. The 2R and 4R alleles appeared to affect eating behavior as well, the former being more frequent in UE children, the latter less frequent in UE and OE children.
DRD4 is a postsynaptic GPCR that lowers the amount of endogenous cAMP, producing neuronal repression. Since alleles with 7 or more repeats have reduced expression when compared with shorter ones [25, 50], in the presence of the 7R allele both in homozygous and heterozygous carriers, neurons may be not efficiently inhibited by DA release. This would represent the possible functional effect of this allele, as a risk factor, on the UE phenotype. According to Volkow et al. (2004) [51], a decrease in DA response is central to attentional impairment in ADHD and altered neuronal repression in 7R allele carriers is among the causes of hyperactivity [52, 53]. We favor this functional hypothesis on impaired neuronal inhibition produced by the 7R DRD4 allele and suggest that the resulting increased activity of DA neurons is also among the neurobiological causes of reduced feeding in small children, via an attentional impairment and easy distractibility.
Presence of the 2R allele also appears to behave as a moderate risk factor for UE, in agreement with the observation that the 2R and 7R alleles provide reduced efficiency in signal transduction and neuronal inhibition when compared to the 4R allele [54]. Additional investigation on this allele is warranted to better understand its functional effect.
Conversely, the 4R allele is strongly suggested to represent a protective factor for both UE and OE. Interestingly, this allele appears to represent a case of incomplete dominance, its effects being stronger in homozygotes then in heterozygotes.
Effects of DAT1 alleles on UE and OE behavior
Our analyses suggest that both 9R and 10R alleles of DAT1 have a role in early EDs, the former as a protective factor, the latter as a risk factor. DAT1 has been associated with attention, since amounts of synaptic DA appear to influence neuronal inhibition necessary to reach a “normal” attentional phenotype [55], regardless of the efficiency of receptors. Due to its reduced expression [36], the DAT1 9R allele, by keeping synaptic DA levels high, may affect attention positively and this may contribute to normal feeding. On the contrary, the DAT1 10R allele, producing increased DA reuptake [37, 38], may further compromise the response of postsynaptic neurons, thus representing a risk factor for EDs. Interestingly, both DAT1 alleles affect OE. Since striatal DA pathways may be involved in altered neural bases of reward process in EDs, DAT1 10R allele carriers, due to reduced amounts of synaptic DA, would be more sensitive to reward reinforcement of food. DAT1 10R carriers with low-quality mother/child feeding interactions (see Table 7) might therefore consume higher amounts of food to regulate DA availability and reward, leading to overeating. Anyhow, the functional mechanism for these effects needs further investigation.
Interaction of DRD4 and DAT1 alleles on UE and OE behavior
The DRD4 4R allele appeared to interact with DAT1 9R and 10R alleles, supporting previous observations on DA mechanisms in the pathogenesis of psychopathology [56]. Present observations reinforce the role of DRD4 [40, 57, 58] and DAT1 [59] in psychopathology and further support the idea that activity of DRD4 is influenced by DAT-dependent synaptic DA availability, with visible behavioral effects.
Quality of mother–child interaction and effects of DRD4 and DAT1 alleles
Consistent with previous literature that demonstrates how the low quality of parent–child exchanges during feeding is closely associated with child’s disordered eating [60], SVIA and CBCL scores showed that dyads with undereating and overeating children have maladaptive and clinically at-risk interactions during feeding. Noteworthy, dyads with undereating children display more maladaptive SVIA scores than the other groups, also confirms that undereating in offspring can be associated with severe impairment in the relationship with parents, particularly with the mother [61]. Moreover, UE and OE children showed significantly higher internalizing, externalizing and dysregulation symptoms than control children, UE children having the highest scores. It is recognized that children with eating disturbances have higher risk for psychopathology, or other psychopathological symptoms in comorbidity [61, 62]. Our results extend comorbidity to symptoms of dysregulation and strongly suggest that UE children have more severe relational problems than OE children.
Association of DRD4 2R and 7R alleles with higher scores in both the SVIA and CBCL scales suggests that they also represent a risk factor for these relational aspects. On the contrary, association of the DRD4 4R allele with lower scores in both the SVIA and CBCL scales suggests a protective role for this allele. In agreement with results on undereating or overeating behavior, the DAT1 9R allele appears to be associated with lower SVIA and CBCL scores, suggesting a protective role, while the DAT1 10R allele is associated with higher scores and is a putative risk factor.
In sum, our study presents associations between variants of the DRD4 and DAT1 genes, children’s eating behavior and mother–child interactions. Although no causal conclusions can be drawn, we would hypothesize that DRD4 and DAT1 have a role in children’s neuronal responses necessary for a normal relationship with their mothers during feeding and on their eating behavior.
Strength and limits
Although not performed on a large sample, this study has two major strengths: (a) all enrolled children had a clinical diagnosis performed by pediatricians, based on the DC:0–5 Classification criteria; (b) mother–child interactions during feeding were objectively investigated by the SVIA and analyzed by clinicians; (c) the consistent direction in the association of the genetic data with different behavioral features, all representing markers of psychopathology, makes the possibility that they are due to spurious correlations highly unlikely.
What is already known on this subject?
While a growing body of information is now available on the genetics of major EDs in adults, data on young children are still missing. Studies on genetic and psychological variables associated with abnormal behavior in infancy would represent new tools for Developmental Psychologists, considering their interest in children’s EDs.
What this study adds?
This study provides a first evidence of a role for DRD4 and DAT1 genetic polymorphisms, in addition to specific psychological variables, in abnormal eating behavior in the first years of life, with a potential strong neurobiological and clinical impact in early psychopathology.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Manikam R, Perman JA (2000) Pediatric feeding disorders. J Clin Gastroenterol 30:34–46. https://doi.org/10.1097/00004836-200001000-00007
Bryant-Waugh R (2013) Feeding and eating disorders in children. Curr Opin Psychiatry 26:537–542. https://doi.org/10.1016/j.psc.2018.10.005
Bahr D, Johanson NA (2013) A family-centered approach to feeding disorders in children (Birth to 5-Years). Perspect Swallowing Swallowing Disord (Dysphagia) 22(4):161–171
Kedesdy JH, Budd KS (1998) Childhood feeding disorders: Biobehavioral assessment and intervention. Paul H Brookes Publishing, Baltimore
Murray L, Halligan SL, Goodyer I, Herber J (2010) Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: a preliminary study. J Affect Disord 122(3):218–223. https://doi.org/10.1016/j.jad.2009.06.034
Tarbell MC, Allaire JH (2002) Children with feeding tube dependency: treating the whole child. Infants Young Child 15(1):29–41
Stanley C, Murray L, Stein A (2004) The effect of postnatal depression on mother-infant interaction, infant response to the still-face perturbation, and performance on an instrumental learning task. Dev Psychopathol 16:1–18. https://doi.org/10.1017/s0954579404044384
Leckman JF (2011) Variations in maternal behavior–oxytocin and reward pathways–peripheral measures matter? Neuropsychopharmacol 36(13):2587–2588. https://doi.org/10.1038/npp.2011.201
Di Santis KI, Hodges EA, Johnson SL, Fisher JO (2011) The role of responsive feeding in overweight during infancy and toddlerhood: a systematic review. Int J Obes 35(4):480–492. https://doi.org/10.1038/ijo.2011.3
Thompson LA, Zhang S, Black E, Das R, Ryngaert M, Sullivan S, Roth J (2013) The association of maternal pre-pregnancy body mass index with breastfeeding initiation. Matern Child Health J 17(10):1842–1851. https://doi.org/10.1007/s10995-012-1204-7
Sadeh-Sharvit S, Zubery E, Mankovski E, Steiner E, Lock JD (2016) Parent-based prevention program for the children of mothers with eating disorders: feasibility and preliminary outcomes. Eat Disord 24(4):312–325. https://doi.org/10.1080/10640266.2016.1153400
Chatoor I, Hirsch R, Ganiban J, Persinger M, Hamburger E (1998) Diagnosing infantile anorexia: the observation of mother-infant interactions. J Am Acad Child Adolesc Psichiatry 37:959–967. https://doi.org/10.1097/00004583-199809000-00016
Berrettini W (2004) The genetics of eating disorders. Psychiatry (Edgmont) 1(3):18–25
Watson HJ, Yilmaz Z, Thornton LM et al (2019) Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 51:1207–1214. https://doi.org/10.1038/s41588-019-0439-2
Lampard AM, Franckle RL, Davison KK (2014) Maternal depression and childhood obesity: a systematic review. Prev Med 59:60–67. https://doi.org/10.1016/j.ypmed.2013.11.020
Bello NT, Hajnal A (2010) Dopamine and binge eating behaviors. Pharmacol Biochem Behav 97:25–33. https://doi.org/10.1016/j.pbb.2010.04.016
Broft AI, Berner LA, Martinez D, Walsh BT (2011) Bulimia nervosa and evidence for striatal dopamine dysregulation: a conceptual review. Physiol Behav 104:122–127. https://doi.org/10.1016/j.physbeh.2011.04.028
Sodersten P, Bergh C, Leon M, Zandian M (2016) Dopamine and anorexia nervosa. Neurosci Biobehav Rev 60:26–30. https://doi.org/10.1016/j.neubiorev.2015.11.003
Kontis D, Theochari E (2012) Dopamine in anorexia nervosa: a systematic review. Behav Pharmacol 23:496–515. https://doi.org/10.1097/FBP.0b013e328357e115
Rask-Andersen M, Olszewski PK, Levine AS, Schioth HB (2010) Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev 62:147–164. https://doi.org/10.1016/j.brainresrev.2009.10.007
Geeraerts SG, Deutz MH, Dekovic M, Bunte T, Schoemaker K, Espy KA, Prinzie P, van Baar A, Matthys W (2015) The child behavior checklist dysregulation profile in preschool children: a broad dysregulation syndrome. J Am Acad Child Adolesc Psychiatry 54:595–602. https://doi.org/10.1016/j.jaac.2015.04.012
Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS (1996) Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381:245–248. https://doi.org/10.1038/381245a0
Ariano MA, Larson ER, Noblett KL, Sibley DR, Levine MS (1997) Coexpression of striatal dopamine receptor subtypes and excitatory amino acid subunits. Synapse 26:400–414. https://doi.org/10.1002/(SICI)1098-2396(199708)26:4%3c400::AID-SYN8%3e3.0.CO;2-A
Tarazi FI, Zhang K, Baldessarini RJ (2004) Dopamine D4 receptors: beyond schizophrenia. J Recept Signal Transduct Res 24:131–147. https://doi.org/10.1081/rrs-200032076
Schoots O, Van Tol HH (2003) The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics J 3:343–348. https://doi.org/10.1038/sj.tpj.6500208
Bachner-Melman R, Lerer E, Zohar AH, Kremer I, Elizur Y, Nemanov L, Golan M, Blank S, Gritsenko I, Ebstein RP (2007) Anorexia nervosa, perfectionism, and dopamine D4 receptor (DRD4). Am J Med Genet B Neuropsychiatr Genet 144B(6):748–756. https://doi.org/10.1002/ajmg.b.30505
Gervasini G, Gordillo I, García-Herráiz A, Flores I, Jiménez M, Monge M, Carrillo JA (2013) Influence of dopamine polymorphisms on the risk for anorexia nervosa and associated psychopathological features. J Clin Psychopharmacol 33(4):551–555. https://doi.org/10.1097/JCP.0b013e3182970469
Steiger H, Thaler L, Gauvin L, Joober R, Labbe A, Israel M, Kucer A (2016) Epistatic interactions involving DRD2, DRD4, and COMT polymorphisms and risk of substance abuse in women with binge purge eating disturbances. J Psychiatr Res 77:8–14. https://doi.org/10.1016/j.jpsychires.2016.02.011
Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AJ (1999) Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 409:38–56. https://doi.org/10.1002/(sici)1096-9861(19990621)409:1%3c38::aid-cne4%3e3.0.co;2-1
Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, Ono Y, Kanba S (2004) Eating disorders with binge-eating behavior are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci 29:134–137
Köhnke MD, Batra AK, Kohnke AM, Lutz U, Schick S, Gaertner I (2005) Association of the dopamine transporter gene with alcoholism. Alcohol Alcohol 40:339–342. https://doi.org/10.1093/alcalc/agh179
Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jablonski M, Rommelspacher H, Samochowiec A, Sznabowich M, Horodnicki J, Sagan L, Pelka-Wysiecka J (2006) Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neurosci Lett 410:1–5. https://doi.org/10.1016/j.neulet.2006.05.005
Sano A, Kondoh K, Kakimoto Y, Kondo I (1993) A 40-nucleotide repeat polymorphism in the human dopamine transporter gene. Hum Genet 91:405–406. https://doi.org/10.1007/BF00217369
Costa A, Riedel M, Müller U, Möller HJ, Ettinger U (2011) Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse 65:998–1005. https://doi.org/10.1002/syn.20927
Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J (2014) Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol Psychiatry 19:880–889. https://doi.org/10.1038/mp.2013.126
Heinz A, Goldman D, Jones DW, Plamour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR (2000) Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacol 22:133–139. https://doi.org/10.1016/S0893-133X(99)00099-8
Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S (2001) The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 1:152–156. https://doi.org/10.1038/sj.tpj.6500026
Felten A, Montag C, Markett S, Walter NT, Reuter M (2011) Genetically determined dopamine availability predicts disposition for depression. Brain Behav 1:109–118. https://doi.org/10.1002/brb3.20
Congdon E, Lesch KP, Canli T (2008) Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet 147B(1):27–32. https://doi.org/10.1002/ajmg.b.30557
Carrasco X, Rothhammer P, Moraga M, Henríquez H, Chakraborty R, Aboitiz F, Rothhammer F (2006) Genotypic interaction between DRD4 and DAT1 loci is a high risk factor for attention deficit/hyperactivity disorder in Chilean families. Am J Med Genet B Neuropsychiatr Genet 141B(1):51–54. https://doi.org/10.1002/ajmg.b.30259
Lucarelli L, Cimino S, Perucchini P, Speranza AM, Ammaniti M, Ercolani AP (2002) I disturbi alimentari nella prima infanzia: Validazione di uno strumento osservativo dell’interazione madre-bambino. Infanzia E Adolescenza 2:113–124
Kim J, Carlson GA, Meyer SE, Bufferd SJ, Dougherty LR, Dyson MW, Laptook RS, Olino TM, Klein DN (2012) Correlates of the CBCL-dysregulation profile in preschool-aged children. J Child Psychol Psychiatry 53(9):918–926. https://doi.org/10.1111/j.1469-7610.2012.02546.x
Zeanah CH, Lieberman A (2016) Defining relational pathology in early childhood: The diagnostic classification of mental health and developmental disorders of infancy and early childhood DC: 0–5 approach. Infant Ment Health J 37(5):509–520. https://doi.org/10.1002/imhj.21590
Achenbach T, Rescorla L (2001) Manual for the ASEBA school-ages forms and profiles. University of Vermont, Burlington
Fadda R, Lucarelli L (2017) Mother-infant and extra-dyadic interactions with a new social partner: developmental trajectories of early social abilities during play. Front Psychol 8:436. https://doi.org/10.3389/fpsyg.2017.00436
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge, New York
Pourhoseingholi MA, Vahedi M, Rahimzadeh M (2013) Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench Winter 6(1):14–17
Barr CL, Feng Y, Wigg KG, Schachar R, Tannock R, Roberts W, Malone M, Kennedy JL (2001) 5′-untranslated region of the dopamine D4 receptor gene and attention-deficit hyperactivity disorder. Am J Human Genet 105:84–90
Miller GM, Madras BK (2002) Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry 7(1):44–55. https://doi.org/10.1038/sj.mp.4000921
Volkow ND, Fowler JS, Wang GJ (2004) The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47:3–13. https://doi.org/10.1016/j.neuropharm.2004.07.019
Swanson JM, Posner M, Fusella J, Wasdell M, Sommer T, Fan J (2001) Genes and attention deficit hyperactivity disorder. Curr Psychiatry Rep 3:92–100. https://doi.org/10.1007/s11920-001-0005-2
Kluger AN, Siegfried Z, Ebstein RP (2002) A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Mol Psychiatry 7:712–717. https://doi.org/10.1038/sj.mp.4001082
Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptors variants. J Neurochem 65:1157–1165. https://doi.org/10.1046/j.1471-4159.1995.65031157.x
Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, Posner M (2000) Dopamine genes and ADHD. Neurosci Biobehav Rev 24:21–25. https://doi.org/10.1016/s0149-7634(99)00062-7
Asherson P, IMAGE Consortium (2004) Attention-deficit hyperactivity disorder in the post-genomic era. Eur Child Adolesc Psychiatry 13:I50-70. https://doi.org/10.1007/s00787-004-1006-6
Roman T, Schmitz M, Polanczyk G, Eizirik M, Rohda LA, Hutz MH (2001) Attention-deficit hyperactivity disorder. A study of association with both the dopamine transporter gene and the dopamine D4 receptor gene. Am J Med Genet 105:471–478. https://doi.org/10.1002/ajmg.1408
Henríquez BH, Henríquez HM, Carrasco CX, Rothhammer AP, Llop RE, Aboitiz F, Rothhammer EF (2008) Combination of DRD4 and DAT1 genotypes is an important risk factor for attention deficit disorder with hyperactivity families living in Santiago, Chile. Rev Med Chil 136(6):719–724
Kim JW, Kim BN, Cho SC (2006) The dopamine transporter gene and the impulsivity phenotype in attention deficit hyperactivity disorder: a case-control association study in a Korean sample. J Psychiatr Res 40(8):730–737. https://doi.org/10.1016/j.jpsychires.2005.11.002
Keren M (2016) Eating and feeding disorders in the first five years of life: Revising the DC: 0–3R diagnostic classification of mental health and developmental disorders of infancy and early childhood and rationale for the new DC: 0–5 proposed criteria. Infant Ment Health J 37(5):498–508. https://doi.org/10.1002/imhj.21588
Kerzner B, Milano K, MacLean WC, Berall G, Stuart S, Chatoor I (2015) A practical approach to classifying and managing feeding difficulties. Pediatrics 135:344–353. https://doi.org/10.1542/peds.2014-1630
Davies WH, Satter E, Berlin KS, Sato AF, Silverman AH, Fischer EA, Arvedson JC, Rudolph CD (2006) Reconceptualizing feeding and feeding disorders in interpersonal context: the case for a relational disorder. J Fam Psychol 20:409–417. https://doi.org/10.1037/0893-3200.20.3.409
Acknowledgements
The authors are grateful to Dr. Gessica D’Angeli for her technical assistance.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This research was financially supported by Sapienza “Ateneo” Grant No. RG120172B99325BF to S.C.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design, performed material preparation (SC and LC), data collection (SC and LC) and analysis (EP, AB, SC and LC). The first draft of the manuscript was written by AB, and all the authors commented on previous versions of the manuscript. All the authors read and approved the revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Sapienza University (no. 809/2020).
Consent to participate
Written informed consent was obtained from all parents involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add missing OASIS funding note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pascale, E., Cimino, S., Cerniglia, L. et al. Disordered eating in early childhood: DRD4 and DAT1 gene polymorphisms and quality of mother–child interaction. Eat Weight Disord 27, 2605–2616 (2022). https://doi.org/10.1007/s40519-022-01408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-022-01408-4