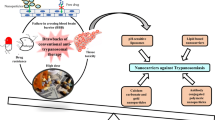
Opinion statement
Human African trypanosomiasis (HAT) or sleeping sickness is one of the neglected tropic diseases caused by Trypanosoma brucei and endemic in sub-saharan Africa. HAT affects half a million people every year in Africa and is fatal, if untreated. Although the number of cases have dropped in recent years, but still there is a strong need to identify and validate new therapeutic targets for trypanosomiasis. Treatment of HAT poses several challenges due to the availability of few drugs and their associated risks like limited efficacy, toxicity, lack of selectivity, stage specificity, and drug resistance. Overcoming these key issues can be explored by identification of some novel targets. Recently, various trypanosomatid biochemical pathways and enzymes have been identified as novel targets for anti-parasitic drug development. New approaches like proteomics and high-throughput phenotypic screening have bridged the gap between the development of new anti-HAT drugs and challenges associated with their development. The present review focuses on novel targets for HAT that hold great promise for elucidating new mechanisms for anti-parasitic action.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, et al. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis. 2012;6(10):e1859. doi:10.1371/journal.pntd.0001859.
Control and surveillance of human African trypanosomiasis. WHO Technical Report Series 984, 2013;1–237. http://www.who.int/iris/handle/10665/95732.
Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin Epidemiol. 2014;6:257–75. doi:10.2147/CLEP.S39728.
Barrett MP, Burchmore RJS, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. The trypanosomiases. Lancet. 2003;362:1469–80. doi:10.2147/CLEP.S39728.
Sternberg JM. Human African trypanosomiasis: clinical presentation and immune response. Parasite Immunol. 2004;26:469–76. doi:10.1111/j.0141-9838.2004.00731.x.
Kennedy PG. Human African trypanosomiasis of the CNS: current issues and challenges. J Clin Invest. 2004;113:496–504. doi:10.1172/JCI200421052.
Burri C. Chemotherapy against human African trypanosomiasis: is there a road to success? Parasitology. 2010;137:1987–94. doi:10.1017/S0031182010001137.
• Horn D, Duraisingh MT. Antiparasitic chemotherapy: from genomes to mechanisms. Annu Rev Pharmacol Toxicol. 2014;54:5.1–5.24. doi:10.1146/annurev-pharmtox-011613-135915. Highlights the existing drugs and approaches that influence development of new drugs for malaria and African trypanosomiasis
Kuepfer I, Schmid C, Allan M, Edielu A, Haary EP, et al. Safety and efficacy of the 10-day melarsoprol schedule for the treatment of second stage rhodesiense sleeping sickness. PLoS Negl Trop Dis. 2012;6:e1695. doi:10.1371/journal.pntd.0001695.
Iten M, Mett H, Evans A, Enyaru JC, Brun R, Kaminsky R. Alterations in ornithine decarboxylase characteristics account for tolerance of Trypanosoma brucei rhodesiense to D,L-α-difluoromethylornithine. Antimicrob Agents Chemother. 1997;41:1922–5.
Simarro PP, Franco J, Diarra A, Postigo JA, Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139:842–6. doi:10.1017/S0031182012000169.
Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, et al. Nifurtimox–eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi:10.1016/S0140-6736(09)61117-X.
Chappuis F, Udayraj N, Stietenroth K, Meussen A, Bovier PA. Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2005;41:748–51. doi:10.1086/432576.
Tagoe DNA, Kalejaiye TD, de Koning HP. The ever unfolding story of cAMP signaling in trypanosomatids: vive la difference! Front Pharmacol. 2015;6:185. doi:10.3389/fphar.2015.00185.
Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, et al. Membrane guanylyl cyclase receptors: an update. Trends EndocrinolMetab. 2006;17:251–8. doi:10.1016/j.tem.2006.06.006.
Salmon D, Bachmaier S, Krumbholz C, Kador M, Gossmann JA, Uzureau P, et al. Cytokinesis of Trypanosoma brucei bloodstream forms depends on expression of adenylyl cyclases of the ESAG4 or ESAG4-like subfamily. Mol Microbiol. 2012;84:225–42. doi:10.1111/j.1365-2958.2012.08013.x.
Huang H. Signal transduction in Trypanosoma cruzi. Adv Parasitol. 2011;75:325–44. doi:10.1016/B978-0-12-385863-4.00015-0.
Shalaby T, Liniger M, Seebeck T. The regulatory subunit of a cGMP-regulated protein kinase A of Trypanosoma brucei. Eur J Biochem. 2001;268:6197–206. doi:10.1046/j.0014-2956.2001.02564.x.
Yamaoka M, Ando T, Terabayashi T, Okamoto M, Takei M, Nishioka T, Kaibuchi K, Matsunaga K, Ishizaki R, Izumi T, et al. PI3K regulates endocytosis after insulin secretion via signaling crosstalk between Arf6 and Rab27a. J Cell Sci. 2015;129:637–49. doi:10.1242/jcs.180141.
Seeds AM, Tsui MM, Sunu C, Spana EP, York JD. Inositol phosphate kinase 2 is required for imaginal disc development in Drosophila. Proc Natl Acad Sci. 2015;112:15660–5. doi:10.1073/pnas.1514684112.
Steger DJ, Haswell ES, Miller AL, Wente SR, O’Shea EK. Regulation of chromatin remodeling by inositol polyphosphates. Science. 2003;299:114–6. doi:10.1126/science.1078062.
Wickramasinghe VO, Savill JM, Chavali S, Jonsdottir AB, Rajendra E, Gruner T, Laskey RA, Babu MM, Venkitaraman AR. Human inositol polyphosphate multikinase regulates transcript-selective nuclear mRNA export to preserve genome integrity. Mol Cell. 2013;51:737–50. doi:10.1016/j.molcel.2013.08.031.
Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann SN. Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell. 2015;34:338–50. doi:10.1016/j.devcel.2015.06.016.
Cestari I, Stuart K. Inositol phosphate pathway controls transcription of telomeric expression sites in trypanosomes. Proc Natl Acad Sci. 2015;112:E2803–12. doi:10.1073/pnas.1501206112.
Cestari I, Haas P, Moretti NS, Schenkman S, Stuart K. Chemogenetic characterization of inositol phosphate metabolic pathway reveals druggable enzymes for targeting kinetoplastid parasites. Cell Chemical Biology. 2016;23:1–10. doi:10.1016/j.chembiol.2016.03.015.
Andrews KT, Haque A, Jones MK. HDAC inhibitors in parasitic diseases. Immunol Cell Biol. 2012;90:66e77. doi:10.1038/icb.2011.97.
Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85e94. doi:10.1038/icb.2011.100.
Engel JA, Jones AJ, Avery VM, Sumanadasa SDM, Ng SS, Fairlie DP, et al. Profiling the anti-protozoal activity of anti-cancer HDAC inhibitors against Plasmodium and Trypanosoma parasites. Int J Parasitology: Drugs and Drug Resistance. 2015;5:117–26. doi:10.1016/j.ijpddr.2015.05.004.
Zhao C, Ma S. Recent advances in the discovery of N-myristoyltransferase inhibitors. Chem Med Chem Minirev. 2014;9(11):2425–37. doi:10.1002/cmdc.201402174.
Frearson JA, Brand S, McElory SP, Cleghorn LAT, Smid O, Stojanovski L, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–34. doi:10.1038/nature08893.
Spinks D, Smith V, Thompson S, Robinson DA, Luksch T, Smith A, et al. Development of small-molecule Trypanosoma brucei N-myristoyltransferase inhibitors: discovery and optimisation of a novel binding mode. Chem Med Chem. 2015;10:1821–36. doi:10.1002/cmdc.201500301.
Pizarro JC, Hills T, Senisterra G, Wernimont AK, Mackenzie C, et al. Exploring the Trypanosoma brucei Hsp83 potential as a target for structure guided drug design. PLoS Negl Trop Dis. 2013;7(10):e2492. doi:10.1371/journal.pntd.0002492.
Abdeen S, Salim N, Mammadova N, Summers CM, Goldsmith-Pestana K, McMahon-Pratt D, Schultz PG, Horwich AL, Chapman E, Johnson SM. Targeting the HSP60/10 chaperonin systems of Trypanosoma brucei as a strategy for treating African sleeping sickness. Bioorg Med Chem Lett. 2016; doi:10.1016/j.bmcl.2016.09.051.
Manta B, Comini M, Medeiros A, Hugo M, Trujillo M, Radi R. Trypanothione: a unique bis-glutathionyl derivative in trypanosomatids. Biochim Biophys Acta. 2013;1830:3199–216. doi:10.1016/j.bbagen.2013.01.013.
Krauth-Siegel RL, Leroux AE. Low-molecular-mass antioxidants in parasites. Antioxid Redox Signal. 2012;17(4):583–607. doi:10.1089/ars.2011.4392.
Zimmermann S, Oufir M, Leroux A, Krauth-Siegel RL, Becker K, Kaiser M, et al. Cynaropicrin targets the trypanothione redox system in Trypanosoma brucei. Bioorg Med Chem. 2017;21(22):7202–9. doi:10.1016/j.bmc.2013.08.052.
Mackey ZB, Koupparis K, Nishino M, McKerrow JH. High-throughput analysis of an RNAi library identifies novel kinase targets in Trypanosoma brucei. Chem Biol Drug Des. 2011;78:454–63. doi:10.1111/j.1747-0285.2011.01156.x.
Mercer L, Bowling T, Perales J, Freeman J, Nguyen T, et al. 2,4-Diaminopyrimidines as potent inhibitors of Trypanosoma brucei and identification of molecular targets by a chemical proteomics approach. PLoS Negl Trop Dis. 2011;5(2):e956. doi:10.1371/journal.pntd.0000956.
Tu X, Wang CC. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. J Biol Chem. 2004;279(19):20519–28. doi:10.1074/jbc.M312862200.
Valenciano AL, Ramsey AC, Santos WL, Mackey ZB. Discovery and antiparasitic activity of AZ960 as a Trypanosoma brucei ERK8 inhibitor. Bioorg and Med Chem. 2016; doi:10.1016/j.bmc.2016.07.069.
Katiyar S, Kufareva I, Behera R, Thomas SM, Ogata Y, et al. Lapatinib-binding protein kinases in the African trypanosome: identification of cellular targets for kinase-directed chemical scaffolds. PLoS One. 2013;8(2):e56150. doi:10.1371/journal.pone.0056150.
Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–22. doi:10.1126/science.1112642.
Albert MA, Haanstra JR, Hannaert V, Van Roy J, Opperdoes FR, Bakker BM, Michels PA. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. J Biol Chem. 2005;280(31):28306–15. doi:10.1074/jbc.M502403200.
Brimacombe KR, Walsh MJ, Liu L, Vásquez-Valdivieso MG, Morgan HP, McNae I, et al. Identification of ML251, a potent inhibitor of T. brucei and T. cruzi phosphofructokinase. Med Chem Lett. 2014;5:12–7. doi:10.1021/ml400259d.
Cross GAM, Kim H-S, Wickstead B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol Biochem Parasitol. 2014;195:59–73. doi:10.1016/ j.molbiopara.2014.06.004.
• Schulz D, Mugnier MR, Paulsen E-M, Kim H-S, Chung C-wW, Tough DF, et al. Bromodomain proteins contribute to maintenance of bloodstream form stage identity in the African trypanosome. PLoS Biol. 2015;13(12):e1002316. doi:10.1371/journal.pbio.1002316. Describes the role of bromodomain proteins in maintaining the identity of parasite in its bloodstream form.
• Ettari R, Tamborini L, Angelo IC, Micale N, Pinto A, De Micheli C, et al. Inhibition of rhodesain as a novel therapeutic modality for human African trypanosomiasis. J Med Chem. 2013;56(14):5637–58. doi:10.1021/jm301424d. Reveals rhodesain as novel therapeutic target for HAT and describes different classes of rhodesain inhibitors.
Scory S, Caffrey CR, Stierhof Y-D, Ruppel A, Steverding D. Trypanosoma brucei: killing of bloodstream forms in vitro and in vivo by the cysteine proteinase inhibitor Z-Phe-Ala-CHN2. Exp Parasitol. 1999;91:327–33. doi:10.1186/1475-9292-6-2.
Willert EK, Phillips MA. Regulated expression of an essential allosteric activator of polyamine biosynthesis in African trypanosomes. PLoS Pathog. 2008;4:e1000183. doi:10.1371/journal.ppat.1000183.
Chang KP, Steiger RF, Dave C, Cheng YC. Effects of methylglyoxal bis (ganylhydrazone) on trypanosomatid flagellates: inhibition of growth and nucleoside incorporation in Trypanosoma brucei. J Protozool. 1978;25:145–9.
Bacchi CJ, Brun R, Croft SL, Alicea K, Buhler Y. In vivo trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob Agents Chemother. 1996;40:1448–53.
Barker RH, Liu JR, Hirth B, Celatka CA, Fitzpatrick R, Xiang Y. Novel S-adenosylmethionine decarboxylase inhibitors for the treatment of human african trypanosomiasis. Antimicrob Agents Chemother. 2009;53(5):2052–8.
Lu J, Vodnala SK, Gustavsson A-L, Gustafsson TN, Sjöberg B, Johansson HA, Kumar S, et al. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J Biol Chem. 2013;288:27456–68. doi:10.1074/jbc.M113.495101.
Lazarin-Bidóia D, Desoti VC, Ueda-Nakamura T, BPD F, Nakamura CV, Silva SO. Further evidence of the trypanocidal action of eupomatenoid-5: confirmation of involvement of reactive oxygen species and mitochondria owing to a reduction in trypanothione reductase activity. Free Radic Biol Med. 2013;60:17–28. doi:10.1016/j.freeradbiomed.2013.01.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shikha Girdhar declares that she has no conflict of interest. Amit Girdhar declares that he has no conflict of interest. Viney Lather declares that he has no conflict of interest. Deepti Pandita declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neglected Tropical Diseases
Rights and permissions
About this article
Cite this article
Girdhar, S., Girdhar, A., Lather, V. et al. Novel Therapeutic Targets for Human African Trypanosomiasis. Curr Treat Options Infect Dis 9, 200–209 (2017). https://doi.org/10.1007/s40506-017-0120-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-017-0120-1