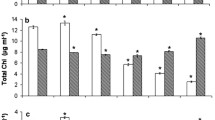
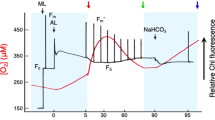
Abstract
Photosynthetic oxygen evolution (measured by polarographic oxygen rate electrode) and pulse amplitude modulated chlorophyll fluorescence were used to assess the effect of sanosil-induced oxidative stress on photosystem II (PSII) in the green alga Chlorella vulgaris and the cyanobacterium Synechocystis salina isolated from Antarctic and mesophilic environments. This study revealed a relatively stronger influence of sanosil (especially its main component, hydrogen peroxide) on the donor site (oxygen evolving complex) compared to the acceptor side of the PSII in both green algae and cyanobacteria. The inhibition of the oxygen evolution results mainly from a decrease in the fast operating PSII centers. In addition, the obtained data showed that the effects of the oxidative stress on the cyanobacterium and the green alga strongly depend on the antenna size of PSII.


Similar content being viewed by others
References
Anderson, J. M., & Aro, E.-M. (1994). Grana stacking and protection of photosystem II in thylakoid membranes of higher plant leaves under sustained high irradiation: An hypothesis. Photosynthesis Research, 41, 315–326.
Apostolova, E. L., Dobrikova, A. G., Ivanova, P. I., Petkanchin, I. B., & Taneva, S. G. (2006). Relationship between the organization of the supercomplex and the functions of the photosynthetic apparatus. Journal Photochemistry and Photobiology B: Biology, 83, 114–122.
Apostolova, E. L., Domonkos, I., Dobrikova, A. G., Sallai, A., Bogos, B., Wada, H., et al. (2008). Effect of phosphatidylglycerol depletion on the surface electric properties and fluorescence emission of thylakoid membranes. Journal Photochemistry and Photobiology B: Biology, 91, 51–57.
Apostolova, E. L., Pouneva, I. D., Grigorova, I., Minkova, K. M., Nikolaeva, N., & Rashkov, G. (2010). Differential response of the photosynthetic apparatus of Antarctic algae Synechocystis salina (Cyanophyta) and Chlorella vulgaris (Chlorophyta) to UV-B radiation. Comptes rendus de l’Académie bulgare des Sciences, 63, 1009–1016.
Apostolova, E. L., Pouneva, I., Rashko, G., Dankov, K., Grigorova, I., & Misra, A. N. (2014). Effect of UV-B radiation on Photosystem II functions in Antarctic and mesophilic strains of a green alga Chlorella vulgaris and a cyanobacterium Synechocystis salina. Indian Journal of Plant Physiology, 19, 111–118.
Arora, A., Sairam, R. K., & Srivastara, G. C. (2002). Oxidative stress and antioxidative system in plants. Current Scienc, 82, 1227–1238.
Bald, D., Kruip, J., & Roegner, M. (1996). Supramolecular architecture of cyanbacterial thylakoid membranes: How is the phicobilisome connected with the photosystems? Photosynthesis Research, 49, 103–118.
Barrington, D. J., & Ghadouai, A. (2008). Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environmental Science and Technology, 42, 8916–8921.
Dankov, K., Busheva, M., Stefanov, D., & Apostolova, E. L. (2009). Relationship between the degree of carotinoid depletion and function of photosynthetic apparatus. Journal Photochemistry and Photobiology B: Biology, 96, 49–56.
Dankov, K. G., Dobrikova, A., Ughy, B., Bogos, B., Gombos, Z., & Apostolova, E. L. (2011). LHCII organization and thylakoid lipids affect the sensitivity of the photosynthetic apparatus to high-light treatment. Plant Physiology Biochemistry, 49, 629–635.
Dankov, K., Rashkov, G., Misra, A. N., Apostolova E. L. (2015). Temperature sensitivity of photosystem II in isolated thylakoid membranes from fluridone-treated pea leaves. Turkish Journal of Botany, 39, 420–428.
Dobrikova, A. G., Domonkos, I., Sözer, Ö., Laczkó-Dobos, H., Kis, M., Párduc, Á., et al. (2013a). Effect of partial or complete elimination of light-harvesting complexes on the surface electric properties and the functions of cyanobacterial photosynthetic membranes. Physiology Plantarum, 147, 248–260.
Dobrikova, A. G., Krasteva, V., & Apostolova, E. L. (2013b). Damage and protection of the photosynthetic apparatus from UV-B radiation. I. Effect of ascorbate. Journal Plant Physiology, 170, 251–257.
Drávkova, M., Admiral, W., & Maršálek, B. (2007). Combined exposure to hydrogen peroxide and light—Selective effects on cyanobacteria, green algae and diatoms. Environmental Science and Technology, 41, 309–314.
Georgiev, D., Dilov, H., & Avramova, S. (1978). Millieu nutritif tamponne et méthode de culture intensive des microalgues vertes. Hydrobiology (Bulgaria), 7, 14–23. [In Bulgarian].
Ivanova, P. I., Dobrikova, A. G., Taneva, S. G., & Apostolova, E. L. (2008). Sensitivity of the photosynthetic apparatus to UV-A radiation: A role of light-harvesting complex II—Photosystem II supercomplex organization. Radiation and Environmental Biophysics, 47, 169–177.
Kitajima, M., & Butler, W. (1975). Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochiica et Biophysica Acta, 376, 105–115.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Lin, Z. F., Liu, N., Lin, G. Z., & Peng, C. L. (2011). Factors altering the membrane fluidity of spinach thylakoid as determined by fluorescence polarization. Acta Physiologiae Plantarum, 33, 1019–1024.
MacColl, R. (1998). Cyanobacterial phycobilisomes. Journal of Structural Biology, 124, 311–334.
Maksimov, E. G., Kuzminov, F. I., Konyuhov, I. V., Elanskaya, I. V., & Paschenkoet, V. Z. (2011). Photosystem 2 effective fluorescence cross-section of cyanobacterium Synechocystis sp. PCC6803 and its mutants. Journal Photochemistry and Photobiology B: Biology, 104, 285–291.
Matthijs, H. C. P., Visser, P. M., Reeze, B., Meeuse, J., Slot, P. C., Wijn, G., et al. (2012). Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research, 46, 1460–1472.
Pekárková, B., Hindák, F., Šmarda, J. (1988) Morphological characteristics and physiological properties of a coccoid rhodophycean alga Rhodella grisea from thermal springs at Pieštany CzechoslovakiArchive für Protistenkund. 135, 69–83.
Pospíšil, P. (2009). Production of reactive oxygen species by photosystem II. Biochiica et Biophysica Acta, 1787, 1151–1160.
Pospíšil, P. (2012). Molecular mechanism of production and scavenging of reactive oxygen species by photosystem II. Biochiica et Biophysica Acta, 1817, 218–231.
Pospíšil, P. (2014). The role of metals in production and scavenging of reactive oxygen species in photosytem II. Plant and Cell Physiology, 55, 1233–1244.
Samuilov, V. D., Bezryadnov, D. B., Gusev, M. V., Kitashov, A. V., & Fedorenko, T. A. (2001). Hydrogen peroxide inhibits photosynthetic electron transport in cells of cyanobacterium. Biochemistry (Moscow), 66, 640–645.
Sandusky, P. O., & Yocum, C. F. (1988). Hydrogen peroxide oxidation catalyzed by chloride-depleted thylakoid membranes. Biochiica et Biophysica Acta, 936, 149–156.
Sanosil disinfectants—for better disinfection: “SANOSIL BG” Ltd [http://sanosilbg.com].
Setlik, I. (1967). Contamination of algal cultures by heterotrophic microorganisms and its prevention. Annual Report Algology for the Year 1966, Trebon, CSAV, Inst Microbiology (pp. 89–100).
Siegelman, H., & Kucia, J. (1978). Algal biliproteins. In J. Hellebuts & J. Craisie (Eds.), Handbook of phycological methods (pp. 71–79). Cambridge: Cambridge University Press.
Zeinalov, Y. (2002). An equipment for investigations of photosynthetic oxygen production reactions. Bulgarian Journal of Plant Physiology., 28, 57–67.
Acknowledgments
This work was supported by the Bulgarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apostolova, E.L., Rashkov, G., Dankov, K. et al. Influence of the sanosil-induced oxidative stress on the photosynthetic apparatus of different strains of green algae and cyanobacteria . Ind J Plant Physiol. 20, 333–338 (2015). https://doi.org/10.1007/s40502-015-0183-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0183-2