Abstract
Purpose of Review
Here, we systematically review the published literature indicating that aberrant regulation of Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling was associated with the autoimmune disorders, rheumatoid arthritis, systemic lupus erythematosus, psoriasis/psoriatic arthritis, multiple sclerosis, inflammatory bowel diseases, and ankylosing spondylitis.
Pertinent Findings
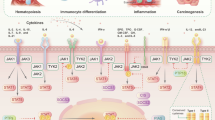
The autoimmune disorders discussed in this review are characterized by several alterations resulting in abnormal JAK-STAT signaling. These abnormalities in JAK-STAT signaling include (1) constitutive activation of both the interleukin-6(IL-6)/interleukin-6 receptor (IL-6R) canonical and IL-6 trans-signaling pathway, the latter involving soluble IL-6R; (2) the hyperactivation of JAK/STAT signaling as a response to the significantly elevated levels of pro-inflammatory “immunocytokines”, exemplified by IL-6, IL-15, IL-17, IL-23, interferon-γ; and (3) the reduced activity of the negative regulators of JAK-STAT signaling, including suppressor of cytokine signaling and protein inhibitor of activated STATs as well as protein tyrosine phosphatases-1, -2, which was shown to inhibit STAT-signaling.
Summary
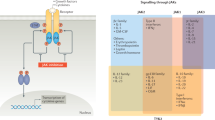
The involvement of abnormal JAK-STAT signaling in autoimmune disorders has led to the development of JAK small molecule inhibitors (SMIs), such as tofacitinib, ruxolitinib, and baricitinib for the therapy of rheumatoid arthritis, psoriasis/psoriatic arthritis, and Crohn’s disease. However, the extent to which treatment of these diseases with JAK SMIs will result in blunting the “cross-talk” between the JAK-STAT signaling pathway and the other signaling pathways known to participate in autoimmune disorders remains to be determined, involving the mitogen-activated protein kinase pathway and the phosphatidylinositide/Akt/mechanistic target of rapamycin signaling pathway.

Similar content being viewed by others
References
Darnell JR Jr. STATS and gene regulation. Science. 1997;277:1630–5.
Leonard WJ, O’Shea JJ. JAKS and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322.
Ivashkiv LB, Hu X. Signaling by STATs. Arthritis Res Ther. 2004;6:159–68.
Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9.
Malemud CJ, Pearlman E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr Signal Transduction Ther. 2009;4:201–21.
Stark GR, Darnell JE Jr. The JAK-STAT pathway at 20. Immunity. 2012;36:503–14.
Müller-Ladner U, Judex M, Ballhorn W, et al. Activation of the IL-4 STAT pathway in rheumatoid arthritis. J Immunol. 2000;164:3894–901.
Yokota A, Narazaki N, Shima Y, et al. Preferential and persistent activation of the STAT1 pathway in rheumatoid synovial fluid cells. J Rheumatol. 2001;28:1952–9.
Kasperkovitz PV, Verbeet NL, Smeets TJ. Activation of the STAT1 pathway in rheumatoid arthritis. Ann Rheum Dis. 2006;65:149–56.
Walker JG, Ahern MG, Coleman M, Weedon H, Papangelis V, Beroukas D, et al. Expression of Jak3, STAT1, STAT4 and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells and seropositive rheumatoid arthritis. Ann Rheum Dis. 2006;65:149–56.
Walker JG, Ahern MG, Coleman M, et al. Characterisation of a dendritic cell subset in synovial tissue which strongly expresses Jak/STAT transcription factors from patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:992–9.
Isomäki P, Alanärä T, Isohanni P, et al. The expression of SOCS is altered in rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1538–46.
Malemud CJ, Meszaros EC, Wylie MA, et al. Matrix metalloproteinase-9 production in immortalized human chondrocyte lines. J Clin Cell Immunol. 2016;7:422.
Malemud CJ. Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int J Mol Sci. 2017;18:484.
Kawasaki M, Fujishiro M, Yamaguchi A, Nozawa K, Kaneko H, Takasaki Y, et al. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus. 2011;20:1231–9.
Meshaal S, El Refai B, El Saie A, et al. Signal transducer and activator of transcription 5 is implicated in disease activity in adult and juvenile onset systemic lupus erythematosus. Clin Rheumatol. 2016;35:1515–20.
Goropevšek A, Holcar M, Avčin T. The role of STAT signaling pathways in the pathogenesis of systemic lupus erythematosus. Clin Rev Allergy Immunol. 2017;52:164–81.
Welsch K, Holstein J, Laurence A, Ghoreschi K. Targeting JAK/STAT signaling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47:1096–107.
Calautti E, Avalle L, Poli V. Psoriasis: a STAT3-centric view. Int J Mol Sci. 2018;19:E171.
Fiocco U, Accordi B, Martini V, Oliviero F, Facco M, Cabrelle A, et al. JAK/STAT/PKCδ molecular pathways in synovial T lymphocytes reflect the in vivo T helper-17 expansion in psoriatic arthritis. Immunol Res. 2014;58:61–9.
Costa L, Del Puente A, Peluso R, et al. Small molecule therapy for managing moderate to severe psoriatic arthritis. Expert Opin Pharmacother. 2017;18:1557–67.
Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol. 2004;55:46–57.
Egwuagu CE, Larkin J III. Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAKSTAT. 2013;2:e24134.
Liu Y, Gibson SA, Benveniste EN, Qin H. Opportunities for translation from the bench: therapeutic intervention of the JAK/STAT pathway in neuroinflammatory diseases. Crit Rev Immunol. 2015;35:505–27.
Yan Z, Gibson SA, Buckley JA, Qin H, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol. 2018;189:4–13.
Hatami M, Salmani T, Arsang-Jang S, et al. STAT5A and STAT6 gene expression levels in multiple sclerosis patients. Cytokine. 2018;106:108–13.
Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J Biol Chem. 2003;278:16777–81.
Mitsuyama K, Matsumoto S, Masuda J, Yamasakii H, Kuwaki K, Takedatsu H, et al. Therapeutic strategies for targeting IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–56.
Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8.
Galien R. Janus kinases in inflammatory bowel disease: four kinases for multiple purposes. Pharmacol Rep. 2016;68:789–96.
Flamant M, Rigaill J, Paul S, Roblin X. Advances in the development of Janus kinase inhibitors in inflammatory bowel disease: future prospects. Drugs. 2017;77:1057–68.
Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, et al. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the JAK-STAT pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat Res. 2010;690:108–15.
Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:291–7.
Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196–202.
Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signaling pathway. Semin Cell Dev Biol. 2008;19:414–22.
Yin Y, Liu W, Dai Y. SOCS3 and its role in associated diseases. Hum Immunol. 2015;76:775–80.
Malemud CJ. Suppressor of cytokine signaling and rheumatoid arthritis. Integr Mol Med. 2016;3:17–20.
Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–82.
Lao M, Shi M, Zou Y, Huang M, Ye Y, Qiu Q, et al. Protein inhibitor of activated STAT3 regulates migration, invasion and activation of fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol. 2016;196:596–606.
Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. 2016;75:311–5.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–46.
Izzo R, Bevivino G, Monteleone G. Tofacitinib for the treatment of ulcerative colitis. Expert Opin Investig Drugs. 2016;25:991–7.
Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–44.
Mattar M, Jandali B, Malemud CJ, et al. Atherosclerosis and rheumatic diseases. Rheumatology (Sunnyvale). 2015;5:147.
Nowell MA, Richards PJ, Fielding CA. Regulation of pre-B cell colony enhancing factor by STAT3-interleukin-6 trans-signaling. Implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–95.
Malemud CJ. Suppression of pro-inflammatory cytokines via targeting of STAT-responsive genes. In: El-Shemy H, editor. Drug discovery. Rijeka: InTech Publishing; 2013. p. 373–411.
Kimura A, Naka T, Kishimoto T. IL-6 dependent and –independent pathways in the development of interleukin-17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–104.
Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, et al. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19:695–702.
Ahmad R, Malemud CJ, Askari AD. Treatment of SLE and secondary Sjögren’s syndrome with belimumab. J Immuno Biol. 2016;1:3.
Taherian E, Rao A, Malemud CJ, Askari AD. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr Rheumatol Rev. 2013;9:45–62.
Ramanujam M, Davidson A. BAFF blockade for systemic lupus erythematosus: will the promise be fulfilled? Immunol Rev. 2008;223:156–74.
Navarra SV, Gusmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled phase 3 trial. Lancet. 2011;377:721–31.
Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A phase III, randomized placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with system lupus erythematosus. Arthritis Rheum. 2011;63:3918–30.
Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;64:2328–37.
Frieri M, Heuser W, Bliss J. Efficacy of novel monoclonal antibody belimumab in the treatment of lupus nephritis. J Pharmacol Pharmacother. 2015;6:71–6.
Malemud CJ, Gillespie HJ. The role of apoptosis in arthritis. Curr Rheumatol Rev. 2005;1:131–42.
Andrade F, Casciloa-Rosen L, Rosen L. Apoptosis in systemic lupus erythematosus. Clinical implications. Rheum Dis Clin N Am. 2000;26:215–27.
Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, et al. The apoptotic ligands TRAIL, TWEAK and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–9.
de la Varga Martinez R, Rodriguez-Bayona B, Aῆez GA, et al. Clinical relevance of circulating anti- ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol. 2017;47:1211–9.
Wang L, Tassiulas I, Park-Min KH, Reid AC, Gil-Henn H, Schlessinger J, et al. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9:186–93.
Tagoe C, Putterman C. JAK2 inhibition in systemic lupus erythematosus. Immunotherapy. 2012;4:369–72.
Lu LD, Stump KL, Wallace NH, Dobrzanski P, Serdikoff C, Gingrich DE, et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol. 2011;187:3840–53.
Wang S, Yang N, Zhang L, Huang B, Tan H, Liang Y, et al. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19:1171–80.
Azevedo A, Torres T. Tofacitinib: a new oral therapy for psoriasis. Clin Drug Investig. 2018;38:101–12.
Hald A, Andrés RM, Salskov-Iversen ML, Kjellerup RB, Iversen L, Johansen C. STAT1 expression and activation is increased in lesional psoriatic skin. Br J Dermatol. 2013;168:302–10.
Andrés RM, Hald A, Johansen C, Kragballe K, Iversen L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol. 2013;22:323–8.
Johansen C, Rittig AH, Mose M, Bertelsen T, Weimar I, Nielsen J, et al. STAT2 is involved in the pathogenesis of psoriasis by promoting CXCL11 and CCL5 production by keratinocytes. PLoS One. 2017;12:e0176994.
Di Lernia V, Bardazzi F. Profile of tofacitinib citrate and its potential in the treatment of moderate- to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016;10:533–9.
Korman AM, Hill D, Alikhan A et al. Oral tofacitinib for the treatment of adults with moderate to severe plaque psoriasis. Expert Rev Clin Pharmacol. 2016:1–15.
Wcisło-Dziadecka D, Zbiciak-Nylec M, Brzeziňska-Wcisło L, et al. Newer treatments of psoriasis regarding IL-23 inhibitors, phosphodiesterase 4 inhibitors, and Janus kinase inhibitors. Dermatol Ther. 2017; https://doi.org/10.1111/dth.12555.
Vazquez ML, Kaila N, Strohbach JW, Trzupek JD, Brown MF, Flanagan ME, et al. Identification of N-{cis-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J Med Chem. 2018;61:1130–52.
Peeva E, Hodge MR, Kieras E, et al. Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: a phase 1, randomized, placebo-controlled, dose-escalation study. Br J Clin Pharmacol. 2018; https://doi.org/10.1111/bcp.13612.
Meszaros EC, Dahoud W, Mesiano S, Malemud CJ. Blockade of recombinant human IL-6 with tocilizumab suppresses matrix metalloproteinase-9 production in the C28/I2 immortalized human chondrocyte cell line. Integr Mol Med. 2015;2:304–10.
Malemud CJ. Regulation of chondrocyte matrix metalloproteinase gene expression. In: Dhalla NS, Chakraborti S, editors. Role of proteases in cellular dysfunction. United Kingdom: Springer Science; 2013. p. 63–77.
Meszaros EC, Malemud CJ. Phosphorylation of STAT proteins by recombinant human IL-6 in immortalized human chondrocyte lines, T/C28a2 and C28/I2. J Inflamm Res. 2017;10:143–50.
Yang EJ, Sanchez IM, Beck K, Sekhon S, Wu JJ, Bhutani T. Guselkumab for the treatment of moderate-to-severe plaque psoriasis. Expert Rev Clin Pharmacol. 2018;11:333–44.
Megna M, Balato A, Raimondo A, Balato N. Guselkumab for the treatment of psoriasis. Expert Opin Biol Ther. 2018;18:459–68.
Camporeale A, Poli V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci (Landmark Ed). 2012;17:2306–26.
Lei X, Cai S, Chen Y, Cui J, Wang Y, Li Z, et al. Down-regulator of interleukin 7 receptor (IL-7R) contributes to central nervous system demyelination. Oncotarget. 2017;8:28395–407.
Sanchez-Muňoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–8.
West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin M derives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579–89.
Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9.
Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human inflammatory bowel diseases. Gut. 2002;51:379–85.
Mudter A, Dentelli P, Carlino A, et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72.
Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relationship to circulating interleukin-6. Gut. 1995;36:45–9.
Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81.
Olivera P, Danese S, Peyrin-Biroulet L. JAK inhibition in inflammatory bowel disease. Expert Rev Clin Immunol. 2017;13:693–703.
Zhao HM, Xu R, Huang XY, et al. Curcumin suppressed activation of dendritic cells via JAK/STAT/SOCS signal in mice with experimental colitis. Front Pharmacol. 2016;7:455.
Tetreault MP, Alrabaa R, McGeehan M, Katz JP. Krüppel-like factor 5 protects against murine colitis and activates JAK-STAT signaling in vivo. PLoS One. 2012;7:e38338.
Nieminen JK, Niemi M, Sipponen T, Salo HM, Klemetti P, Färkkilä M, et al. Dendritic cells from Crohn’s disease patients show aberrant STAT1 and STAT3 signaling. PLoS One. 2013;8:e70738.
Mahajan S, Gollob JA, Ritz J, Frank DA. CD2 stimulation leads to delayed and prolonged activation of STAT1 in T cells but not NK cells. Exp Hematol. 2001;29:209–20.
Boysen P, Olsen I, Berg I, Kulberg S, Johansen GM, Storset AK. Bovine CD2-/NKp46+ cells are fully functional natural killer cells with a high activation status. BMC Immunol. 2006;7:10.
Sasada T, Yang H, Reinherz EL. CD2 facilitates differentiation of CD4 Th cells without affecting Th1/Th2 polarization. J Immunol. 2002;168:1113–22.
Gonsky R, Deem RL, Bream J, Young HA, Targan SR. Enhancer role of STAT5 in CD2 activation of IFN-γ expression. J Immunol. 2004;173:6241–7.
Gonsky R, Deem RL, Young HA, et al. CD2 mediates activation of IFN-γ intronic STAT binding region in mucosal T cells. Eur J Immunol. 2003;33:1152–62.
Alkim C, Balci M, Alkim H, Dağli U, Parlak E, Tezel A, et al. The importance of peripheral immune cells in inflammatory bowel disease. Turk J Gastroenterol. 2007;18:82–8.
Khalili A, Ebrahimpour S, Maleki I, et al. CD4+CD25+CD127lowFoxP3+ regulatory T cells in Crohn’s disease. Rom J Intern Med. 2018; https://doi.org/10.2478/rjm-2018-0006.
van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New Engl J Med. 2012;367:509–19.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507.
Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41:837–52.
Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORALStrategy): a phase 3b/4 double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–68.
Kremer JM, Schiff M, Muram M, et al. Response to baricitinib therapy in patients with rheumatoid arthritis with inadequate response to csDMARDs as a function of baseline characteristics. RMD Open. 2018;4:e000581.
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–36.
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–50.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36.
Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–59.
Thomas S, Fisher KH, Snowden JA, Danson SJ, Brown S, Zeidler MP. Methotrexate is a JAK/STAT inhibitor. PLoS One. 2015;10:e0130078.
Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35.
Raychaudhuri SK, Raychaudhuri SP. Janus kinase/signal transducer and activator of transcription pathways in spondyloarthritis. Curr Opin Rheumatol. 2017;29:311–6.
Muraro D, Simmons A. An integrative analysis of gene expression and molecular interaction data to identify dys-regulated sub-networks in inflammatory bowel disease. BMC Bioinformatics. 2016;17:42.
Zhang Q, Pulheti P, Zhou Q, et al. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–56.
Ferretti E, Corcione A, Pistoia V. The IL-31/IL-31 receptor axis: general features and role in tumor microenvironment. J Leukoc Biol. 2017;102:711–7.
Hermanns HM. Oncostatin M and interleukin-31. Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26:545–58.
Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–56.
Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012;91:552–66.
Malemud CJ. PI3K/Akt/PTEN/mTOR signaling: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7:1137–47.
Malemud CJ, Sun Y, Pearlman E, et al. Monosodium urate and tumor necrosis factor-α increase apoptosis in human chondrocyte cultures. Rheumatology (Sunnyvale). 2012;2:113.
Acknowledgements
The experimental results reported in Meszaros and Malemud (references 73 and 75) and Malemud et al. (references 13 and 118) were supported, in part, by contracts between Takeda Pharmaceuticals of North America and CWRU, and, Genentech/Roche and CWRU, the Visual Sciences Research Center funded by the National Eye Institute (P30-EY11373) and the National Institute of Childhood & Human Development (R01-HD061819; PI: Sam Mesiano, PhD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Kinase Inhibitor
Rights and permissions
About this article
Cite this article
Malemud, C.J. Defective JAK-STAT Pathway Signaling Contributes to Autoimmune Diseases. Curr Pharmacol Rep 4, 358–366 (2018). https://doi.org/10.1007/s40495-018-0151-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-018-0151-4