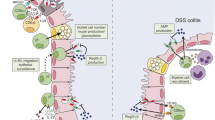
Abstract
Purpose of Review
Intestinal mucosal immunity is tightly regulated to ensure effective host defense against invasive microorganisms while limiting the potential for aberrant damage. In inflammatory bowel disease (IBD), an imbalance between effector and regulatory T cell populations results in an uncontrolled inflammatory response to commensal bacteria. Intraepithelial lymphocytes (IEL) are perfectly positioned within the intestinal epithelium to provide the first line of mucosal defense against luminal microbes or rapidly respond to epithelial injury. This review will highlight how IELs promote protective intestinal immunity and discuss the evidence indicating that altered IEL responses contribute to the pathogenesis of IBD.
Recent Findings
Although the role of IELs in mucosal homeostasis has been largely underappreciated, many of the same factors that contribute to the dysregulation of host defense in IBD also adversely affect IELs. For example, IL-23 and the endoplasmic reticulum stress response can enhance IEL lytic activity toward enterocytes. Microbial dysbiosis or defective microbial recognition results in the loss of regulatory IELs, further amplifying these pro-inflammatory effects. Migration of T cells into or within the intraepithelial compartment has a profound effect on their differentiation or effector function demonstrating that IELs are exquisitely sensitive to changes in the local intestinal microenvironment.
Summary
Enhanced mechanistic insight into the regulation of IEL survival, differentiation, and effector function may provide useful tools to modulate IEL surveillance or enhance IEL regulatory function. Elucidation of these processes may result in the development of novel therapeutics to reduce intestinal inflammation and reinforce the mucosal barrier in IBD.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. https://doi.org/10.1038/nature11582.
Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140(6):1729–37. https://doi.org/10.1053/j.gastro.2011.02.012.
Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. https://doi.org/10.1038/nrmicro3552.
Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F. Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front Immunol. 2016;7:290. https://doi.org/10.3389/fimmu.2016.00290.
• Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14(5):269–78. https://doi.org/10.1038/nrgastro.2016.208. A recent review of T cell signaling pathways currently being targeted for IBD therapeutic development.
Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152(2):327–39 e4. https://doi.org/10.1053/j.gastro.2016.10.012.
Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341(8858):1437–9.
D'Inca R, Di Leo V, Corrao G, Martines D, D'Odorico A, Mestriner C, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94(10):2956–60.
May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104(6):1627–32.
Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136(2):551–63. https://doi.org/10.1053/j.gastro.2008.10.081.
• Edelblum KL, Sharon G, Singh G, Odenwald MA, Sailer A, Cao S, et al. The microbiome activates CD4 T-cell-mediated immunity to compensate for increased intestinal permeability. Cell Mol Gastroenterol Hepatol. 2017;4(2):285–97. https://doi.org/10.1016/j.jcmgh.2017.06.001. This study is the first demonstration that the microbiota stimulates protective immunity against acute bacterial invasion in response to tight junction-mediated increases in intestinal permeability.
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. https://doi.org/10.1038/nri2515.
Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–46. https://doi.org/10.1146/annurev.immunol.22.012703.104522.
Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, et al. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16(3):355–64.
Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25(4):631–41. https://doi.org/10.1016/j.immuni.2006.08.018.
Suzuki K, Oida T, Hamada H, Hitotsumatsu O, Watanabe M, Hibi T, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13(5):691–702.
Oida T, Suzuki K, Nanno M, Kanamori Y, Saito H, Kubota E, et al. Role of gut cryptopatches in early extrathymic maturation of intestinal intraepithelial T cells. J Immunol. 2000;164(7):3616–26.
Nonaka S, Naito T, Chen H, Yamamoto M, Moro K, Kiyono H, et al. Intestinal gamma delta T cells develop in mice lacking thymus, all lymph nodes, Peyer's patches, and isolated lymphoid follicles. J Immunol. 2005;174(4):1906–12.
Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11(7):445–56. https://doi.org/10.1038/nri3007.
Chennupati V, Worbs T, Liu X, Malinarich FH, Schmitz S, Haas JD, et al. Intra- and intercompartmental movement of gammadelta T cells: intestinal intraepithelial and peripheral gammadelta T cells represent exclusive nonoverlapping populations with distinct migration characteristics. J Immunol. 2010;185(9):5160–8. https://doi.org/10.4049/jimmunol.1001652.
Sugahara S, Shimizu T, Yoshida Y, Aiba T, Yamagiwa S, Asakura H, et al. Extrathymic derivation of gut lymphocytes in parabiotic mice. Immunology. 1999;96(1):57–65.
Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151(4):1765–76.
Bonneville M, Itohara S, Krecko EG, Mombaerts P, Ishida I, Katsuki M, et al. Transgenic mice demonstrate that epithelial homing of gamma/delta T cells is determined by cell lineages independent of T cell receptor specificity. J Exp Med. 1990;171(4):1015–26.
Jarry A, Cerf-Bensussan N, Brousse N, Selz F, Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990;20(5):1097–103. https://doi.org/10.1002/eji.1830200523.
Lundqvist C, Baranov V, Hammarstrom S, Athlin L, Hammarstrom ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7(9):1473–87.
Suzuki H. Differences in intraepithelial lymphocytes in the proximal, middle, distal parts of small intestine, cecum, and colon of mice. Immunol Investig. 2009;38(8):780–96. https://doi.org/10.3109/08820130903258800.
Hirata I, Berrebi G, Austin LL, Keren DF, Dobbins WO III. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31(6):593–603.
Cerf-Bensussan N, Brousse N, Jarry A, Goulet O, Revillon Y, Ricour C, et al. Role of in vivo activated T cells in the mechanisms of villous atrophy in humans: study of allograft rejection. Digestion. 1990;46(Suppl 2):297–301.
Guy-Grand D, Cuenod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. 1996;26(9):2248–56. https://doi.org/10.1002/eji.1830260942.
Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15(1):83–93.
Ebert EC. IL-15 converts human intestinal intraepithelial lymphocytes to CD94 producers of IFN-gamma and IL-10, the latter promoting Fas ligand-mediated cytotoxicity. Immunology. 2005;115(1):118–26. https://doi.org/10.1111/j.1365-2567.2005.02132.x.
Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191(3):435–44.
Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21(3):357–66. https://doi.org/10.1016/j.immuni.2004.06.020.
Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, et al. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology. 2015;149(3):681–691 e10. https://doi.org/10.1053/j.gastro.2015.05.013.
Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195(11):1491–7.
Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100(9):5324–9. https://doi.org/10.1073/pnas.0831037100.
Kuhl AA, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Hoffmann JC. Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J Leukoc Biol. 2007;81(1):168–75. https://doi.org/10.1189/jlb.1105696.
Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro T, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196(12):1563–74.
Chang JS, Ocvirk S, Berger E, Kisling S, Binder U, Skerra A, et al. Endoplasmic reticulum stress response promotes cytotoxic phenotype of CD8alphabeta+ intraepithelial lymphocytes in a mouse model for Crohn’s disease-like ileitis. J Immunol. 2012;189(3):1510–20. https://doi.org/10.4049/jimmunol.1200166.
Kawaguchi-Miyashita M, Shimada S, Kurosu H, Kato-Nagaoka N, Matsuoka Y, Ohwaki M, et al. An accessory role of TCRgammadelta (+) cells in the exacerbation of inflammatory bowel disease in TCRalpha mutant mice. Eur J Immunol. 2001;31(4):980–8.
Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99(22):14338–43. https://doi.org/10.1073/pnas.212290499.
Hoffmann JC, Peters K, Henschke S, Herrmann B, Pfister K, Westermann J, et al. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48(4):489–95.
Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173(2):1390–8.
Nancey S, Holvoet S, Graber I, Joubert G, Philippe D, Martin S, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131(2):485–96. https://doi.org/10.1053/j.gastro.2006.05.018.
Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–98.
Apostolaki M, Manoloukos M, Roulis M, Wurbel MA, Muller W, Papadakis KA, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn's disease. Gastroenterology. 2008;134(7):2025–35. https://doi.org/10.1053/j.gastro.2008.02.085.
Kaser A, Martinez-Naves E, Blumberg RS. Endoplasmic reticulum stress: implications for inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2010;26(4):318–26. https://doi.org/10.1097/MOG.0b013e32833a9ff1.
Jabri B, Ebert E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol Rev. 2007;215:202–14. https://doi.org/10.1111/j.1600-065X.2006.00481.x.
Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–31. https://doi.org/10.1111/j.0105-2896.2005.00284.x.
Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11(8):656–65. https://doi.org/10.1038/ni.1905.
Fuchs A, Colonna M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr Opin Gastroenterol. 2013;29(6):581–7. https://doi.org/10.1097/MOG.0b013e328365d339.
Liu Z, Yadav PK, Xu X, Su J, Chen C, Tang M, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol. 2011;89(4):597–606. https://doi.org/10.1189/jlb.0810456.
Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20(2):185–91. https://doi.org/10.1016/j.coi.2008.03.009.
Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8alphabeta versus CD8alphaalpha expression. J Immunol. 2001;167(5):2577–84.
Sydora BC, Mixter PF, Holcombe HR, Eghtesady P, Williams K, Amaral MC, et al. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993;150(6):2179–91.
Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294(5548):1936–9. https://doi.org/10.1126/science.1063564.
Teitell M, Mescher MF, Olson CA, Littman DR, Kronenberg M. The thymus leukemia antigen binds human and mouse CD8. J Exp Med. 1991;174(5):1131–8.
Olivares-Villagomez D, Mendez-Fernandez YV, Parekh VV, Lalani S, Vincent TL, Cheroutre H, et al. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2008;105(46):17931–6. https://doi.org/10.1073/pnas.0808242105.
Hoffmann JC, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Kuhl AA. Gammadelta T lymphocytes: a new type of regulatory T cells suppressing murine 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Int J Color Dis. 2008;23(10):909–20. https://doi.org/10.1007/s00384-008-0535-8.
Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity. 2001;15(3):419–34.
Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14(3):281–9. https://doi.org/10.1038/ni.2523.
•• Sujino T, London M, Hoytema van Konijnenburg DP, Rendon T, Buch T, Silva HM, et al. Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science. 2016;352(6293):1581–6. https://doi.org/10.1126/science.aaf3892. This report is the first to demonstrate the conversion of lamina propria Foxp3 + Tregs into Foxp3 - CD4 + CD8 + IELs.
• Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quevrain E, et al. CD4CD8alphaalpha lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol. 2014;12(4):e1001833. https://doi.org/10.1371/journal.pbio.1001833. This report identifies a protective role for CD4 + CD8 + T cells in IBD patients.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. https://doi.org/10.1126/science.1198469.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. https://doi.org/10.1016/j.cell.2009.09.033.
Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67(7):3504–11.
• Tada A, Zelaya H, Clua P, Salva S, Alvarez S, Kitazawa H, et al. Immunobiotic Lactobacillus strains reduce small intestinal injury induced by intraepithelial lymphocytes after Toll-like receptor 3 activation. Inflamm Res. 2016;65(10):771–83. https://doi.org/10.1007/s00011-016-0957-7. This study demonstrates that commensal Lactobacillus reduces the licensing of IEL cytolytic activity in response to TLR3 activation.
Roselli M, Finamore A, Nuccitelli S, Carnevali P, Brigidi P, Vitali B, et al. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm Bowel Dis. 2009;15(10):1526–36. https://doi.org/10.1002/ibd.20961.
•• Cervantes-Barragan L, Chai JN, Tianero MD, DiLuccia B, Ahern PP, Merriman J et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8alphaalpha+ T cells. Science. 2017. https://doi.org/10.1126/science.aah5825. This report shows that colonization or fecal transfer of L. reuteri can induce the differentiation of CD4+ CD8+ IELs through an AhR-dependent mechanism.
Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, et al. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci U S A. 1993;90(18):8591–4.
Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108(21):8743–8. https://doi.org/10.1073/pnas.1019574108.
Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210(11):2465–76. https://doi.org/10.1084/jem.20122490.
Qiu Y, Pu A, Zheng H, Liu M, Chen W, Wang W, et al. TLR2-dependent signaling for IL-15 production is essential for the homeostasis of intestinal intraepithelial lymphocytes. Mediat Inflamm. 2016;2016:4281865. https://doi.org/10.1155/2016/4281865.
Kaneko M, Mizunuma T, Takimoto H, Kumazawa Y. Development of TCR alpha beta CD8 alpha alpha intestinal intraepithelial lymphocytes is promoted by interleukin-15-producing epithelial cells constitutively stimulated by gram-negative bacteria via TLR4. Biol Pharm Bull. 2004;27(6):883–9.
Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol. 2006;176(10):6180–5.
•• Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41(2):311–24. https://doi.org/10.1016/j.immuni.2014.06.015. This is the first report linking pro-inflammatory changes in IEL effector function to an expansion of a specific commensal B. vulgatus as a result of Nod2 deficiency.
•• Kober OI, Ahl D, Pin C, Holm L, Carding SR, Juge N. Gammadelta T-cell-deficient mice show alterations in mucin expression, glycosylation, and goblet cells but maintain an intact mucus layer. Am J Physiol Gastrointest Liver Physiol. 2014;306(7):G582–93. https://doi.org/10.1152/ajpgi.00218.2013. This report demonstrates that γδ T cell-deficiency adversely affects goblet cell number and mucus production.
Sheng YH, Hasnain SZ, Florin TH, McGuckin MA. Mucins in inflammatory bowel diseases and colorectal cancer. J Gastroenterol Hepatol. 2012;27(1):28–38. https://doi.org/10.1111/j.1440-1746.2011.06909.x.
Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6(3):451–63. https://doi.org/10.1038/mi.2013.13.
Lee J, Geddes K, Streutker C, Philpott DJ, Girardin SE. Role of mouse peptidoglycan recognition protein PGLYRP2 in the innate immune response to Salmonella enterica serovar Typhimurium infection in vivo. Infect Immun. 2012;80(8):2645–54. https://doi.org/10.1128/IAI.00168-12.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42; quiz e30. https://doi.org/10.1053/j.gastro.2011.10.001.
• Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152(2):398–414 e6. https://doi.org/10.1053/j.gastro.2016.10.019. This recent review discusses the impact of diet and bacterial metabolism on IBD development and therapies.
Menezes JS, Mucida DS, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, et al. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2003;15(3):447–55.
Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40. https://doi.org/10.1016/j.cell.2011.09.025.
Franco Robles E, Pérez Vázquez V, Ramírez Emiliano J, González Amaro R, López BS. High fat diet induces alterations to intraepithelial lymphocyte and cytokine mRNA in the small intestine of C57BL/6 mice. Royal Society of Chemistry. 2017;7(9):5322–30. https://doi.org/10.1039/C6RA24689C.
Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151(1):130–8. https://doi.org/10.1111/j.1365-2249.2007.03530.x.
Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–38. https://doi.org/10.1016/j.immuni.2004.08.011.
Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21(1):8–13. https://doi.org/10.1016/j.smim.2008.09.002.
Lee H, Ko G. Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Sci Rep. 2016;6:25835. https://doi.org/10.1038/srep25835.
Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47(2):211–4.
Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8(4):417–20. https://doi.org/10.1089/gte.2004.8.417.
Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230–3.
Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–74.
Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105(52):20834–9. https://doi.org/10.1073/pnas.0808700106.
Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol. 2007;102(9):2016–25. https://doi.org/10.1111/j.1572-0241.2007.01411.x.
Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. https://doi.org/10.1038/nri3384.
Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol. 2015;296(1):31–40. https://doi.org/10.1016/j.cellimm.2015.04.008.
Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, et al. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171(10):5507–13.
Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182(5):3047–54. https://doi.org/10.4049/jimmunol.0802705.
•• Edelblum KL, Singh G, Odenwald MA, Lingaraju A, El Bissati K, McLeod R, et al. Gammadelta intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology. 2015;148(7):1417–26. https://doi.org/10.1053/j.gastro.2015.02.053. This is the first report demonstrating that γδ IEL motility and surveillance of the epithelium is required for protection against acute microbial invasion.
Kabelitz D, Lettau M, Janssen O. Immunosurveillance by human gammadelta T lymphocytes: the emerging role of butyrophilins. F1000Res. 2017;6. https://doi.org/10.12688/f1000research.11057.1.
Walker CR, Hautefort I, Dalton JE, Overweg K, Egan CE, Bongaerts RJ, et al. Intestinal intraepithelial lymphocyte-enterocyte crosstalk regulates production of bactericidal angiogenin 4 by Paneth cells upon microbial challenge. PLoS One. 2013;8(12):e84553. https://doi.org/10.1371/journal.pone.0084553.
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8. https://doi.org/10.1126/science.1209791.
Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109(18):7097–102. https://doi.org/10.1073/pnas.1112519109.
Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR II, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(1):111–26. https://doi.org/10.1083/jcb.200902153.
Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266(5188):1253–5.
• Meehan TF, Witherden DA, Kim CH, Sendaydiego K, Ye I, Garijo O, et al. Protection against colitis by CD100-dependent modulation of intraepithelial gammadelta T lymphocyte function. Mucosal Immunol. 2014;7(1):134–42. https://doi.org/10.1038/mi.2013.32. This is the first demonstration that a direct ligand/receptor interaction between γδ IELs and epithelial cells is required to promote epithelial regeneration following DSS-induced injury.
Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75(2):274–82.
Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, et al. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183(3):847–56.
Takahashi I, Iijima H, Katashima R, Itakura M, Kiyono H. Clonal expansion of CD4+ TCRbetabeta+ T cells in TCR alpha-chain- deficient mice by gut-derived antigens. J Immunol. 1999;162(3):1843–50.
Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2011;184(4):1675–9. https://doi.org/10.4049/jimmunol.0903539.
•• Do JS, Kim S, Keslar K, Jang E, Huang E, Fairchild RL, et al. Gammadelta T cells coexpressing gut homing alpha4beta7 and alphaE integrins define a novel subset promoting intestinal inflammation. J Immunol. 2017;198(2):908–15. https://doi.org/10.4049/jimmunol.1601060. This study shows that a subset of inflammatory γδ T cells within the lamina propria and mesenteric lymph node promotes chronic intestinal inflammation.
• Catalan-Serra I, Sandvik AK, Bruland T, Andreu-Ballester JC. Gammadelta T cells in Crohn’s disease: a new player in the disease pathogenesis? J Crohns Colitis. 2017; https://doi.org/10.1093/ecco-jcc/jjx039. This review provides a detailed overview of the current knowledge regarding the role of γδ T cells in the pathogenesis of Crohn’s disease and the potential of these cells as a therapeutic target for immunotherapy.
Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120(11):2269–79. https://doi.org/10.1182/blood-2012-05-430470.
McCarthy NE, Bashir Z, Vossenkamper A, Hedin CR, Giles EM, Bhattacharjee S, et al. Proinflammatory Vdelta2+ T cells populate the human intestinal mucosa and enhance IFN-gamma production by colonic alphabeta T cells. J Immunol. 2013;191(5):2752–63. https://doi.org/10.4049/jimmunol.1202959.
•• Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, Eberl M. Antigen-presenting human gammadelta T cells promote intestinal CD4+ T cell expression of IL-22 and mucosal release of calprotectin. J Immunol. 2017;198(9):3417–25. https://doi.org/10.4049/jimmunol.1700003. This report demonstrates that Vγ9/Vδ2 T cells promote local barrier defense through the production of antimicrobial proteins.
McCarthy NE, Hedin CR, Sanders TJ, Amon P, Hoti I, Ayada I, et al. Azathioprine therapy selectively ablates human Vdelta2(+) T cells in Crohn’s disease. J Clin Invest. 2015;125(8):3215–25. https://doi.org/10.1172/JCI80840.
Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991;21(4):1053–9. https://doi.org/10.1002/eji.1830210429.
•• Di Marco BR, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific gammadelta T cell compartments. Cell. 2016;167(1):203–18 e17. https://doi.org/10.1016/j.cell.2016.08.030. This is the first demonstration that epithelial butyrophilin expression shapes local intestinal γδ T cell development and function.
•• Lebrero-Fernandez C, Wenzel UA, Akeus P, Wang Y, Strid H, Simren M, et al. Altered expression of Butyrophilin (BTN) and BTN-like (BTNL) genes in intestinal inflammation and colon cancer. Immun Inflamm Dis. 2016;4(2):191–200. https://doi.org/10.1002/iid3.105. This report provides the first indication that butryophilin gene expression is altered in ulcerative colitis.
• Rogoz A, Reis BS, Karssemeijer RA, Mucida D. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J Immunol Methods. 2015;421:89–95. https://doi.org/10.1016/j.jim.2015.03.014. This is the first report demonstrating IEL migration in a novel murine IEL/enteroid co-culture model.
Nozaki K, Mochizuki W, Matsumoto Y, Matsumoto T, Fukuda M, Mizutani T, et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol. 2016;51(3):206–13. https://doi.org/10.1007/s00535-016-1170-8.
Acknowledgements
The authors would like to thank Dr. Tessa Bergsbaken for her critical review and thoughtful suggestions regarding the manuscript.
Funding
This work is supported by funding from the National Institutes of Health K01 DK093627, R03 DK106484 and the Feldstein Medical Foundation (KLE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Immunology and Inflammation
Rights and permissions
About this article
Cite this article
Hu, M.D., Edelblum, K.L. Sentinels at the Frontline: the Role of Intraepithelial Lymphocytes in Inflammatory Bowel Disease. Curr Pharmacol Rep 3, 321–334 (2017). https://doi.org/10.1007/s40495-017-0105-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-017-0105-2