Abstract
Introduction
The phase III randomized KEYNOTE-522 trial demonstrated that pembrolizumab in combination with chemotherapy as neoadjuvant treatment followed by adjuvant pembrolizumab (pembrolizumab + chemotherapy) provided significant improvements in event-free survival (EFS) and overall survival (OS) for patients with high-risk early-stage triple-negative breast cancer (eTNBC). The objective was to assess the cost-effectiveness of pembrolizumab + chemotherapy compared to neoadjuvant chemotherapy alone (chemotherapy) in patients with high-risk eTNBC from a Hong Kong third-party payer perspective.
Methods
A multistate transition model with four health states (event-free), locoregional recurrence, distant metastases, and death) was developed to assess the lifetime medical costs and health outcomes (3% annual discount), along with incremental cost-effectiveness ratios (ICERs) using efficacy and safety data from the KEYNOTE-522 trial. The health state utilities were derived from KEYNOTE-522 Euro-QoL-five-dimension five-level questionnaire (EQ-5D-5L) data. Costs were expressed in 2022 Hong Kong dollars (HKD). Scenario and sensitivity analyses were performed to assess the robustness of results.
Results
Over a 32-year time horizon, base case results showed that pembrolizumab + chemotherapy was associated with a 3.42 year longer EFS and expected gains of 3.05 life years (LYs) and 2.45 quality-adjusted life years (QALYs) compared to chemotherapy. The resultant ICERs were HKD 135,200 per QALY gained and HKD 108,463 per LY gained, which were lower than the World Health Organization (WHO) cost-effectiveness threshold of three times gross domestic product (GDP) per capita for Hong Kong of HKD 1,171,308 per QALY. The one-way sensitivity analyses (OWSA) and probabilistic sensitivity analysis (PSA) showed the results were robust across various inputs and alternative scenarios.
Conclusion
On the basis of the analysis conducted for a 56-year-old cohort with high-risk eTNBC and assumptions in the model, pembrolizumab + chemotherapy represents a cost-effective proposition (as the ICER is approximately 35% of the GDP per capita in Hong Kong) for patients with high-risk eTNBC in Hong Kong.
Plain Language Summary
The manuscript outlines the methods and results of a health economic model to estimate the cost-effectiveness of pembrolizumab + chemotherapy compared to neoadjuvant chemotherapy alone (chemotherapy) in patients with high-risk early-stage triple-negative breast cancer in the Hong Kong setting. The results of the manuscript show that the combination of pembrolizumab and chemotherapy provides an additional 3.05 life years and 2.45 quality-adjusted life years. Despite the potential drawbacks of employing gross domestic product (GDP) as a proxy for cost-effectiveness thresholds, the incremental cost-effectiveness ratio was less than three times GDP per capita in Hong Kong. Therefore, the new intervention was cost-effective. However, like any similar study, the results rely substantially on extrapolation of trial outcomes and thus sensitivity analysis has also been performed to handle the uncertainty associated with results. The one-way sensitivity analyses and probabilistic sensitivity analysis showed the results were robust. The manuscript provides economic evidence of the new intervention and provides new clinical insights.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
The KEYNOTE-522 trial demonstrated that pembrolizumab in combination with chemotherapy as neoadjuvant treatment followed by adjuvant pembrolizumab (pembrolizumab + chemotherapy) improved event-free survival (EFS) along with overall survival (OS) in high-risk patients with high-risk early-stage triple-negative breast cancer (eTNBC). |
The study aimed to assess whether pembrolizumab + chemotherapy against chemotherapy in patients with high-risk eTNBC is cost-effective from a Hong Kong third-party payer perspective. |
The results from current analysis reflect that at the given willingness to pay (WTP) threshold for Hong Kong, pembrolizumab + chemotherapy was cost-effective. |
Patients receiving pembrolizumab + chemotherapy were projected to experience fewer recurrences and thereby accrue fewer costs in the locoregional recurrence (LR) and distant metastases (DM) health states compared to chemotherapy. |
Additionally, life years (LYs) and quality-adjusted life years (QALYs) were gained because patients treated with pembrolizumab + chemotherapy spent more time in the EF state, and fewer of them progressed to DM as compared to chemotherapy. |
Introduction
Breast cancer (BC) has become the third most common cancer after lung (15.9%) and colorectum (14.9%) cancer in Hong Kong, with an annual growth rate of 4.1% in 2020 [1]. It is the leading cancer for women in Hong Kong and accounts for 28.4% of all new cancers [2]. It is the third leading cause of cancer deaths in women. According to Hong Kong Cancer Registry, Hospital Authority, there were 4956 new cases of BC and 751 BC-related deaths among Hong Kong women in 2020 [1]. The lifetime risk of BC is 1 in every 14 women in Hong Kong [2].
Definitive surgical resection is considered as the primary treatment for early-stage BC. For patients with high-risk early-stage triple negative BC (eTNBC), neoadjuvant chemotherapy has been the standard of care [3, 4], with adjuvant chemotherapy being an optional treatment for this cohort [5]. These patients nonetheless face significant mortality and recurrence rates, highlighting the unmet need for new therapeutics that can improve the efficacy of chemotherapy [6].
Pembrolizumab is a high-affinity monoclonal antibody that blocks the interaction between programmed cell-death (PD-1) receptor and programmed cell-death ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2). On the basis of the results of the phase III KEYNOTE-522 trial (ClinicalTrials.gov identifier NCT03036488), the US Food and Drug Administration and the European Medicines Agency both approved pembrolizumab for the treatment of high-risk eTNBC in combination with chemotherapy as neoadjuvant therapy followed by single-agent adjuvant therapy after surgery in July 2021 and May 2022, respectively [7,8,9].
The comparator chemotherapy was the control group in the KEYNOTE-522 trial, which was administered as a management strategy for high-risk eTNBC in the neoadjuvant phase to reflect local clinical practice in Hong Kong. The patients then received definitive surgery after 3–6 weeks of neoadjuvant treatment completion. In the adjuvant phase, radiation therapy as indicated or placebo was administered Q3W for 9 cycles.
The first interim analysis of KEYNOTE-522 at a median follow-up of 15.5 months showed that the proportion of patients with pathological complete response (having no invasive cancer in the breast and negative nodes) was significantly higher in the pembrolizumab + chemotherapy group compared to chemotherapy (64.8% vs. 51.2%, p < 0.001) [10]. With a median follow-up of 39.1 months, the fourth interim analysis of KEYNOTE-522 resulted in a statistically significant and clinically meaningful improved EFS in the pembrolizumab + chemotherapy group compared to the chemotherapy group with high-risk eTNBC (84.5% vs. 76.8%, p < 0.001). Furthermore, the estimated OS at 36 months was 89.7% for pembrolizumab + chemotherapy and 86.9% for chemotherapy (hazard rate (HR) 0.72; 95% confidence interval (CI) [0.51, 1.02]). However, the median overall survival was not reached in either group but the trial follow-up is still ongoing. Also, the addition of pembrolizumab to neoadjuvant chemotherapy was typically well tolerated [11]. Table 1 compares the modelled OS against the internal and external sources.
Both National Institute for Health and Care Excellence (NICE) and Canadian Agency for Drugs and Technologies in Health (CADTH) have published their positive reviews of pharmacoeconomic models for pembrolizumab + chemotherapy in high-risk eTNBC, providing further support of the cost-effectiveness of pembrolizumab + chemotherapy in those settings [12, 13]. Further, an economic model has been published from the US perspective, indicating evidence for pembrolizumab + chemotherapy being cost-effective for high-risk patients with eTNBC and another one based on the Swiss perspective is under review for journal publication.
This study’s goal was to assess the cost-effectiveness of pembrolizumab + chemotherapy versus chemotherapy in patients with high-risk eTNBC from a Hong Kong third-party payer perspective. The KEYNOTE-522 data and treatment patterns and healthcare resource utilization specific to Hong Kong clinical practice served as the basis for this cost-effectiveness analysis (CEA).
Methods
Population and Patient Characteristics
In this analysis, patients with high-risk eTNBC were considered as the target population, consistent with the population in the KEYNOTE-522 trial [7]. The baseline characteristics of patients, including starting age, body weight, and body surface area (BSA), were obtained from Hong Kong Breast Cancer Study (HKBCS). Only female patients were considered in the model for analysis (Table 2). WTP willingness-to-pay, QALY quality-adjusted life year.
Model Structure and Analysis
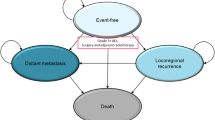
The state transition cohort model was developed using Microsoft Excel® 2016. Figure 1 illustrates the health states and allowable transitions in the cost-effectiveness model. The model consists of four mutually exclusive health states (event-free [14], locoregional recurrence [LR], distant metastases [DM], and death) to track patients’ disease course and death over time.
In the KEYNOTE-522 trial, patients were randomized to the pembrolizumab + chemotherapy arm or chemotherapy arm. In the neoadjuvant phase of the pembrolizumab + chemotherapy arm, pembrolizumab (200 mg administered once every 3 weeks (Q3W) on day 1 of cycles 1–8) in combination with chemotherapy (4 cycles of paclitaxel plus carboplatin followed by 4 cycles of doxorubicin or epirubicin plus cyclophosphamide) was administered to the patients. The patients then received definitive surgery after 3–6 weeks of neoadjuvant treatment completion. In the adjuvant phase, radiation therapy as indicated or pembrolizumab as a single agent was administered Q3W for 9 cycles. Chemotherapy is considered as a base case comparator to pembrolizumab + chemotherapy as a management strategy for high-risk eTNBC in the neoadjuvant phase to reflect local clinical practice in Hong Kong. The patients then received definitive surgery after 3–6 weeks of neoadjuvant treatment completion. In the adjuvant phase, radiation therapy as indicated or placebo was administered Q3W for 9 cycles [7]. The details of dosing schedule of different regimens in both the pembrolizumab + chemotherapy arm and chemotherapy arm, based on the KEYNOTE-522 trial, are provided in Table 3, whereas the details for adjuvant pembrolizumab are provided in Table 4.
This economic evaluation was conducted from a Hong Kong third-party payer perspective. Therefore, only direct healthcare costs were considered, expressed in Hong Kong dollars (HKD). The original cost values were inflated to 2022 prices using the consumer price index (CPI) obtained from the Census and Statistics Department, Hong Kong [15].
A time horizon of 32 years was used in the analysis to capture all relevant costs and benefits. The cohort of patients were followed up until 88 years of age, which is the average life expectancy of female citizens in Hong Kong as per the World Bank data [16], from the starting age of 56 years of the patient cohort at the model entry based on HKBCS. The present model used a weekly cycle length to allow for precise calculation of drug acquisition and administration costs. Also, half-cycle correction was applied to costs and effectiveness for additional precision in the base case. Costs and effectiveness were discounted at 3% annually, as recommended by the annual report on CPI, Census and Statistics Department, Hong Kong [17]. The outcomes of the model included aggregated and disaggregated incremental costs, quality-adjusted life years (QALYs) and life years (LYs) estimated for each treatment arm, along with the incremental cost-effectiveness ratio (ICER) expressed as incremental cost per QALY gained and per LY gained.
A series of scenario and one-way sensitivity analyses (OWSA) were conducted to assess the robustness of results through changes in parameters and under alternative model settings. A probabilistic sensitivity analysis (PSA) was also conducted to estimate the probability of pembrolizumab + chemotherapy being cost-effective relative to chemotherapy, based on the willingness-to-pay (WTP) threshold of HKD 1,171,308 per QALY, which is three times of gross domestic product (GDP) per capita for Hong Kong as of June 2023 [18]. For the PSA, a Monte Carlo simulation with 1000 iterations was conducted, and in each iteration, the model inputs were randomly drawn from the specified distributions (Table 2). The standard errors of the selected distributions were obtained directly from the same data source that informed the mean value, if available. Otherwise, the standard error for each cost parameter was assumed to be equal to 50% of the mean value. Tables S1 and S2 in the Supplementary Materials provide more information on the scenario analyses, OWSA and PSA.
Clinical Inputs
Transition Probabilities
The transition probabilities in the Markov model were based on patient-level data from the KEYNOTE-522 trial, with extrapolation using fitted parametric functions. Patients enter the model in the EF state, and at the end of each cycle, patients may remain in the EF state or transition to LR, DM, or death state. Also, patients who are in the LR state may stay in the same state or transition to DM, or death state, but could not transition back to the EF state. Patients in the DM state may stay in the DM or transition to death state but could not transition back to the EF or LR state.
The survival curve fitting for different parametric functions was carried out in line with the NICE Decision Support Unit guidelines [19]. Standard one-piece and two-piece parametric models were extrapolated using exponential, Weibull, log-normal, log-logistic, Gompertz, gamma, and generalized gamma distributions. For the base case, Akaike information criterion (AIC) and Bayesian information criterion (BIC) of statistical tests combined with visual inspection were used to obtain the best fit parametric distributions. The clinical plausibility of the long-term extrapolations was also evaluated for the selected distributions. Alternative parametric functions at different cutoff points were tested in scenario analyses. Table S3 in the Supplementary Materials presents detailed information regarding all health state transitions and data sources.
Transition Probabilities from EF State
The estimated probability of an EFS event and the probabilities of first EFS event being LR, DM, or death in each treatment arm were used to compute the cause-specific probability of each transition from the EF state (EF → LR, EF → DM, or EF → death) for each model cycle. Data from the KEYNOTE-522 trial were used to estimate the probability of the first EFS event being LR, DM, or death whereas the time to LR, time to DM, and time to death were analyzed using Gray’s method for competing risks [20]. The probability of the EFS event was capped by the all-cause natural mortality based on Hong Kong-specific life tables [21].
Different parametric models were fitted to the patient-level data from the KEYNOTE-522 trial to extrapolate the EFS throughout the modelled time horizon [22]. The base case parametric survival models were selected on the basis of the statistical fit, visual inspection, and clinical plausibility of the extrapolated model for both the pembrolizumab + chemotherapy and chemotherapy arms. Week 50 was selected as a suitable cutoff point for two-piece models. Generalized gamma and log-normal distributions were chosen as the best-fit for base case analysis for the pembrolizumab + chemotherapy arm and the chemotherapy arm, respectively [23]. Alternative parametric functions (log-normal and generalized gamma) at different cutoff points (week 43, 50, and 68) were tested in scenario analyses (Table 2). Figure 2a presents the Kaplan–Meier curves and the base case extrapolated curves for EFS. The base case curves and alternative parametric functions are shown in Fig. S1 in the Supplementary Materials and the estimated cumulative incidence of the three transitions EF → LR, EF → DM, and EF → death along with the actual cumulative incidence derived from the KEYNOTE-522 trial data is presented in Fig. S2 in the Supplementary Materials.

Source for observed survival: Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. New England Journal of Medicine. 2022 Feb [11]
Predicted long-term outcomes under base case parametric distribution assumptions versus observed data from the KEYNOTE-522 trial. a EFS. b OS. EFS event-free survival, KM Kaplan–Meier, OS overall survival.
Transition Probabilities from LR State
The KEYNOTE-522 trial data for time from LR to DM or death were fitted using parametric models, and the best fit was an exponential distribution [22]. As a result of the limited number of patients with confirmed LR in the trial, pooled data from both the treatment arms were used to estimate transition probabilities of LR → DM or death. The transition probabilities of LR → DM and LR → death were obtained for each model cycle using estimated probability of LR → DM or death, and the proportions of DM and death, respectively, obtained from the KEYNOTE-522 trial.
Transition Probabilities from DM State
The transition probabilities from DM to death were calculated using the duration of survival for patients with documented DM in the KEYNOTE-522 trial [22]. Parametric models were fitted on patient-level data for each treatment arm, providing an exponential distribution as the best fit. As a result, the fitted exponential distribution was used to estimate the time-constant transition probability of DM → death.
Adverse Events
The model accounted for medical costs and health disutilities associated with all-cause grade 3+ adverse events (AEs) with an incidence of at least 5% from the combined neoadjuvant and adjuvant phases in either arm. KEYNOTE-522 trial data were used to determine the incidence rates, mean durations (Table 2), and hospitalization rates of AEs (provided in Table S5 in the Supplementary Materials) [22].
Health Utilities
Utility inputs used in the base case and scenario analyses were derived through primary analyses of the EuroQoL-five-dimension questionnaire (EQ-5D-5L) data collected in the KEYNOTE-522 trial [22]. The generic health status assessed from the EQ-5D questionnaires were converted to population-based utility values using the UK algorithm [24]. Utilities based on the UK EQ-5D-5L value set were used in the base case analysis. The impact of the UK five-level (5L) crosswalk to three-level (3L) utility algorithm was tested in the scenario analysis. Utilities were examined by health state, treatment status (on- or off-treatment), and AE status. Pooled data were used because of insignificant differences between treatment arms. Furthermore, disutility related to aging was applied on the basis of a published model with mean health state utility values from the general population [25]. Coefficients used for age-related disutility in the model are presented in Table S4 in the Supplementary Materials.
Costs
Different cost categories considered in the analysis are presented in Fig. 3 and their corresponding costs are provided in Table 2. The costs were obtained from Hospital Authority, Hong Kong and were inflated to 2022 on the basis of CPI obtained from the Census and Statistics Department, Hong Kong [15].
Neoadjuvant and Adjuvant Drug Cost
Drug acquisition costs for neoadjuvant and adjuvant treatment were calculated as a function of the list price per drug unit (Table 5), defined dosing for the medication, relative dose intensity (RDI), and time on treatment (TOT) (Tables 3 and 4 for neoadjuvant and adjuvant treatment, respectively). The list price per drug unit were retrieved from Hospital Authority, Hong Kong. The dosing schedules for all drugs were consistent with the treatment protocol used in the KEYNOTE-522 trial [7]. The TOT for neoadjuvant and adjuvant treatments was estimated using observed Kaplan–Meier curves from the KEYNOTE-522 trial which were used to determine the number of treatment cycles in each arm (Fig. S3 in the Supplementary Materials depicts the TOT in neoadjuvant and adjuvant setting). The projected TOT was then adjusted according to the RDI observed in the KEYNOTE-522 trial, to account for any delay or interruptions in administration [22].
Pembrolizumab is administered at a fixed dose of 200 mg, once Q3W on day 1 of cycles 1 through 8 and is available in 100-mg vials only. The unit price of pembrolizumab 100 mg vial (HKD 19,800) was obtained from Hospital Authority, Hong Kong. The average number of vials per administration for each BSA-based or body weight-based regimens was estimated using a log-normal distribution of the BSA (using mean as 1.92 m2 and SD as 0.23) or body weight distribution (using mean value as 76.37 kg and SD as 17.84), respectively, of patients in Hong Kong. An optimal vial mix algorithm was employed to reduce vial wastage in the base case, where the lowest cost per milligram vial size was selected.
A unit cost of HKD 2400.00 for chemotherapy preparation and administration, obtained from the Hospital Authority, List of Private Services, Hong Kong, was included as an administration cost for each treatment combination, and then applied to the weekly model cycles according to the dosing schedule of each individual drug (Table 2) [26].
Subsequent Therapy Cost
After the documented DM in the KEYNOTE-522 trial, about 62.5% of patients in the pembrolizumab + chemotherapy arm and 70.3% in the chemotherapy arm received systemic anticancer therapy. When patients entered the DM state, the expenses of drug acquisition and administration for first- to fourth-line metastatic TNBC treatments were included. The KEYNOTE-522 trial data were used for distribution and duration of subsequent treatments in the model [22]. The average cost for post-DM therapy was estimated to be HKD 308,030.97 per patient for the pembrolizumab + chemotherapy arm, whereas it was calculated to be HKD 206,854.46 per patient for the chemotherapy arm. Detailed dosing schedule, relative dose intensity, and treatment allocation in first-line metastatic treatment are provided in Table 6.
Surgery and Radiation Cost
The KEYNOTE-522 protocol stated that when neoadjuvant therapy was completed, patients would undergo definitive surgery 3–6 weeks later [7]. Following the procedure, radiation therapy and adjuvant therapies were administered as prescribed. Costs for surgery were calculated on the basis of the weighted average unit costs of lumpectomy (HKD 24,000.00) and mastectomy (35,750.00) obtained from Queen Mary Hospital Private Clinic (Table 2) [27]. The unit cost of radiation, HKD 150,000.00 (taken as an average of HKD 100,000.00 and HKD 200,000.00, the end-points of the range of cost), was obtained from Queen Mary Hospital Private Clinic, which estimated the mean radiation costs among patients who had breast-conserving surgery [27]. The percentages of patients who underwent surgery and radiation therapy in the pembrolizumab + chemotherapy arm (98.00% and 75.90%, respectively) and chemotherapy arm (97.70% and 78.50%, respectively) were obtained from KEYNOTE-522 trial data [22].
Disease Management Cost
The disease management cost comprised recurring and one-off disease management costs. The recurring disease management costs in EF state across various time periods were based on the average cost of consultation for each subsequent follow-up, and were obtained from the Hospital Authority, List of Private Services, Hong Kong [26]. The frequency of use of these resources and the respective costs were used to estimate the average recurring disease management costs and these were then converted to costs per week for EF state, which are shown in Table 2. Recurring disease management costs for the LR and DM states include the cost of clinical visits and the cost of medical interventions (cost of tumor markers, positron emission tomography/computed tomography (PET/CT) whole body, magnetic resonance imaging (MRI) brain, and standard lab tests). Just like in the EF state, the average recurring disease management costs were estimated and further converted into costs per week (Table 2) for both the LR and DM state.
After entering LR or DM stage, a one-time disease management cost was included as an average cost for biopsy of recurrence sites. These costs were taken from the Hospital Authority’s List of Private Services, Hong Kong (Operations) (Table 2) [28].
Terminal Care Cost
Terminal care cost was applied as a one-time cost prior to death, under the assumption that the cost was equal for death from EF, LR, or DM states. It includes expenses in the last 6 months of life and was obtained from Wong et al. [29]. The cost was inflated to 2022 using CPI indices from the Census and Statistics Department, Hong Kong, and then converted to from USD to HKD using currency exchange rate to include in the CEA [21].
Adverse Event Management Cost
The one-time AE costs were computed as a function of the AE rates, the proportion hospitalized for each AE event, and the unit costs of medical management for each AE in the inpatient or outpatient setting, for each treatment arm.
The unit costs of AE management were calculated by multiplying the number of hospitalized days and outpatient visits required for each AE, obtained from Hospital Authority, Hong Kong, with the unit cost of hospitalization (HKD 6650) and outpatient visit (HKD 1990), respectively, obtained from the List of Private Services, Hospital Authority, Hong Kong [26]. Table 2 displays the medical management unit costs for each AE. It was assumed that the patients with neutropenia, neutrophil count decreased, and decreased white blood cell count did not require hospitalization, and thus the hospitalization cost per unit for the aforementioned AEs was zero.
Ethical Approval
This article is based on the data drawn from trials that had already been completed and from published literature and does not contain any studies with human participants or animals performed by any of the authors.
Model Validation
The observed OS from the KEYNOTE-522 trial serves as the initial validation for the modelled OS [22]. The projected OS curves for both the arms closely resemble the observed Kaplan–Meier curve, as seen in Fig. 2b. Following that, external data from Walsh et al. [30] and Sikov et al. [14] were used to compare the projected OS curve for chemotherapy (Fig. S4 in the Supplementary Materials). Independent clinical experts also confirmed the clinical plausibility of projected long-term outcomes. External data were not available to validate the modelled OS for the patients with eTNBC who received pembrolizumab treatment. The panel of key opinion leaders (KOLs), consisting of eight medical oncologists and two health economists from Europe, validated the intervention extrapolation in terms of the clinical plausibility and in comparison, with the comparator extrapolation in terms of the expected improvement with immuno-oncology therapies. The panel of KOLs supported the validity of the long-term OS estimate for the pembrolizumab + chemotherapy arm.
Results
Base Case
Over the 32-year time horizon, the total costs were HKD 1,040,120 (discounted costs HKD 967,743) for the pembrolizumab + chemotherapy arm and HKD 751,012 (discounted costs HKD 636,550) for the chemotherapy arm, whereas total QALYs were estimated to be 19.09 (discounted QALYs 13.15) for pembrolizumab + chemotherapy and 15.04 (discounted QALYs 10.70) for chemotherapy. Total LYs were estimated to be 24.03 (discounted LYs 16.33) and 18.92 (discounted LYs 13.28) for the pembrolizumab + chemotherapy arm and chemotherapy arm, respectively. Therefore, the patients treated with pembrolizumab + chemotherapy were projected to gain 5.12 LYs (discounted incremental LYs 3.05) and 4.04 QALYs (discounted incremental QALYs 2.45) relative to those in the chemotherapy arm. The total time spent in the EF state was 23.68 years (discounted value 16.03 years) in the pembrolizumab + chemotherapy arm compared with 18.08 years (discounted value 12.61 years) in the chemotherapy arm. The discounted base case results are presented in Table 7.
The treatment with pembrolizumab + chemotherapy had a projected increase of HKD 331,192 in the total discounted costs, driven mostly by the drug acquisition costs in the neoadjuvant and adjuvant phases, and offset by disease management cost, metastatic treatment cost, and terminal care cost. The resulting ICERs (based on discounted costs and health outcomes) were HKD 135,200 per QALY gained, and HKD 108,463 per LY gained for pembrolizumab + chemotherapy vs. chemotherapy. As the ICER was below Hong Kong’s WTP threshold, pembrolizumab + chemotherapy is a cost-effective treatment option in Hong Kong.
Scenario Analyses
Scenario analyses were performed to assess uncertainty in the model associated with key model assumptions and specifications. The inputs and settings varied in the scenario analyses are reported in Table S1 in the Supplementary Materials, along with the base case inputs and settings, accompanied by the resulting ICERs.
The resultant ICERs, in terms of incremental cost per QALY gained, for scenario analyses are all below Hong Kong’s WTP threshold of HKD 1,171,308 per QALY, consistent with the findings of the base case CEA. The ICERs from the scenario analyses range from HKD 81,902 per QALY gained (obtained by considering no discount rate for effectiveness) to HKD 664,935 per QALY gained (obtained by considering 10-year time horizon).
OWSA and PSA
Table S2 in the Supplementary Materials report parameters varied in the OWSA and PSA. Figure 4 presents OWSA results with the most influential parameters shown at the top of the tornado diagram. According to to the findings of the OWSA, the ICERs for pembrolizumab + chemotherapy varied from HKD 68,243 to HKD 1,688,127, compared to chemotherapy. The ICER was most sensitive to parameters affecting the extrapolations of EFS for both the treatment arms. The cost-effectiveness results were also moderately sensitive to exponential rate of DM to death, and the total cost of treating metastatic disease.
Tornado diagram for the incremental cost-effectiveness ratio of pembrolizumab + chemotherapy vs. chemotherapy. AE adverse event, DM distant metastases, EF event-free, EFS event-free survival, HKD Hong Kong dollar, ICER incremental cost-effectiveness ratio, LR locoregional recurrence, QALY quality-adjusted life year
Figure 5 presents the scatterplot of simulated incremental cost and QALY pairs from the PSA for the pembrolizumab + chemotherapy vs. chemotherapy arms. Across the 1000 iterations of the PSA, the average incremental costs were HKD 268,805, and the average incremental QALY gain was 2.24 for pembrolizumab + chemotherapy vs. chemotherapy. The resulting average ICER for pembrolizumab + chemotherapy vs. chemotherapy was HKD 119,901 per QALY gained, approximately 11.32% less than the base case ICER, and also within Hong Kong’s WTP threshold of HKD 1,171,308 per QALY[18]. On the basis of the 1000 replicates shown in Fig. 6, pembrolizumab + chemotherapy had a 98.20% probability of being cost-effective vs. chemotherapy. The cost-effectiveness acceptability curve (CEAC) for pembrolizumab + chemotherapy vs. chemotherapy is shown in Fig. 6
.
Discussion
With the addition of pembrolizumab in both the neoadjuvant and adjuvant phases for high-risk patients with eTNBC, it is anticipated that over 32-year of time horizon, pembrolizumab + chemotherapy yields gains in QALYs (by 2.45) and LYs (by 3.05) at a reasonable incremental cost (HKD 331,192) per patient resulting in an ICER of HKD 135,200 per QALY gained compared to chemotherapy alone. Since the proportion of LYs without recurrence was higher for pembrolizumab + chemotherapy (16.03 years in the pembrolizumab + chemotherapy arm compared with 12.61 years in the chemotherapy arm), the strategy led to reduced/delayed cancer recurrence. This further resulted in lower costs in the advanced setting for the pembrolizumab + chemotherapy arm, with the reductions in the overall costs of metastatic treatments, disease management, and terminal care in this arm. The scenario and sensitivity analysis (OWSA and PSA) results show the robustness of the base case findings.
In the current study, the WTP threshold of HKD 1,171,308 per QALY gained for Hong Kong was calculated on the based of the WHO recommendation [18]. Despite the potential drawbacks of employing GDP as a proxy for cost-effectiveness thresholds [31, 32], it was higher than the ICER for intervention (HKD 135,200), showing that pembrolizumab + chemotherapy is cost-effective. We have also provided the CEAC, reflecting that at a threshold of three times GDP per capita, there is a 98.20% chance that pembrolizumab will be cost-effective.
Strengths
The major strength of this analysis is the reliance upon direct, head-to-head comparative data from the phase III KEYNOTE-522 trial to inform the economic evaluation of pembrolizumab + chemotherapy vs. chemotherapy in high-risk patients with eTNBC. This made it possible to validate the predicted OS against the observed OS in the KEYNOTE-522 trial and use the accurate data for this patient cohort as inputs in the model. External studies were also used to validate the EFS and OS extrapolations for the treatment arm [14, 30].
The economic evaluation is based on a well-established Markov modelling approach that has been commonly used in the published cost-effectiveness analyses and submission of oncology drugs to internationally recognized health technology assessment (HTA) organizations. The same model was also submitted to NICE and CADTH, based on the UK and Canadian perspective for pembrolizumab + chemotherapy in high-risk eTNBC, showing favorable outcomes that align with the results obtained in this analysis. The model’s validity was assessed by comparing modelled efficacy outcomes against the sources that informed the efficacy inputs [12, 13].
Also, we compared the methodology of this study with that of published literature assessing cost-effectiveness of pembrolizumab + chemotherapy in the US setting [23]. The outcomes of the current analysis are consistent with the US study. The ICER obtained in the US analysis was $27,285 per QALY gained, which was cost-effective at the WTP threshold of $50,000 per QALY and is in line with the ICER obtained for Hong Kong.
Utility and AE-related disutility inputs were directly calculated from the KEYNOTE-522 trial, and were measured using the EQ-5D, the utility measure preferred by NICE [33]. The QALY decrement associated with AEs was considered in each treatment arm, accounting for the mean duration of AEs in both arms and the treatment-specific AE rates. In the base case, the same utilities were applied for both treatment arms based on the pooled data from KEYNOTE-522.
The economic evaluation duly accounted for first-line and/or subsequent lines of therapies for metastatic TNBC by incorporating drug acquisition costs, administration costs, and estimated market share for each regimen.
Limitations
As with any pharmacoeconomic evaluation, this model is subject to some limitations. First, the median follow-up for KEYNOTE-522 at the time of the analysis was 39 months at the data cutoff date; and thus, extrapolations were required to model a longer time horizon. This approach is inherently unpredictable. Several scenario analyses were conducted to examine various extrapolation methods for EFS to address this, and sensitivity analyses were conducted to test the parameter uncertainty related to the base case extrapolation. The sensitivity analysis findings confirmed the robustness of the base case ICER. Also, EFS curves from the published literature were digitized and fitted into the model so that the predicted EFS at specific time points could be compared against external data. To verify the model results, however, a longer-term follow-up of the clinical trial will be necessary. Second, the KEYNOTE-522 data representing the treatment pattern over the trial follow-up period were used to estimate the transition from the DM state to death. In the base case, the mean OS following the entry of the DM state was estimated among all patients who had documented distant recurrent/progression, regardless of whether they have received subsequent treatments or not. The predicted mean OS may be understated compared to that in current real-world practice because more efficacious treatments in the metastatic setting may be available. This is because TNBC care is changing quickly, with immunotherapies and targeted therapies emerging as a new treatment paradigm to test uncertainty associated with OS, the model has the flexibility to use KEYNOTE-355 as the source where the mean OS of each 1L metastatic TNBC treatment was estimated on the basis of the predicted OS curves in the CEM of the 1L metastatic TNBC. Furthermore, the model validated the predicted OS of the chemotherapy arm against external sources. Third, the model did not account for the expenditures of grade 3+ AEs with a frequency of less than 5% in both the arms. As a common practice, a 5% inclusion criterion was adopted for AEs; however, excluding the incidents having frequency less than 5% was not anticipated to affect the results.
Lastly, in Hong Kong, there are three types of healthcare services available: public, private, and private in public hospitals. However, cost from a payer perspective across these three different healthcare services will not vary significantly. In the current analysis, the cost data were obtained from the public sector, and the results might not be a full representation of the Hong Kong healthcare system. Despite variable cost data, the base case ICER was within the cost-effectiveness threshold and the sensitivity analysis showed the robustness of the cost-effectiveness results which reflect that the pembrolizumab would be cost-effective under the different healthcare services.
Conclusions
On the basis of the assumptions in the model and the analysis conducted for a 56-year-old cohort with high-risk eTNBC, pembrolizumab + chemotherapy is cost-effective compared to chemotherapy, from the perspective of a third-party payer in Hong Kong, at a WTP of HKD 1,171,308 per QALY [18]. The ICER is less than 35% of the GDP per capita in Hong Kong while the WHO recommendation is to use three times GDP per capita as WTP. Patients receiving pembrolizumab + chemotherapy are projected to incur fewer recurrences and thereby accrue fewer costs in the LR and DM health states compared to chemotherapy. Pembrolizumab + chemotherapy results in an incremental gain in LYs and QALYs compared with chemotherapy in Hong Kong as pembrolizumab delays cancer progression as compared to the chemotherapy arm. The robustness of the base case ICER was further supported by sensitivity analyses (OWSA and PSA) and across various alternative scenarios.
Data Availability
The efficacy, safety, patient-reported outcomes data used in this study were collected in the phase III KEYNOTE-522 trial. Although the patient-level data are not publicly accessible, the trial’s findings have been reported in a number of publications. The trial results supporting the findings of this analysis are available within the article and its electronic supplementary material. Additional data, such as cost information and datasets for model validation, were sourced from published literature, for which references are provided in the article.
References
Overview of Hong Kong Cancer Statistics of 2020. https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202020.pdf. Accessed 16 Feb 2023.
Female Breast Cancer in 2020. https://www3.ha.org.hk/cancereg/pdf/factsheet/2020/breast_2020.pdf. Accessed 16 Feb 2023.
Balmaña J, Díez O, Rubio I, Cardoso F. BRCA in breast cancer: ESMO clinical practice guidelines. Ann Oncol. 2011;22(6):iv31–4. https://doi.org/10.1093/annonc/mdr373.
Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Current Oncol. 2018;25(1):142–50. https://doi.org/10.3747/co.25.3954.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59. https://doi.org/10.1056/nejmoa1612645.
Liedtke C, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. https://doi.org/10.1200/JCO.2007.14.4147.
Study of pembrolizumab (MK-3475) plus chemotherapy vs placebo plus chemotherapy as neoadjuvant therapy and pembrolizumab vs placebo as adjuvant therapy in participants with triple negative breast cancer (TNBC) (MK-3475-522/KEYNOTE-522). ClinicalTrials.gov, U.S. National Library of Medicine (ed.)
Highlights of prescribing information: KEYTRUDA (pembrolizumab). United States Food and Drug Administration. 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
Product information KEYTRUDA (pembrolizumab). European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed 13 May 2024.
Schmid P, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/nejmoa1910549.
Schmid P, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. https://doi.org/10.1056/nejmoa2112651.
Pembrolizumab for neoadjuvant and adjuvant treatment of triple-negative early or locally advanced breast cancer. Technology appraisal guidance [TA851]. National Institute for Health and Care Excellence (NICE). 14 Dec 2022. https://www.nice.org.uk/guidance/ta851/chapter/1-Recommendations.
Reimbursement review: pembrolizumab. Canadian Agency for Drugs and Technologies in Health (CADTH). https://www.cadth.ca/pembrolizumab-3. Accessed 17 Dec 2022.
Sikov WM, et al. CALGB (Alliance) 40603: long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT)+/-carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(15):591. https://doi.org/10.1200/JCO.2019.37.15_suppl.591.
Time series of composite consumer price indices (Table E501–5). Census and Statistics Department, Government of Hong Kong. https://www.censtatd.gov.hk/en/scode270.html. Accessed 23 May 2024.
Life expectancy at birth, female (years)—Hong Kong SAR, China. Life expectancy at birth, female (years)—Hong Kong SAR, China. https://data.worldbank.org/indicator/SP.DYN.LE00.FE.IN?locations=HK. Accessed 16 Feb 2023.
Annual report on the consumer price index. Census and Statistics Department, Hong Kong Special Administrative Region, Hong Kong, 2021.
GDP per Capita (current US$). Accessed 16 Feb 2023. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
National Institute for Health and Care Excellence. Decision Support Unit Technical Support Document 14: Survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data 2013. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 16 Feb 2023.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.2307/2670170.
Census and Statistics Department, The Government of the Hong Kong Special Administrative Region. https://www.censtatd.gov.hk/en/EIndexbySubject.html?pcode=D5320183&scode=190#section3. Accessed 18 Jun 2024.
Merck & Co. Inc, KEYNOTE-522 Clinical Study Report. 2021. Available: Data on file.
Huang M, et al. Cost-effectiveness of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant single-agent pembrolizumab for high-risk early-stage triple-negative breast cancer in the United States. Adv Ther. 2023;40:1153–70.
Hout BV, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15. https://doi.org/10.1016/j.jval.2012.02.008.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
List of private services. https://www.ha.org.hk/haho/ho/cs/238768_en.pdf. Accessed 16 Feb 2023.
Medical services. Accessed 16 Feb 2023. https://www8.ha.org.hk/qmh/patient_and_visitor/medical_services/medical_services.aspx.
List of private services. https://www.3ha.org.hk/fnc/Operations.aspx. Accessed 16 Feb 2023.
Wong IOL, Kuntz KM, Cowling BJ, Lam CLK, Leung GM. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–95. https://doi.org/10.1002/cncr.22848.
Walsh EM, et al. Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy. Breast Cancer Res Treat. 2019;174:1–13. https://doi.org/10.1007/s10549-018-5066-6.
Chi YL, Becker M, Chalkidou K, et al. What next after GDP-based cost-effectiveness thresholds. Gates Open Res. 2020;4:176.
Kazibwe J, Gheorghe A, Wilson D, Ruiz F, Chalkidou K, Chi YL. The use of cost-effectiveness thresholds for evaluating health interventions in low-and middle-income countries from 2015 to 2020: a review. Value Health. 2022;25(3):385–9.
NICE health technology evaluations: the manual. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741. Accessed 31 Oct 2023.
Acknowledgements
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank John Cook, Praveen Dhankhar and Geetika Khatri (Complete Health Economics and Outcomes Research Solutions (CHEORS), North Wales, PA, USA), Amin Haiderali (Merck & Co., Inc., Rahway, NJ, USA), Jason Jen and Felix Choi (MSD Hong Kong) for their review and comments on the manuscript.
Funding
The sponsorship for this study and the journal’s Rapid Service Fee were funded by Merck Sharp & Dohme LLC (Grant no. GMSA/231073), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Ava Kwong, Roland Leung, and Tsz Ching Chan guided the process of adapting the model for Hong Kong, the development of the manuscript and provided guidance towards the collection of Hong Kong specific input. Anvi Khandelwal and Kshama Mishra adapted the model, conducted the analyses, interpreted the results, and wrote the manuscript. Min Huang developed the model and provided strategic guidance to the model adaptation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Prof. Ava Kwong works with Department of Surgery, The University of Hong Kong, HKSAR, China and has received consultancy honorarium from MSD for this project. Roland Leung works with Division of Hematology and Medical Oncology, Department of Medicine, Queen Mary Hospital, HKSAR, China. Tsz Ching Chan works with Department of Surgery, The University of Hong Kong, HKSAR, China and has no conflicts of interest that are directly relevant to the content of this article. CHEORS received funds from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, during the conduct of the study; Anvi Khandelwal and Kshama Mishra are employees of CHEORS. Min Huang is employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Ethical Approval
This article is based on the data drawn from trials that had already been completed and from published literature and does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kwong, A., Leung, R., Chan, T.C. et al. Cost-effectiveness of Pembrolizumab in Combination with Chemotherapy as Neoadjuvant Treatment and Continued as a Single Agent Adjuvant Treatment for High-Risk Early-Stage Triple-Negative Breast Cancer in Hong Kong. Oncol Ther (2024). https://doi.org/10.1007/s40487-024-00285-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40487-024-00285-4