Abstract
Anaplastic large cell lymphoma (ALCL) and inflammatory myofibroblastic tumor (IMT) are rare cancers observed predominantly in children and young adults. ALCL accounts for 10–15% of all pediatric non-Hodgkin lymphomas and is commonly diagnosed at an advanced stage of disease. In children, 84–91% of cases of ALCL harbor an anaplastic lymphoma kinase (ALK) gene translocation. IMT is a rare mesenchymal neoplasm that also tends to occur in children and adolescents. Approximately 50–70% of IMT cases involve rearrangements in the ALK gene. A combination of chemotherapeutic drugs is typically used for children with ALK-positive ALCL, and the only known curative therapy for ALK-positive IMT is complete surgical resection. Crizotinib, a first-generation ALK inhibitor, was approved in the USA in 2021 for pediatric patients and young adults with relapsed or refractory ALK-positive ALCL; however, its safety and efficacy have not been established in older adults. In 2022, crizotinib was approved for adult and pediatric patients with unresectable, recurrent, or refractory ALK-positive IMT. This podcast provides an overview of ALK-positive ALCL and IMT. We discuss the current treatment landscape, the role of ALK tyrosine kinase inhibitors, and areas of future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anaplastic large cell lymphoma (ALCL) and inflammatory myofibroblastic tumor (IMT) are rare cancers observed predominantly in children and young adults. |
Recently, anaplastic lymphoma kinase (ALK) inhibitors have demonstrated efficacy and acceptable safety in patients with relapsed/refractory ALK-positive ALCL or IMT, which historically have had limited treatment options. |
However, additional research is needed on the first-line use of ALK inhibitors in these two diseases. |
This podcast begins with a brief overview of each disease followed by the current treatment landscape, efficacy of targeted therapies, and areas of future research for patients with ALK-positive ALCL or IMT. |
Podcast Discussion. (MP4 575654 kb)
Digital Features
This article is published with digital features, including podcast audio, to facilitate understanding of the article. To view digital features for this article, go to: https://doi.org/10.6084/m9.figshare.25470082
Podcast Transcript
Welcome to the podcast. This podcast was supported by Pfizer, with editorial support provided by Jill Fountain of Nucleus Global, and funded by Pfizer.
EL: Hello, my name is Eric Lowe. I’m a pediatric hematologist-oncologist at the Children's Hospital of the King’s Daughters in Norfolk, Virginia.
YM: Hello, my name is Yael Mossé. I’m also a pediatric hematologist-oncologist at the Children's Hospital of Philadelphia.
Eric and I will be discussing two challenging and rare diseases: anaplastic large cell lymphoma, or ALCL, and inflammatory myofibroblastic tumor, or IMT. We will begin with a brief overview of each disease. Then, we will discuss the current treatment landscape for patients with anaplastic lymphoma kinase–positive—or ALK-positive—disease, the efficacy of targeted therapies, and areas of future research. Eric, would you please give us an overview of ALCL?
EL: Absolutely. ALCL is a peripheral T-cell non-Hodgkin lymphoma, or NHL, that accounts for about 10–15% of pediatric NHLs [1, 2]. This cancer is primarily observed in children and adolescents, with a median age of approximately 10 to 11 years at diagnosis, and has a male predominance. Patients often present with advanced stage III or IV disease with extranodal involvement [2, 3]. Swollen lymph nodes, fever, and weight loss are common presenting symptoms. The hallmark tumor cell is a large, CD30-positive T cell with a kidney-shaped nucleus [4, 5].
The World Health Organization defines two main subtypes of systemic ALCL: systemic ALK positive and systemic ALK negative [4, 6]. For the purpose of this discussion, we will be focusing on systemic ALK-positive ALCL, which is the most common subtype. Among the pediatric ALCL population, 84–91% of cases are ALK positive [7]. In most cases of ALK-positive ALCL, a chromosomal translocation produces an ALK fusion with the gene that encodes nucleophosmin [3]. The resulting nucleophosmin-ALK fusion protein is a constitutively active tyrosine kinase that promotes tumorigenesis. The prognosis is generally better for ALK-positive ALCL than for ALK-negative ALCL [8, 9]. Considering only pediatric cases, overall survival rates are higher in ALK-positive than in ALK-negative patients, with an event-free survival rate of 65–75% compared with 15–46%, respectively.
YM: Thank you for that background, Eric. So, what is the current treatment landscape for patients with ALK-positive ALCL?
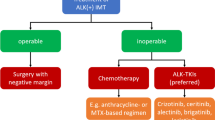
EL: A combination of chemotherapeutic drugs is typically used to treat children with ALK-positive ALCL, and this tends to be effective. Between 65% and 80% of pediatric patients are cured using this approach [9]. While adult patients with ALCL tend to receive CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) [10], pediatric patients usually receive six cycles of multiagent chemotherapy similar to CHOP, as shown in the ALCL99 and the ANHL12P1 studies [11, 12]. These patients undergo computed tomography/positron emission tomography (CT/PET) imaging 1 month after completion of therapy to assess response.
Among other treatment strategies, vinblastine monotherapy has shown promising results in pediatric patients with relapsed or refractory ALCL who were ALK positive upon testing [13]. Complete remission (CR) was observed in 83% of patients, 36% of whom remain in complete remission. The phase 3 ALCL-vinblastine study is investigating the efficacy and safety of vinblastine monotherapy compared to standard chemotherapy, which is an ALCL99 protocol [14] in pediatric patients with standard risk, minimal disseminated disease or MDD-negative, treatment-naive, ALK-positive ACLC [15]. Patients who are MDD positive will continue to be treated with the ALCL99 protocol without vinblastine, and patients, depending on the residual disease after the first course of ALCL99, could be considered refractory to treatment and eligible for inclusion in a different trial [16]. All of these are trials that are currently ongoing in Europe for ALK-positive ALCL. In some cases, ALK-positive ALCL is also resistant to chemotherapy, and there really is no standard of care for relapsed or refractory ALK-positive ALCL. Many patients receive chemotherapy followed by an allogeneic stem cell transplant [9]. Although the optimal treatment for chemotherapy-resistant ALK-positive ALCL has not been established, the use of targeted therapies, such as ALK inhibitors, may be beneficial [9, 17].
YM: Thanks so much for that, Eric. EL: Absolutely. Yael, will you please describe an overview of IMT, which is the other rare disease that we are discussing today?
YM: IMTs are rare mesenchymal neoplasms that, like ALCL, occur primarily in children and young adults [18,19,20]. The prevalence ranges from 0.04% to 0.7% [18]. The primary location is in the lungs, but other areas of the body can be involved, such as the head, neck, and gastrointestinal and gynecologic tracts [19]. IMTs are usually locally invasive and rarely metastatic [21]. Cellular morphology is diverse, but IMTs usually involve myofibroblastic spindle cells mixed with inflammatory cells, such as lymphocytes and eosinophils [18, 20]. Symptoms vary depending on the site of the tumor, but many patients present with fevers, cough, dyspnea, fatigue, and hemoptysis [18, 19]. Computed tomography is the most common diagnostic method to detect the precise location of the tumor. However, final diagnosis of IMT depends on pathological and immunohistochemical analysis [21]. The 10-year survival is around 80% [18].
Approximately 50% but up to 70% of IMTs harbor an ALK gene rearrangement that results in overexpression and constitutive activation of the ALK protein [22, 23]. ALK-negative IMTs tend to be more aggressive, with a higher frequency of metastasis [22, 24]. More than ten different genes have been identified as ALK fusion partners in IMT; the clinical implications of this molecular heterogeneity are unclear, but it may play a role in the diverse pathology of IMT and the differential responses to ALK inhibitors [22, 25].
EL: Yael, thank you for that. Given the background, what is the current treatment landscape for patients with ALK-positive IMT?
YM: The treatment of IMT depends on the location, size, and extent of the tumor [18], and surgery is the mainstay of treatment, with complete surgical resection and clean margin being the only known curative therapy for this entity. These tumors can be multifocal (for example, multiple tumors can coexist in the lungs), but they rarely metastasize beyond the site of origin. The rate of recurrence varies widely depending on the site of the lesion and extent of surgery if amenable to this approach. So, for example, pulmonary tumors have a recurrence rate of less than 2%, while tumors originating outside the lungs have a recurrence rate of up to 25% [21]. After surgery, there is a low risk of local or metastatic recurrence so long as the primary tumor is completely resected with wide margins that show no involvement with the tumor [26].
Chemotherapy is reserved for patients with inoperable or recurrent IMT and is largely ineffective; similarly, these tumors are usually resistant to external beam radiation [20]. ALK inhibitors really represent an important treatment option for patients with unresectable, recurrent, or refractory ALK-positive IMT because conventional modalities of cancer treatment, as we have just discussed, such as cytotoxic chemotherapy and radiation, are largely ineffective [20, 21]. Eric, what is the evidence for using ALK inhibitors in patients with ALK-positive ALCL?
EL: So, in current guidelines, crizotinib and alectinib are recommended as options for patients with relapsed or refractory ALK-positive ALCL [27]. Crizotinib was approved in the USA in 2021 for pediatric patients 1 year of age and older and young adults with relapsed or refractory ALK-positive ALCL; however, its safety and efficacy have not been established in older adults [28, 29]. This approval was based on results from the CRISP trial, a multicenter, single-arm, phase 1/2 ADVL0912 clinical trial, which was conducted by the Children's Oncology Group or COG. The trial enrolled 26 pediatric patients between 1 and 21 years of age with relapsed or refractory ALK-positive ALCL [28, 30]. In this study, patients responded to both doses of crizotinib, both the lower starting dose, 165 mg/m2, as well as 280 mg/m2, indicating sensitivity to crizotinib at both doses. Objective response rates were 83% with the lower dose and 90% with the higher dose. Of the 26 patients, 23 had a complete or partial response, and 18 of those patients had the response within 4 weeks of initiating treatment. The most common adverse event in this trial was decrease in neutrophil count.
In Japan, alectinib was approved in 2020 for recurrent or refractory ALK-positive ALCL [31]. In an open-label phase 2 trial with pediatric and adult patients, an objective response to alectinib was observed in eight of ten patients, with six complete responses. The 1-year progression-free survival and overall survival rates were 58% and 70%, respectively. Common adverse events in this trial included diarrhea, upper respiratory tract infection, rash, and increased blood alkaline phosphatase levels. The most common grade 3 or higher adverse event was a decrease in neutrophil count occurring in two patients. Therefore, in pediatric patients with ALK-positive ALCL, both crizotinib and alectinib have demonstrated efficacy in clinical trials. Given the favorable efficacy and safety profiles of both crizotinib and vinblastine as monotherapies, 13 patients with relapsed ALK-positive ALCL were treated with the combination of crizotinib and vinblastine [32]. The treatment combination was efficacious, with two of 13 patients relapsing; however, severe toxicities occurred in 11 of the 13 patients, including one fatal infection. Yael, do we have similar evidence for using ALK inhibitors in patients with ALK-positive IMT?
YM: Yes, we do, Eric. Current guidelines list alectinib, brigatinib, ceritinib, crizotinib, and lorlatinib as potential regimens in the treatment of ALK-positive IMT [33]. However, crizotinib is the only ALK inhibitor treatment approved by the US Food and Drug Administration (FDA) [34]. The phase 2 ADVL0912 trial conducted by the Children’s Oncology Group, which we just discussed, also included pediatric patients with ALK-positive IMT [30]. The study enrolled 14 patients with metastatic, inoperable, or recurrent ALK-positive IMT. The objective response rate with crizotinib was 86%. Five of 14 patients, or 36%, achieved a complete response. Seven patients achieved the first response within 4 weeks. The median duration of therapy was 1.6 years. Similarly to ALCL, the most common adverse event was a decrease in neutrophil count, which occurred in 43% of patients. Based on these results, the FDA approved crizotinib for pediatric patients with unresectable, recurrent, or refractory ALK-positive IMT [29, 34].
In a phase 1 study that included pediatric patients with malignancies harboring genetic ALK alterations, ten patients with advanced ALK-positive refractory or recurrent IMT were treated with ceritinib [35]. At a median follow-up of 33.2 months, the objective response rate was 70%, with a median duration of response not reached. Overall, grade 3 or 4 adverse events occurred in 81% of patients, mostly due to elevated aminotransferase levels; however, adverse events led to treatment discontinuation in only 11% of patients, indicating that ceritinib had a manageable safety profile overall. Therefore, in these patients, both crizotinib and ceritinib have shown objective benefit and manageable adverse events. Since these patients are likely to benefit from ALK inhibition with crizotinib, it is essential that all such tumors be subjected to next-generation sequencing platforms, especially since we now know that traditional immunohistochemistry and fluorescence in situ hybridization or FISH may miss identifying a subset of these rare tumors due to the nature and heterogeneity of the underlying ALK translocations. Eric, given that we have limited treatment options for patients with ALK-positive ALCL or IMT, what are the unmet needs and future areas of research for ALCL?
EL: I would start by saying that additional research is needed on the first-line or front-line use of crizotinib and next-generation ALK inhibitors. For example, in the phase 2 COG ANHL12P1 trial, we examined the efficacy and safety of crizotinib plus chemotherapy in pediatric patients with newly diagnosed ALK-positive ALCL in the first-line setting [12, 36]. The study included 66 patients with a median (age) of 14 years. We observed a complete response in 92% of patients after two cycles, and the 2-year event-free survival rate was 77%, with a 2-year overall survival rate of 95%. Twenty percent of patients in the crizotinib arm experienced a grade 2 or higher thromboembolic event. Among 41 patients who then received prophylactic anticoagulation, 11 experienced grade 2 or higher thromboembolic adverse events.
In the same trial, the efficacy of brentuximab vedotin, an antibody–drug conjugate to CD30, plus chemotherapy was assessed in pediatric patients with newly diagnosed ALK-positive ALCL [36, 37]. The addition of brentuximab vedotin to the chemotherapy regimen was tolerable. Median time to relapse for brentuximab vedotin plus chemotherapy was 7.5 months, and no patients progressed or relapsed while receiving therapy. In the chemotherapy-only arm of ALCL99, median time to relapse was 6.5 months, and six patients or 5.6% progressed or relapsed during therapy [11, 37]. The 2-year event-free survival rate for patients receiving brentuximab vedotin plus chemotherapy was 79% compared with 70% for patients receiving chemotherapy only in ALCL99. This study demonstrated that additional treatment options may be available in the future for first-line treatment of pediatric patients with ALK-positive ALCL.
Future research really needs to examine the use of crizotinib in combination with other agents in the first-line setting and determine whether prophylactic anticoagulation treatment is necessary with other combinations. For example, a combination that would be interesting is crizotinib with brentuximab vedotin [38]. This combination has shown efficacy in relapsed ALCL in a single case report [39]. With respect to next-generation ALK inhibitors, a phase 2 trial in Europe is assessing the efficacy and safety of third-generation lorlatinib in patients with ALK-positive ALCL resistant to ALK inhibitors [40].
Lorlatinib penetrates the blood–brain barrier and plays an important role in the treatment of adult patients with ALK-positive, metastatic non-small cell lung cancer and central nervous system or CNS metastases [41,42,43]. In the phase 3 CROWN study, treatment with first-line lorlatinib showed improved systemic and intracranial efficacy versus crizotinib in adult patients with advanced ALK-positive non-small cell lung cancer, 24.8% of whom had baseline CNS metastases [43]. Another randomized phase 2 trial, NIVO-ALCL, is investigating the efficacy of nivolumab in pediatric and adult patients with relapsed or refractory ALK-positive ALCL [15]. Yael, what are the key research areas in IMT?
YM: Thanks, Eric. In IMT, the Briga-PED study, a phase 1/2 trial in the European Union, is currently assessing the safety and efficacy of the second-generation ALK inhibitor brigatinib in pediatric and young adult patients with ALK-positive IMT [16]. This leads me to another important issue regarding the long-term effects of ALK inhibitors in children.
The duration of therapy with ALK inhibition remains an open question. Although monotherapy with ALK inhibitors yields high response rates, concern exists about disease recurrence after stopping therapy [44, 45]. Also, the need for long-term or perhaps lifelong therapy is hindered by the potential for toxicity. Future trials are really needed to determine the risk–benefit ratio of prolonged ALK inhibitor treatment in children and risk of recurrence to be able to provide more guidance to the community about duration of therapy [44]. In addition, the long-term effectiveness of ALK inhibitors can be limited by the development of acquired resistance. We need more information on longer courses of ALK inhibitor treatment to identify resistance mechanisms and ways to mitigate them [46]. To date, very little is known about the frequency and mechanisms of resistance to crizotinib in the specific setting of IMTs. Finally, to optimally manage patients throughout the course of their disease, there is a need for minimally invasive methods to detect residual disease or emerging resistance. Such methods may be similar to the use of PCR in chronic myeloid leukemia [47]. Eric, are there other areas of research on ALCL that you wish to mention?
EL: Yes, thank you. I think another important data gap that needs to be closed is how to incorporate ALK inhibitors in the treatment of patients with ALK-positive ALCL who are at high risk of relapse and are candidates for transplant. The prognosis for relapsed or refractory ALCL is poor [48], especially for those patients who relapsed earlier during treatment. In these cases, consolidation chemotherapy followed by an allogeneic stem cell transplant is usually recommended. Unfortunately, conventional consolidation chemotherapy using multidrug combinations is not very effective. We need to establish whether ALK inhibitors are effective bridging agents to autologous or allogeneic stem cell transplant. In this context, we should be asking questions like:
-
o
Does the use of an ALK inhibitor avoid the need for a transplant?
-
o
Does the use of an ALK inhibitor for 2 years prior to allogeneic transplant decrease or increase survival versus up-front transplant?
-
o
Can ALK inhibitors safely and effectively be used as maintenance therapy after transplant?
And while there is scientific merit for these ideas [48, 49], there are limited data.
YM: Thanks very much, Eric. In closing, ALK inhibitors have demonstrated efficacy and acceptable safety for patients with relapsed, refractory ALK-positive ALCL and as first-line treatment of patients with ALK-positive inoperable IMT and can perhaps obviate the need for surgery altogether for patients with IMTs. ALK inhibitors have made a positive impact in managing patients with these diseases, who historically have had limited treatment options.
EL: Yeah, more research is needed on the first-line use of ALK inhibitors and on how to position ALK inhibitors in conjunction with transplant therapy for ALCL.
YM: Thank you very much. EL: Thank you.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed.
References
Kinney MC, Higgins RA, Medina EA. Anaplastic large cell lymphoma: twenty-five years of discovery. Arch Pathol Lab Med. 2011;135:19–43.
Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013;30:509–19.
Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–67.
Irshaid L, Xu ML. ALCL by any other name: the many facets of anaplastic large cell lymphoma. Pathology. 2020;52:100–10.
Lymphoma Research Foundation. Understanding lymphoma: anaplastic large cell lymphoma. 2022. https://lymphoma.org/wp-content/uploads/2022/07/LRF_Anaplastic_Large_Cell_Lymphoma_Fact_Sheet.pdf. Accessed 28 Feb 2024.
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49.
Falini B, Martelli MP. Anaplastic large cell lymphoma: changes in the World Health Organization classification and perspectives for targeted therapy. Haematologica. 2009;94:897–900.
Prokoph N, Larose H, Lim MS, Amos Burke GA, Turner SD. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): current standard and beyond. Cancers (Basel). 2018;10:99.
Gromowsky MJ, D’Angelo CR, Lunning MA, Armitage JO. ALK-positive anaplastic large cell lymphoma in adults. Fac Rev. 2023;12:21.
Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28:3987–93.
Lowe EJ, Reilly AF, Lim MS, et al. Crizotinib in combination with chemotherapy for pediatric patients with ALK+ anaplastic large-cell lymphoma: the results of Children’s Oncology Group trial ANHL12P1. J Clin Oncol. 2022;41:2043–53.
Brugières L, Pacquement H, Le Deley MC, et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27:5056–61.
Brugières L, Le Deley MC, Rosolen A, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27:897–903.
Beishuizen A, Mellgren K, Andrés M, et al. Improving outcomes of childhood and young adult non-Hodgkin lymphoma: 25 years of research and collaboration within the framework of the European Intergroup for Childhood Non-Hodgkin Lymphoma. Lancet Haematol. 2023;10:e213–24.
ClinicalTrials.gov. Brigatinib in pediatric and young adult patients with ALK+ ALCL, IMT or other solid tumors (Briga-PED). 2022. https://clinicaltrials.gov/study/NCT04925609. Accessed 28 Feb 2024.
Dana-Farber Cancer Institute. Childhood anaplastic large cell lymphoma. 2024. https://www.dana-farber.org/cancer-care/types/childhood-anaplastic-large-cell-lymphoma. Accessed 28 Feb 2024.
Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis. 2015;7:908–11.
Sachdev R, Mohapatra I, Goel S, Ahlawat K, Sharma N. Core biopsy diagnosis of ALK positive inflammatory myofibroblastic tumor of lung: an interesting case. Turk Patoloji Derg. 2020;36:173–7.
Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory myofibroblastic tumour: state of the art. Cancers (Basel). 2022;14:3662.
Da M, Qian B, Mo X, et al. Inflammatory myofibroblastic tumors in children: a clinical retrospective study on 19 cases. Front Pediatr. 2021;9: 543078.
Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–67.
Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889–95.
Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–20.
Mahajan P, Casanova M, Ferrari A, Fordham A, Trahair T, Venkatramani R. Inflammatory myofibroblastic tumor: molecular landscape, targeted therapeutics, and remaining challenges. Curr Probl Cancer. 2021;45:100768.
Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–33.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for T-Cell Lymphomas V.1.2024. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed March 1, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org.
US Food and Drug Administration. FDA approves crizotinib for children and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma. 2021. News release. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-children-and-young-adults-relapsed-or-refractory-systemic-anaplastic-large. Accessed 28 Feb 2024.
Pfizer Laboratories Div Pfizer Inc. Xalkori (crizotinib) capsules, for oral use. Prescribing information. 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=676. Accessed 1 Mar 2024.
Mossé YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group study. J Clin Oncol. 2017;35:3215–21.
Fukano R, Mori T, Sekimizu M, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: an open-label phase II trial. Cancer Sci. 2020;111:4540–7.
Knörr F, Schellekens KPJ, Schoot RA, et al. Combination therapy with crizotinib and vinblastine for relapsed or refractory pediatric ALK-positive anaplastic large cell lymphoma. Haematologica. 2023;108:1442–6.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma V.3.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed March 1, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org.
US Food and Drug Administration. FDA approves crizotinib for ALK-positive inflammatory myofibroblastic tumor. 2022. News release. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-alk-positive-inflammatory-myofibroblastic-tumor. Accessed 28 Feb 2024.
Fischer M, Moreno L, Ziegler DS, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1764–76.
ClinicalTrials.gov. Brentuximab vedotin or crizotinib and combination chemotherapy in treating patients with newly diagnosed stage II-IV anaplastic large cell lymphoma. https://clinicaltrials.gov/study/NCT01979536, Accessed 28 Feb 2024.
Lowe EJ, Reilly AF, Lim MS, et al. Brentuximab vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: results of COG trial ANHL12P1. Blood. 2021;137:3595–603.
Burke GAA. Brentuximab vedotin: frontline help in ALCL. Blood. 2021;137:3581–2.
Stumme H, Lang P, Woessmann W, et al. Combination therapy with crizotinib/brentuximab vedotin in chemorefractory ALK-positive ALCL is feasible and highly effective: a case report [abstract]. Oncol Res Treat. 2015;38:212.
ClinicalTrials.gov. A study of oral lorlatinib in patients with relapsed ALK positive lymphoma (CRU3). https://clinicaltrials.gov/study/NCT03505554. Accessed 28 Feb 2024.
Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–9.
Solomon BJ, Bauer TM, Ignatius Ou SH, et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J Clin Oncol. 2022;40:3593–602.
Solomon BJ, Bauer TM, Mok TSK, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11:354–66.
Pearson ADJ, Barry E, Mossé YP, et al. Second Paediatric Strategy Forum for anaplastic lymphoma kinase (ALK) inhibition in paediatric malignancies: ACCELERATE in collaboration with the European Medicines Agency with the participation of the Food and Drug Administration. Eur J Cancer. 2021;157:198–213.
Gambacorti-Passerini C, Mussolin L, Brugieres L. Abrupt relapse of ALK-positive lymphoma after discontinuation of crizotinib. N Engl J Med. 2016;374:95–6.
Wang Z, Geng Y, Yuan LY, et al. Durable clinical response to ALK tyrosine kinase inhibitors in epithelioid inflammatory myofibroblastic sarcoma harboring PRRC2B-ALK rearrangement: a case report. Front Oncol. 2022;12:761558.
Shanmuganathan N, Hughes TP. Molecular monitoring in CML: how deep? How often? How should it influence therapy? Blood. 2018;132:2125–33.
Nakai R, Fukuhara S, Maeshima AM, et al. Alectinib, an anaplastic lymphoma kinase (ALK) inhibitor, as a bridge to allogeneic stem cell transplantation in a patient with ALK-positive anaplastic large-cell lymphoma refractory to chemotherapy and brentuximab vedotin. Clin Case Rep. 2019;7:2500–4.
Rigaud C, Knörr F, Brugières L, Woessmann W. Diagnosis and management of ALK-positive anaplastic large cell lymphoma in children and adolescents. Best Pract Res Clin Haematol. 2023;36: 101444.
Medical Writing, Editorial, and Other Assistance
Editorial support was provided by Jill Fountain of Nucleus Global and was funded by Pfizer.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This podcast, and journal’s Rapid Service fees, were sponsored by Pfizer.
Author information
Authors and Affiliations
Contributions
Eric Lowe and Yael P Mossé made substantial contributions to the development of the podcast, critically reviewed, and revised the podcast, and provided final approval of the podcast as submitted.
Corresponding author
Ethics declarations
Conflict of Interest
Eric Lowe participated on the data safety monitoring board for the European study of ALK inhibitors. Yael P Mossé reports consulting fees from Pfizer, served as a principal investigator for the COG ADVL0912 trial, received crizotinib and lorlatinib via an MTA for preclinical work in the laboratory, and is a member of the ASCO TAPUR DSMB.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lowe, E., Mossé, Y.P. Podcast on Emerging Treatment Options for Pediatric Patients with ALK-Positive Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumors. Oncol Ther 12, 247–255 (2024). https://doi.org/10.1007/s40487-024-00275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-024-00275-6