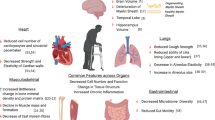
Abstract
Purpose of review
To discuss how T, natural killer (NK), and B cell aging modifies outcomes of organ transplantation, the transplant recipient’s efficacy to vaccines, susceptibility to reactivation of latent viral infections, as well as organ quality and function.
Recent findings
The aged immune system is characterized by a decline in naïve T cells and an increase of memory T cells, many of which also express innate markers. NK cells demonstrate a decline in cytokine secretion, cytotoxicity, and immune recognition. A population of pro-inflammatory B lymphocytes termed age-associated B cells (ABCs) expands with age. Functional changes in the aged immune cells induce inflammation and are less effective at controlling infection, mounting vaccine responses, and contributing to allorejection.
Summary
Optimization of care for organ transplantation in older patients requires an improved understanding of immune cell aging and its pleotropic effects.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687–98.
Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–83.
Minato N, Hattori M, Hamazaki Y. Physiology and pathology of T-cell aging. Int Immunol. 2020;32(4):223–31.
Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42(5):559–72.
Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46.
Rodrigues LP, Teixeira VR, Alencar-Silva T, Simonassi-Paiva B, Pereira RW, Pogue R, et al. Hallmarks of aging and immunosenescence: connecting the dots. Cytokine Growth Factor Rev. 2021;59(9):–21.
Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–20.
Huang H, Sikora MJ, Islam S, Chowdhury RR, Chien Y hsiu, Scriba TJ, et al. Select sequencing of clonally expanded CD8 + T cells reveals limits to clonal expansion. Proc Natl Acad Sci.. 2019 116(18):8995-9001
Fali T, Papagno L, Bayard C, Mouloud Y, Boddaert J, Sauce D, et al. New insights into lymphocyte differentiation and aging from telomere length and telomerase activity measurements. J Immunol Baltim Md. 1950;202(7):1962–9.
Martínez‐Zamudio RI, Dewald HK, Vasilopoulos T, Gittens‐Williams L, Fitzgerald‐Bocarsly P, Herbig U. Senescence‐associated β‐galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell. 2021;20(5):e13344.
Bektas A, Schurman SH, Gonzalez-Freire M, Dunn CA, Singh AK, Macian F, et al. Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging. 2019;11(21):9234–63.
Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 2018;17(1):e12675.
Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22.
Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age‐related loss of naive T cells and dysregulation of T‐cell/B‐cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43.
Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47–56.
Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8+ cells. J Exp Med. 2002;195(3):283–93.
Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37(2-3):445–53.
Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. 2016;17(8):966–75.
Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939–51.
Martin-Ruiz C, Hoffmann J, Shmeleva E, T von Z, Richardson G, Draganova L, et al. CMV-independent increase in CD27D2739393 1 aging and respos inversely related to mortality in octogenarians. NPJ Aging Mech Dis. 2020;21(6):3.
Choremi-Papadopoulou H, Panagiotou N, Samouilidou E, Kontopidou F, Viglis V, Antoniadou A, et al. CD28 costimulation and CD28 expression in T lymphocyte subsets in HIV-1 infection with and without progression to AIDS. Clin Exp Immunol. 2000;119(3):499.
Higdon LE, Gustafson CE, Ji X, Sahoo MK, Pinsky BA, Margulies KB, et al. Association of premature immune aging and cytomegalovirus after solid organ transplant. Front Immunol. 2021;12 Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.661551
Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and Convey Enhanced Protective Immunity. Immunity. 2018;48(4):716–729.e8.
Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):30630630.
Seyda M, Elkhal A, Quante M, Falk CS, Tullius SG. T cells going innate. Trends Immunol. 2016;37(8):54654637.
Chen G, Lustig A, Weng N, ping. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;20(4):121.
Gumá M, Busch LK, Salazar‐Fontana LI, Bellosillo B, Morte C, García P, López‐Botet M. The CD94/NKG2C killer lectin‐like receptor constitutes an alternative activation pathway for a subset of CD8+ T cells. Eur J Immunol. 2005;35(7):2071–80.
Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8(6):693–701.
Le Dréan E, Vély F, Olcese L, Cambiaggi A, Guia S, Krystal G, Gervois N, Moretta A, Jotereau F, Vivier E. Inhibition of antigen‐induced T cell response and antibody‐induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP‐1 and SHP‐2 protein‐tyrosine phosphatases. Eur J Immunol. 2005;28(1):264–76.
Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, et al. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79(18):12112–6.
• Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat Immunol. 2020;21(6):684–94. Genetic inhibition of sestrin 2 resulted in decreased expression of NKG2D-DAP12 and restored TCR signaling in CD27− CD28− CD8+ T cells suggesting that sestrins can reverse senescence in T cells.
Fribourg M, Anderson L, Fischman C, Cantarelli C, Perin L, La Manna G, et al. T-cell exhaustion correlates with improved outcomes in kidney transplant recipients. Kidney Int. 2019;96(2):436–49.
Song Y, Wang B, Song R, Hao Y, Wang D, Li Y, et al. T-cell immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell. 2018;17(2):e12716.
Onorati A, Havas AP, Lin B, Rajagopal J, Sen P, Adams PD, et al. Upregulation of PD-L1 in senescence and aging. Mol Cell Biol. 2022;42(10):e0017122.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):17317320.
Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol Orlando Fla. 2006;118(1):1–10.
Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94.
Mariani E, Meneghetti A, Formentini I, Neri S, Cattini L, Ravaglia G, et al. Telomere length and telomerase activity: effect of ageing on human NK cells. Mech Ageing Dev. 2003;124(4):403–8.
Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells. 2022;11(6):1017.
Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell. 2020;180(4):749–763.e13.
Le Garff-Tavernier M, Béziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debré P, Merle‐Beral H, Vieillard V. Tissue Determinants of Human NK Cell Development, Function, and Refunctional changes over the life span. Aging Cell. 2010;9(4):527–35.
Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro A, Falcão RR, Abdelhay E, Bouzas LF, Thuler LC, Ornellas MH, Diamond HR. Diamond HR. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–29.
Krishnaraj R, Svanborg A. Preferential accumulation of mature NK cells during human immunosenescence. J Cell Biochem. 1992;50(4):38638650.
Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11(5):7517511.
Rukavina D, Laskarin G, Rubesa G, Strbo N, Bedenicki I, Manestar D, et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92(7):2410–20.
Müller-Durovic B, Lanna A, Covre LP, Mills RS, Henson SM, Akbar AN. Killer cell lectin-like receptor G1 inhibits NK cell function through activation of adenosine 5iomonophosphate-activated protein kinase. J Immunol Baltim Md. 1950;197(7):2891–9.
Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Peña J, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121(1-3)):77–88.
Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54.
• Smith SL, Kennedy PR, Stacey KB, Worboys JD, Yarwood A, Seo S, et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 2020;4(7):1388–406. Single-cell RNA sequencing of NK cells in CMV+ and CMV− donors revealed an abundance of distinct NK cell subtypes.
Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145.
Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7(297):297ra115.
Krishnaraj R. Senescence and cytokines modulate the NK cell expression. Mech Ageing Dev. 1997;96(1-3):89–101.
Krishnaraj R, Bhooma T. Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2-induced interferon gamma secretion. Immunol Lett. 1996;50(1-2):59–63.
Mariani E, Pulsatelli L, Neri S, Dolzani P, Meneghetti A, Silvestri T, et al. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37(2-3):219–26.
Mariani E, Pulsatelli L, Meneghetti A, Dolzani P, Mazzetti I, Neri S, et al. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech Ageing Dev. 2001;122(13):1383–95.
Mariani E, Meneghetti A, Neri S, Ravaglia G, Forti P, Cattini L, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32(6):1524–9.
Frasca D, Blomberg BB. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun Ageing A. 2020;17(1):37.
de Mol J, Kuiper J, Tsiantoulas D, Foks AC. The dynamics of B cell aging in health and disease. Front Immunol. 2021; [cited 2023 Apr 20];12. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.733566
Blanco E, Pérez-Andrés M, Arriba-Méndez S, Contreras-Sanfeliciano T, Criado I, Pelak O, et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141(6):2208–2219.e16.
Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, et al. Age-associated B cells express a diverse repertoire of VH and Vf genes with somatic hypermutation. J Immunol Baltim Md. 1950;198(5):1921–7.
Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting edge: IL-4, IL-21, and IFN-eiinteract to govern T-bet and CD11c expression in TLR-activated B cells. J Immunol Baltim Md. 1950;197(4):1023–8.
Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, et al. IFNγ induces epigenetic programming of human T-bethi B cells and promotes TLR7/8 and IL-21 induced differentiation, Batista FD, Taniguchi T, Gaya M eLife. 2019, 8:e41641.
Cancro MP. Age-associated B cells. Annu Rev Immunol. 2020;38(3155):197.
Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)–driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–15.
Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice Blood.. 2011 118(5):1294-1304
Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65(4):1085–96.
Glauzy S, Boccitto M, Bannock JM, Delmotte FR, Saadoun D, Cacoub P, et al. Accumulation of antigen-driven lymphoproliferations in complement receptor 2/CD21-/low B cells from patients with Sjlls fro syndrome. Arthritis Rheumatol Hoboken NJ. 2018;70(2):298–307.
Mouat IC, Goldberg E, Horwitz MS. Age-associated B cells in autoimmune diseases. Cell Mol Life Sci. 2022;79(8):402.
Mouat IC, Horwitz MS. Age-associated B cells in viral infection. PLoS Pathog. 2022;18(3):e1010297.
Mouat IC, Allanach JR, Fettig NM, Fan V, Girard AM, Shanina I, et al. Gammaherpesvirus infection drives age-associated B cells toward pathogenicity in EAE and MS. Sci Adv. 2022;8(47):eade6844.
Phalke S, Rivera-Correa J, Jenkins D, Flores Castro D, Giannopoulou E, Pernis AB. Molecular mechanisms controlling age-associated B cells in autoimmunity. Immunol Rev. 2022;1:79–100.
Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, et al. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011;117(11):3104–12.
Riley RL, Khomtchouk K, Blomberg BB. Age-associated B cells (ABC) inhibit B lymphopoiesis and alter antibody repertoires in old age. Cell Immunol. 2017;321:61–7.
Du SW, Arkatkar T, Al Qureshah F, Jacobs HM, Thouvenel CD, Chiang K, et al. Functional characterization of CD11c+ age-associated B cells as memory B cells. J Immunol Baltim Md. 1950;203(11):2817–26.
Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–304.
Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-expressing B cells are located at the T Cell/B cell border in spleen and are potent APCs. J Immunol Baltim Md. 1950;195(1):71–9.
Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting edge: IL-4, IL-21, and IFN-e interact to govern T-bet and CD11c expression in TLR-activated B Cells. J Immunol Baltim Md. 1950;197(4):1023–8.
Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17(10):1226226.
Colonna-Romano G, Bulati M, Aquino A, PellicancanDavis CW, Roskin K, et al. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. 2009;130(10):681–90.
Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun. 2018;9(1):1758.
Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49(4):725–739.e6.
Stone SL, Peel J, Scharer CD, Risley CA, Chisolm DA, Schultz MD, et al. T-bet transcription factor promotes antibody secreting cell differentiation by limiting the inflammatory effects of IFNγ on B cells. Immunity. 2019;50(5):1172–1187.e7.
Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, et al. Cutting edge: B cell–intrinsic T-bet expression is required to control chronic viral infection. J Immunol. 2016;197(4):1017–22.
Macshut M, Kaido T, Yao S, Yagi S, Ito T, Kamo N, et al. Older donor age is a risk factor for negative outcomes after adult living donor liver transplantation using small-for-size grafts. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2019;25(10):1524–32.
Yeow M, Pang NQ, Muthiah MD, Soon G, Yock-Young D, Bonney GK, et al. Impact of donor age on recipient morbidity and mortality after living donor liver transplantation. ANZ J Surg. 2022;92(7-8):1867–72.
Weber DJ, Wang IW, Gracon ASA, Hellman YM, Hormuth DA, Wozniak TC, et al. Impact of donor age on survival after heart transplantation: an analysis of the United Network for Organ Sharing (UNOS) registry. J Card Surg. 2014;29(5):723–8.
Bittle GJ, Sanchez PG, Kon ZN, Claire Watkins A, Rajagopal K, Pierson RN, et al. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2013;32(8):760–8.
Lentine KL, Smith JM, Miller JM, Bradbrook K, Larkin L, Weiss S, et al. OPTN/SRTR 2021 annual data report: kidney. Am J Transplant. 2023;23(2):S21–120.
Poulose N, Raju R. Aging and injury: alterations in cellular energetics and organ function. Aging Dis. 2014;5(2):101–8.
Colvin MM, Smith CA, Tullius SG, Goldstein DR. Aging and the immune response to organ transplantation. J Clin Invest. 2017;127(7):2523–9.
He A, Sarwar A, Thole LML, Siegle J, Sattler A, Ashraf MI, et al. Renal inflamm-aging provokes intra-graft inflammation following experimental kidney transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2022;22(11):2529–47.
Matsunaga T, Iske J, Schroeter A, Azuma H, Zhou H, Tullius SG. The potential of Senolytics in transplantation. Mech Ageing Dev. 2021;200:111582.
Iske J, Seyda M, Heinbokel T, Maenosono R, Minami K, Nian Y, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11(1):4289.
Jonsson AH, Zhang F, Dunlap G, Gomez-Rivas E, Watts GFM, Faust HJ, et al. Granzyme K+ CD8 T cells form a core population in inflamed human tissue. Sci Transl Med. 2022;14(649):eabo0686.
Cuollo L, Antonangeli F, Santoni A, Soriani A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology. 2020;9(12):485.
Sanderson SL, Simon AK. In aged primary T cells, mitochondrial stress contributes to telomere attrition measured by a novel imaging flow cytometry assay. Aging Cell. 2017;16(6):1234–43.
Kim HJ, Kim WJ, Shin HR, Yoon HI, Moon JI, Lee E, et al. ROS-induced PADI2 downregulation accelerates cellular senescence via the stimulation of SASP production and NFκB activation. Cell Mol Life Sci. 2022;79(3):155.
Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, et al. ROS-induced PADI2 downregulation accelerates cellular senescence via the stimulation of SASP pty and premature senescence. Science. 2020;368(6497):1371–6.
Chiu YL, Tsai WC, Hung RW, Chen IY, Shu KH, Pan SY, et al. Emergence of T cell immunosenescence in diabetic chronic kidney disease. Immun Ageing. 2020;17(1):31.
Goplen NP, Wu Y, Son YM, Li C, Wang Z, Cheon IS, et al. Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci Immunol. 2020;5(53):eabc4557.
Delgobo M, Heinrichs M, Hapke N, Ashour D, Appel M, Srivastava M, et al. Terminally differentiated CD4+ T cells promote myocardial inflammaging. Front Immunol. 2021;12:584538.
Moro-García MA, López-Iglesias F, Marcos-Fernández R, Bueno-García E, Díaz-Molina B, Lambert JL, et al. More intensive CMV-infection in chronic heart failure patients contributes to higher T-lymphocyte differentiation degree, Clin Immunol., Orlando Fla 2018, 192, 20–29
Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, et al. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2016;15(2):389–92.
Pontrelli P, Rascio F, Castellano G, Grandaliano G, Gesualdo L, Stallone G. The role of natural killer cells in the immune response in kidney transplantation. Front Immunol. 2020;23(11):1454.
Iske J, Matsunaga T, Zhou H, Tullius SG. Donor and recipient age-mismatches: the potential of transferring senescence. Front Immunol. 2021;28(12):671479.
Schmitz R, Fitch ZW, Schroder PM, Choi AY, Jackson AM, Knechtle SJ, et al. B cells in transplant tolerance and rejection: friends or foes? Transpl Int Off J Eur Soc Organ Transplant. 2020;33(1):30–40.
Heinbokel T, Elkhal A, Liu G, Edtinger K, Tullius SG. Immunosenescence and organ transplantation. Transplant Rev Orlando Fla. 2013;27(3):65–75.
Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339(26):1905–13.
Krenzien F, ElKhal A, Quante M, Rodriguez Cetina Biefer H, Hirofumi U, Gabardi S, et al. A rationale for age-adapted immunosuppression in organ transplantation. Transplantation. 2015;99(11):2258–68.
Nian Y, Minami K, Maenesono R, Iske J, Yang J, Azuma H, et al. Changes of T-cell immunity over a lifetime. Transplantation. 2019;103(11):2227–33.
Thangavelu G, Murphy KM, Yagita H, Boon L, Anderson CC. The role of co-inhibitory signals in spontaneous tolerance of weakly mismatched transplants. Immunobiology. 2011;216(8):918–24.
Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol Baltim Md. 1950;181(8):5313–22.
Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007;37(10):2983–90.
• van der List ACJ, Litjens NHR, Klepper M, MGH B. Expression of senescence marker TIGIT identifies polyfunctional donor-reactive CD4+ T cells preferentially lost after kidney transplantation. Front Immunol. 2023;12 https://doi.org/10.3389/fimmu.2021.656846. Authors identified a donor-reactive memory CD4+ T cell type expressing the senescent and inhibitory marker TIGIT with a highly polyfunctional cytokine expression profile that declined after kidney transplantation. The decrease in this cell type is suggested to contribute to hyporesponsiveness in transplant recipients.
Dugast E, David G, Oger R, Danger R, Judor JP, Gagne K, et al. Broad impairment of natural killer cells from operationally tolerant kidney transplanted patients. Front Immunol. 2017;8 https://doi.org/10.3389/fimmu.2017.01721.
Wang L, Rondaan C, de Joode AAE, Raveling-Eelsing E, Bos NA, Westra J. Changes in T and B cell subsets in end stage renal disease patients before and after kidney transplantation. Immun Ageing. 2021;18(1):43.
Cherukuri A, Mohib K, Rothstein DM. Regulatory B cells: TIM‐1, transplant tolerance, and rejection. Immunol Rev. 299(1):31–44.
Wikby A, Ferguson F, Strindhall J, Forsey RJ, Fulop T, Hadrup SR, et al. Immune risk phenotypes and associated parameters in very old humans: a review of findings in the Swedish NONA immune longitudinal study. In: Pawelec G, editor. Immunosenescence. New York, NY: Springer, Medical Intelligence Unit; 2007. p. 1. https://doi.org/10.1007/978-0-387-76842-7_1.
Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121(1–3):187–201.
Weltevrede M, Eilers R, de Melker HE, van Baarle D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: a systematic review. Exp Gerontol. 2016;77:87–95.
Schaenman JM, Rossetti M, Sidwell T, Groysberg V, Sunga G, Korin Y, et al. Increased T cell immunosenescence and accelerated maturation phenotypes in older kidney transplant recipients. Hum Immunol. 2018;79(9):659–67.
Higdon LE, Schaffert S, Huang H, Montez-Rath ME, Lucia M, Jha A, et al. Evolution of cytomegalovirus-responsive T cell clonality following solid organ transplantation. J Immunol Baltim Md. 1950;207(8):2077–85.
Higdon LE, Trofe-Clark J, Liu S, Margulies KB, Sahoo MK, Blumberg E, et al. Cytomegalovirus-responsive CD8+ T cells expand after solid organ transplantation in the absence of CMV disease. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2017;17(8):2045–54.
Martín-Gandul C, Pérez-Romero P, Mena-Romo D, Molina-Ortega A, González-Roncero FM, Suñer M, et al. Kinetic of the CMV-specific T-cell immune response and CMV infection in CMV-seropositive kidney transplant recipients receiving rabbit anti-thymocyte globulin induction therapy: a pilot study. Transpl Infect Dis Off J Transplant Soc. 2018;20(3):e12883.
Higdon LE, Gustafson CE, Ji X, Sahoo MK, Pinsky BA, Margulies KB, et al. Association of Premature Immune Aging and Cytomegalovirus After Solid Organ Transplant. Front Immunol. 2021;12:661551.
Daniel L, Tassery M, Lateur C, Thierry A, Herbelin A, Gombert JM, et al. Allotransplantation is associated with exacerbation of CD8 T-cell senescence: the particular place of the innate CD8 T-cell component. Front Immunol. 2021;12 https://doi.org/10.3389/fimmu.2021.674016.
Pickering H, Schaenman J, Rossetti M, Ahn R, Sunga G, Liang EC, et al. T cell senescence and impaired CMV-specific response are associated with infection risk in kidney transplant recipients. Hum Immunol. 2022;83(4):273–80.
Hammer Q, Rückert T, Borst EM, Dunst J, Haubner A, Durek P, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19(5):453–63.
López-Botet M, Vilches C, Redondo-Pachón D, Muntasell A, Pupuleku A, Yélamos J, et al. Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front Immunol. 2017;16(8):166.
Redondo-Pachón D, Crespo M, Yélamos J, Muntasell A, Pérez-Sáez MJ, Pérez-Fernández S, et al. Adaptive NKG2C+ NK Cell Response and the Risk of Cytomegalovirus Infection in Kidney Transplant Recipients. J Immunol Baltim Md. 1950;198(1):94–101.
Della Chiesa M, Falco M, Podestà M, Locatelli F, Moretta L, Frassoni F, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410.
Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):4725–32.
Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–74.
Ishibashi K, Yamaguchi O, Suzutani T. Reinfection of cytomegalovirus in renal transplantation. Fukushima J Med Sci. 2011;57(1):1–10.
Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing A. 2019;16:25.
Alter G, Sekaly RP. Beyond adjuvants: antagonizing inflammation to enhance vaccine immunity. Vaccine. 2015;8(33 Suppl 2):B55–9.
Dunn-Walters DK. The ageing human B cell repertoire: a failure of selection? Clin Exp Immunol. 2016;183(1):50–6.
Howard WA, Gibson KL, Dunn-Walters DK. Antibody quality in old age. Rejuvenation Res. 2006;9((1):117–25.
Pritz T, Lair J, Ban M, Keller M, Weinberger B, Krismer M, et al. Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol. 2015;45(3):738–46.
Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He XS, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5(171):171ra19.
Nunzi E, Iorio AM, Camilloni B. A 21-winter seasons retrospective study of antibody response after influenza vaccination in elderly (60-85 years old) institutionalized subjects. Hum Vaccines Immunother. 2017;13(11):2659–68.
• Kugler-Umana O, Zhang W, Kuang Y, Liang J, Castonguay CH, Tonkonogy SL, et al. IgD+ age-associated B cells are the progenitors of the main T-independent B cell response to infection that generates protective Ab and can be induced by an inactivated vaccine in the aged. Aging Cell. 2022;21(10):e13705. In this mouse study an IgD+ ABC subtype has been identified that likely function as naïve B cells with a potential to become an antibody secreting cell in the aged population with decreased B and T cell responses.
• Cox A, Cevik H, Feldman HA, Canaday LM, Lakes N, Waggoner SN. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol Sci. 2021;42(9):789–801. NK cells have recently been identified as key regulators of vaccine-elicited T and B cell responses and as memory cells that contribute to pathogen control. The antibody-dependent cytolytic activity of NK cells is vital to the magnitude and quality of long-lived T and B cell memory. NK cells can restrain the affinity maturation of neutralizing antibodies.
Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, Potekhin A, Staub-Hohenbleicher H, Choi M, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrology. 2021;32(12):3027.
Rincon-Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):–eabj1031.
Malahe SRK, Hartog Y den, Rietdijk WJR, van Baarle D, de Kuiper R, Reijerkerk D, et al. The role of interleukin-21 in COVID-19 vaccinecinen kidney transplant recipients and dialysis patients. Am J Transplant. 2023, Available from: https://www.sciencedirect.com/science/article/pii/S1600613523004926
Furian L, Russo FP, Zaza G, Burra P, Hartzell S, Bizzaro D, et al. Differences in humoral and cellular vaccine responses to SARS-CoV-2 in kidney and liver transplant recipients. Front Immunol. 2022;13 https://doi.org/10.3389/fimmu.2022.853682.
Funding
This work was supported by awards to JSM from the Veterans Administration (1I01CX001971).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
JSM is a member of the scientific advisory board of Qihan Biotec and has a family member who is employed by Iconovir.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rollenhagen, C., Maltzman, J.S. The Effects of Aging on Solid Organ Transplantation—Characteristics and Consequences of Immunosenescence. Curr Transpl Rep 10, 135–146 (2023). https://doi.org/10.1007/s40472-023-00405-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-023-00405-5