Abstract
Purpose of Review
Antibody-mediated rejection (ABMR) is one of the most common causes of renal allograft loss. This review aims to outline the different clinical scenarios of ABMR with an emphasis on the cellular sources of antibody.
Recent Findings
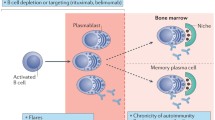
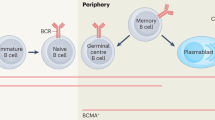
Studies of human plasma cells (PC) and memory B cells have been limited, but existing data suggest that both can contribute to ABMR. Hyperacute ABMR is due to pre-existing anti-HLA antibodies. Early acute ABMR likely involves the stimulation of memory B cells and can lead to new long-lived plasma cells. Late acute ABMR involves the de novo development of new memory B cells and PCs that are resistant to conventional immunosuppression. Chronic active ABMR involves not only antibodies (either pre-existing or de novo) but also effector cells such as NK cells, T cells, and macrophages.
Summary
Cellular processes underlying ABMR involve interactions between memory B cells, plasmablasts, plasma cells, and various effectors. Understanding these cellular processes is needed to improve therapies for ABMR.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–35. https://doi.org/10.1111/j.1600-6143.2008.02519.x.
Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. https://doi.org/10.1111/j.1600-6143.2011.03840.x.
Parajuli S, Aziz F, Garg N, Panzer SE, Joachim E, Muth B, et al. Histopathological characteristics and causes of kidney graft failure in the current era of immunosuppression. World J Transplant. 2019;9(6):123–33. https://doi.org/10.5500/wjt.v9.i6.123.
Hart A, Singh D, Brown SJ, Wang JH, Kasiske BL. Incidence, risk factors, treatment, and consequences of antibody-mediated kidney transplant rejection: a systematic review. Clin Transplant. 2021:e14320. doi: https://doi.org/10.1111/ctr.14320.
Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95(3):410–7. https://doi.org/10.1097/TP.0b013e31827d62e3.
Aubert O, Loupy A, Hidalgo L, Duong van Huyen JP, Higgins S, Viglietti D, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. 2017;28(6):1912–23. https://doi.org/10.1681/ASN.2016070797.
Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation. 2012;94(8):775–83. https://doi.org/10.1097/TP.0b013e31825d1587.
• Seifert M, Kuppers R. Human memory B cells. Leukemia. 2016;30(12):2283–92. https://doi.org/10.1038/leu.2016.226. (This review provides a thorough description of generation, functions, and diversity of memory B cells along with an emphasis on their role in persistent antibody generation.)
Marino J, Paster J, Benichou G. Allorecognition by T lymphocytes and allograft rejection. Front Immunol. 2016;7:582. https://doi.org/10.3389/fimmu.2016.00582.
Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15(3):149–59. https://doi.org/10.1038/nri3802.
Weisel F, Shlomchik M. Memory B cells of mice and humans. Annu Rev Immunol. 2017;35:255–84. https://doi.org/10.1146/annurev-immunol-041015-055531.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. https://doi.org/10.3389/fimmu.2014.00520.
Valenzuela NM, Hickey MJ, Reed EF. Antibody subclass repertoire and graft outcome following solid organ transplantation. Front Immunol. 2016;7:433. https://doi.org/10.3389/fimmu.2016.00433.
Lionaki S, Panagiotellis K, Iniotaki A, Boletis JN. Incidence and clinical significance of de novo donor specific antibodies after kidney transplantation. Clin Dev Immunol. 2013;2013: 849835. https://doi.org/10.1155/2013/849835.
•• Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–67. https://doi.org/10.1111/j.1600-6143.2012.04013.x. (This is a landmark clinical trial that identifies the risk factors for and the clinicopathological outcomes of de novo donor-specific HLA antibody development in kidney recipients.)
•• Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The synergistic effect of Class II HLA epitope-mismatch and nonadherence on acute rejection and graft survival. Am J Transplant. 2015;15(8):2197–202. https://doi.org/10.1111/ajt.13341. (This prospective study demonstrated that Class II HLA mismatch and medication nonadherence synergistically increase the risk of developing de novo DSA and exhibit an exposure-response relationship.)
Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15(11):2921–30. https://doi.org/10.1111/ajt.13347.
•• Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011;91(5):536–41. https://doi.org/10.1097/TP.0b013e3182081333. (This clinical trial found that the proteosome inhibitor bortezomib is efficacious in reducing bone marrow plasma cell number and DSA levels in presensitized kidney recipients.)
Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9(1):201–9. https://doi.org/10.1111/j.1600-6143.2008.02461.x.
DeWolf S, Sykes M. Alloimmune T cells in transplantation. J Clin Invest. 2017;127(7):2473–81. https://doi.org/10.1172/JCI90595.
Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol. 2017;8:170. https://doi.org/10.3389/fimmu.2017.00170.
Schinstock CA, Bentall AJ, Smith BH, Cornell LD, Everly M, Gandhi MJ, et al. Long-term outcomes of eculizumab-treated positive crossmatch recipients: allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant. 2019;19(6):1671–83. https://doi.org/10.1111/ajt.15175.
Lefaucheur C, Viglietti D, Mangiola M, Loupy A, Zeevi A. From humoral theory to performant risk stratification in kidney transplantation. J Immunol Res. 2017;2017:5201098. https://doi.org/10.1155/2017/5201098.
Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35(4):397–406. https://doi.org/10.1016/j.healun.2016.01.1223.
Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. https://doi.org/10.1016/j.healun.2012.09.021.
• Schinstock C, Stegall MD. Acute antibody-mediated rejection in renal transplantation: current clinical management. Curr Transplant Rep. 2014;1(2):78–85. https://doi.org/10.1007/s40472-014-0012-y. (This is a state-of-the art review summarizing the current clinical phenotypes of and therapeutic options in ABMR.)
Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242–51. https://doi.org/10.1056/NEJMoa0707894.
Redfield RR, Jordan SC, Busque S, Vincenti F, Woodle ES, Desai N, et al. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti-CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant. 2019;19(11):3035–45. https://doi.org/10.1111/ajt.15514.
• Dean PG, Park WD, Cornell LD, Gloor JM, Stegall MD. Intragraft gene expression in positive crossmatch kidney allografts: ongoing inflammation mediates chronic antibody-mediated injury. Am J Transplant. 2012;12(6):1551–63. https://doi.org/10.1111/j.1600-6143.2011.03964.x. (According to this study on intragraft gene expression in sensitized kidney recipients, sensitized patients with transplant glomerulopathy had enrichment of inflammatory genes compared to those without transplant glomerulopathy.)
Soares MP, Lin Y, Sato K, Stuhlmeier KM, Bach FH. Accommodation. Immunol Today. 1999;20(10):434–7. https://doi.org/10.1016/S0167-5699(99)01530-3.
Burdorf L, Azimzadeh AM, Pierson RN 3rd. Progress and challenges in lung xenotransplantation: an update. Curr Opin Organ Transplant. 2018;23(6):621–7. https://doi.org/10.1097/MOT.0000000000000582.
Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58). https://doi.org/10.1126/sciimmunol.abi6950
•• Karahan GE, Krop J, Wehmeier C, de Vaal YJH, Langerak-Langerak J, Roelen DL, et al. An easy and sensitive method to profile the antibody specificities of HLA-specific memory B cells. Transplantation. 2019;103(4):716–23. https://doi.org/10.1097/TP.0000000000002516. (This study showed that screening for pretransplant sensitization improves the detection of HLA-specific memory B cells, which according to the study is more sensitively determined via B cell culture followed by IgG isolation rather than supernatant concentration.)
•• Wehmeier C, Karahan GE, Heidt S. HLA-specific memory B-cell detection in kidney transplantation: insights and future challenges. Int J Immunogenet. 2020;47(3):227–34. https://doi.org/10.1111/iji.12493. (This review outlines the strengths and limitations of the assays used to identify HLA-specific memory B cells (i.e., flow cytometry, Luminex single antigen beads, ELISpot).)
•• Lucia M, Luque S, Crespo E, Melilli E, Cruzado JM, Martorell J, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015;88(4):874–87. https://doi.org/10.1038/ki.2015.205. (This paper suggests that memory B cells play a key role in ABMR through showing correlation between sensitization and HLA-specific memory B cell response.)
•• Woodle ES, Tremblay S, Rossi A, Rojas CC, Alloway R, Roskin K, et al. Plasma cell targeting to prevent antibody-mediated rejection. Am J Transplant. 2020;20(Suppl 4):33–41. https://doi.org/10.1111/ajt.15889. (Reviews the results of recent clinical trials on the efficacy of first- and second-generation proteosome inhibitors in eliminating plasmablasts and long-lived plasma cells.)
•• Woodle ES, Tremblay S, Brailey P, Girnita A, Alloway RR, Aronow B, et al. Proteasomal adaptations underlying carfilzomib-resistance in human bone marrow plasma cells. Am J Transplant. 2020;20(2):399–410. https://doi.org/10.1111/ajt.15634. (Transcriptomic profiling of long-lived plasma cells that survived in vivo desensitization therapy with the proteosome inhibitor carfilzomib revealed increased expression of the immunoproteosome.)
Chesneau M, Michel L, Degauque N, Brouard S. Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol. 2013;4:497. https://doi.org/10.3389/fimmu.2013.00497.
Cowan ML, Sciammas R, Chong AS. Experimental models of B cell tolerance in transplantation. Semin Immunol. 2012;24(2):77–85. https://doi.org/10.1016/j.smim.2011.08.018.
Taddeo A, Khodadadi L, Voigt C, Mumtaz IM, Cheng Q, Moser K, et al. Long-lived plasma cells are early and constantly generated in New Zealand Black/New Zealand White F1 mice and their therapeutic depletion requires a combined targeting of autoreactive plasma cells and their precursors. Arthritis Res Ther. 2015;17:39. https://doi.org/10.1186/s13075-015-0551-3.
Moore N, Moreno Gonzales M, Bonner K, Smith B, Park W, Stegall M. Impact of CXCR4/CXCL12 blockade on normal plasma cells in vivo. Am J Transplant. 2017;17(6):1663–9. https://doi.org/10.1111/ajt.14236.
Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun. 2018;9(1):3698. https://doi.org/10.1038/s41467-018-05853-7.
Zhang Z, Schuster SJ, Lacey SF, Milone MC, Monos D, Bhoj VG. Stable HLA antibodies following sustained CD19+ cell depletion implicate a long-lived plasma cell source. Blood Adv. 2020;4(18):4292–5. https://doi.org/10.1182/bloodadvances.2020002435.
•• Moreno Gonzales MA, Gandhi MJ, Schinstock CA, Moore NA, Smith BH, Braaten NY, et al. 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation. 2017;101(6):1222–7. https://doi.org/10.1097/TP.0000000000001330. (A longer course of bortezomib was not found to be associated with desensitization in highly sensitized kidney recipient.)
Pineiro GJ, De Sousa-Amorim E, Sole M, Rios J, Lozano M, Cofan F, et al. Rituximab, plasma exchange and immunoglobulins: an ineffective treatment for chronic active antibody-mediated rejection. BMC Nephrol. 2018;19(1):261. https://doi.org/10.1186/s12882-018-1057-4.
Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, et al. Daratumumab in sensitized kidney transplantation: potentials and limitations of experimental and clinical use. J Am Soc Nephrol. 2019;30(7):1206–19. https://doi.org/10.1681/ASN.2018121254.
Doberer K, Klager J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, et al. CD38 antibody daratumumab for the treatment of chronic active antibody-mediated kidney allograft rejection. Transplantation. 2021;105(2):451–7. https://doi.org/10.1097/TP.0000000000003247.
Spica D, Junker T, Dickenmann M, Schaub S, Steiger J, Rufli T, et al. Daratumumab for treatment of antibody-mediated rejection after ABO-incompatible kidney transplantation. Case Rep Nephrol Dial. 2019;9(3):149–57. https://doi.org/10.1159/000503951.
• Tambur AR, Schinstock C, Maguire C, Lowe D, Smith B, Stegall M. Estimating alloantibody levels in highly sensitized renal allograft candidates: using serial dilutions to demonstrate a treatment effect in clinical trials. Am J Transplant. 2021;21(3):1278–84. https://doi.org/10.1111/ajt.16363. (In this study involving 20 kidney recipients with a calculated panel-reactive antibody (cPRA) greater than 99.9%, cPRA was normalized through serial serum dilutions and was found to have increased sensitivity to detect antibody reductions compared to cPRA alone.)
Stegall MD, Smith B, Bentall A, Schinstock C. The need for novel trial designs, master protocols, and research consortia in transplantation. Clin Transplant. 2020;34(1): e13759. https://doi.org/10.1111/ctr.13759.
Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018;18(7):1783–9. https://doi.org/10.1111/ajt.14721.
Wojciechowski D, Vincenti F. Current status of costimulatory blockade in renal transplantation. Curr Opin Nephrol Hypertens. 2016;25(6):583–90. https://doi.org/10.1097/MNH.0000000000000268.
van der Zwan M, Hesselink DA, van den Hoogen MWF, Baan CC. Costimulation blockade in kidney transplant recipients. Drugs. 2020;80(1):33–46. https://doi.org/10.1007/s40265-019-01226-6.
Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14(8):1817–27. https://doi.org/10.1111/ajt.12810.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Immunology
Rights and permissions
About this article
Cite this article
Mujtahedi, S.S., Yigitbilek, F., Ozdogan, E. et al. Antibody-Mediated Rejection: the Role of Plasma Cells and Memory B Cells. Curr Transpl Rep 8, 272–280 (2021). https://doi.org/10.1007/s40472-021-00342-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-021-00342-1