Abstract
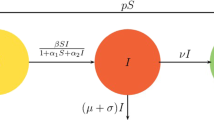
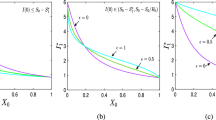
This article explores the global properties of a generalized \(SI_cIRS\) (susceptible-asymptomatically infected-symptomatically infected-recovered-susceptible) epidemic model, which takes into consideration the factors associated with government policies, public responses and social behavioural reactions. Essentially, this study centres on infectious diseases that can be propagated through asymptomatic carriers—individuals who are infected and contagious but do not exhibit any disease symptoms. Additionally, the analysis delves into the effects of environmental fluctuations on the dynamics of disease transmission. For the deterministic model, it has been observed that disease invasion occurs in the system when the basic reproduction number exceeds 1. On the other hand, if we raise the noise intensities in case of the stochastic model, the disease extinction happens quickly. Also, we have proved that the system undergoes transcritical bifurcation in the vicinity of the infection-free equilibrium. Also, two-parameter bifurcation provides the regions where stability of both the equilibrium points are analysed. This work, in particular, highlights the significance of nonlinear dynamic analyses on epidemic models. It also emphasizes the substantial impact that social behaviour and governmental action have on disease transmission by asymptomatically infected carriers and symptomatically infected individuals. The numerical data and simulations illustrate the critical role of government interventions in managing an epidemic, and the system tends to achieve an infection-free state more rapidly when the government takes early action during the onset of a disease outbreak.









Similar content being viewed by others
Data availability
The data used to support the findings of the study are available within the article.
References
Das M, Samanta GP (2022) Optimal control of a fractional order epidemic model with carriers. Int J of Dyn Control 10(2):598–619. https://doi.org/10.1007/s40435-021-00822-3
Oran DP, Topol EJ (2021) Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med 174(2):286–287
Samanta GP (2014) Analysis of a delayed epidemic model with pulse vaccination. Chaos Solit Fract 66:74–85. https://doi.org/10.1016/j.chaos.2014.05.008
Aguilar JB, Faust JS, Westafer LM, Gutierrez JB (2020) Modeling the impact of asymptomatic carriers on COVID-19 transmission dynamics during lockdown, MedRxiv 2020–03
Kalajdzievska D, Li MY (2011) Modeling the effects of carriers on transmission dynamics of infectious diseases. Math Biosci Eng 8(3):711–722
Luo S-H, Liu W, Liu Z-J, Zheng X-Y, Hong C-X, Liu Z-R, Liu J, Weng J-P (2020) A confirmed asymptomatic carrier of 2019 novel coronavirus. Chin Med J 133(09):1123–1125
Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, Wu J, Du H, Chen T, Li R et al (2020) Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 3(3):e203976–e203976
Meyerowitz EA, Richterman A, Gandhi RT, Sax PE (2021) Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 174(1):69–79
Gauld JS, Hu H, Klein DJ, Levine MM (2018) Typhoid fever in Santiago, Chile: insights from a mathematical model utilizing venerable archived data from a successful disease control program. PLoS Negl Trop Dis 12(9):e0006759
Ahmad Z, Bonanomi G, di Serafino D, Giannino F (2023) Transmission dynamics and sensitivity analysis of pine wilt disease with asymptomatic carriers via fractal-fractional differential operator of Mittag-Leffler kernel. Appl Numer Math 185:446–465. https://doi.org/10.1016/j.apnum.2022.12.004
Bernoulli D, Chapelle D. Essai d’une nouvelle analyse de la mortalité causée par la petite vérole, et des avantages de l’inoculation pour la prévenir
Kermack WO, McKendrick AG (1927) A contribution to the mathematical theory of epidemics. Proc Royal Soc London Ser A Contain Papers Math Phys Character 115(772):700–721. https://doi.org/10.1098/rspa.1927.0118
Gaff H, Schaefer E (2009) Optimal control applied to vaccination and treatment strategies for various epidemiological models. Math Biosci Eng 6(3):469–492
Lee S, Chowell G, Castillo-Chávez C (2010) Optimal control for pandemic influenza: the role of limited antiviral treatment and isolation. J Theor Biol 265(2):136–150
Ahmed M, Masud MAB, Sarker MMA (2023) Bifurcation analysis and optimal control of discrete SIR model for COVID-19. Chaos Solit Fract 174:113899. https://doi.org/10.1016/j.chaos.2023.113899
Tomochi M, Kono M (2021) A mathematical model for COVID-19 pandemic-SIIR model: effects of asymptomatic individuals. J General Family Med 22(1):5–14
De la Sen M, Alonso-Quesada S, Ibeas A (2015) On the stability of an SEIR epidemic model with distributed time-delay and a general class of feedback vaccination rules. Appl Math Comput 270:953–976. https://doi.org/10.1016/j.amc.2015.08.099
Turkyilmazoglu M (2021) Explicit formulae for the peak time of an epidemic from the SIR model. Physica D 422:132902
Mamis K, Farazmand M (2023) Stochastic compartmental models of the COVID-19 pandemic must have temporally correlated uncertainties. Proc Royal Soc A 479(2269):20220568
Britton T (2010) Stochastic epidemic models: a survey. Math Biosci 225(1):24–35
Mao X, Marion G, Renshaw E (2002) Environmental Brownian noise suppresses explosions in population dynamics. Stoch Process Appl 97(1):95–110. https://doi.org/10.1016/S0304-4149(01)00126-0
Ross R (1911) The prevention of malaria. John Murray, London
Medley GF, Lindop NA, Edmunds WJ, Nokes DJ (2001) Hepatitis-B virus endemicity: heterogeneity, catastrophic dynamics and control. Nat Med 7(5):619–624
Naresh R, Pandey S, Misra A (2008) Analysis of a vaccination model for carrier dependent infectious diseases with environmental effects. Nonlinear Anal Modell Control 13(3):331–350. https://doi.org/10.15388/NA.2008.13.3.14561
Trotter CL, Gay NJ, Edmunds WJ (2005) Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol 162(1):89–100
Zhao S, Xu Z, Lu Y (2000) A mathematical model of hepatitis B virus transmission and its application for vaccination strategy in China. Int J Epidemiol 29(4):744–752
Zhou W, Wang A, Xia F, Xiao Y, Tang S (2020) Effects of media reporting on mitigating spread of COVID-19 in the early phase of the outbreak. Math Biosci Eng 17(3):2693–2707
Wang C (2023) A simple epidemic model for semi-closed community reveals the hidden outbreak risk in nursing homes, prisons, and residential universities. Int J Dyn Control 11(4):1506–1517. https://doi.org/10.1007/s40435-022-01068-3
Wang C, Kavak H (2022) A general epidemic model and its application to mask design considering different preferences towards masks. Complexity. https://doi.org/10.1155/2022/1626008
Dutta P, Samanta G, Nieto JJ (2024) Periodic transmission and vaccination effects in epidemic dynamics: a study using the SIVIS model. Nonlinear Dyn 112:2381–2409. https://doi.org/10.1007/s11071-023-09157-4
Svirezhev I, Logofet DO (1983) Stability of biological communities MIR Publishers, Moscow, Russia
Nisar KS, Srinivas M, Murthy B, Madhusudanan V, Gul N, Abdulrehman J, Zeb A (2023) Exploring the dynamics of white noise and spatial temporal variations on hearing loss due to mumps virus, Results Phys. p 106584
Sabbar Y (2021) Mathematical Analysis of Some Stochastic Infectious Disease Models with White Noises and Lévy Jumps, Ph.D. thesis, Université Sidi Mohamed Ben Abdellah de Fès (Maroc)
Horsthemke W (1984) Noise induced transitions. In: Non-equilibrium dynamics in chemical systems: proceedings of the international symposium, Bordeaux, France, September 3–7, 1984, Springer, pp 150–160
Kwuimy C, Nazari F, Jiao X, Rohani P, Nataraj C (2020) Nonlinear dynamic analysis of an epidemiological model for COVID-19 including public behavior and government action. Nonlinear Dyn 101:1545–1559
Saha S, Dutta P, Samanta G (2022) Dynamical behavior of SIRS model incorporating government action and public response in presence of deterministic and fluctuating environments. Chaos Solit Fract 164:112643. https://doi.org/10.1016/j.chaos.2022.112643
Jana D, Banerjee A, Samanta GP (2017) Degree of prey refuges: control the competition among prey and foraging ability of predator. Chaos Solit Fract 104:350–362. https://doi.org/10.1016/j.chaos.2017.08.031
Maiti A, Samanta GP (2005) Deterministic and stochastic analysis of a prey-dependent predator-prey system. Int J Math Educ Sci Technol 36(1):65–83. https://doi.org/10.1080/00207390412331314980
Maiti A, Samanta GP (2006) Deterministic and stochastic analysis of a ratio-dependent prey-predator system. Int J Syst Sci 37(12):817–826. https://doi.org/10.1080/00207720600879252
Manna D, Maiti A, Samanta G (2019) Deterministic and stochastic analysis of a predator-prey model with Allee effect and herd behaviour. Simulation 95(4):339–349. https://doi.org/10.1177/0037549718779445
Jana D, Samanta GP (2014) Role of multiple delays in ratio-dependent prey-predator system with prey harvesting under stochastic environment. Neural Parallel Sci Comput 22:205–222
Samanta GP (1991) Stochastic analysis of a noisy oscillator. Appl Math Lett 4(2):61–63. https://doi.org/10.1016/0893-9659(91)90170-Z
Bera SP, Maiti A, Samanta G (2016) Stochastic analysis of a prey-predator model with herd behaviour of prey. Nonlinear Anal Modell Control 21(3):345–361. https://doi.org/10.15388/NA.2016.3.4
Das A, Samanta GP (2021) Modelling the effect of resource subsidy on a two-species predator-prey system under the influence of environmental noises, International Journal of. Dyn Control 9:1800–1817. https://doi.org/10.1007/s40435-020-00750-8
Athreya A, Kolba T, Mattingly J (2012) Propagating Lyapunov functions to prove noise-induced stabilization. Electron J Probab 17:1–38
Li X, Mao X (2009) Population dynamical behavior of non-autonomous Lotka–Volterra competitive system with random perturbation. Disc Contin Dyn Syst Ser A 24(2):523–593
Mao X (2007) Stochastic differential equations and applications. Elsevier, Amsterdam
Castillo-Chavez C, Song B (2004) Dynamical models of tuberculosis and their applications. Math Biosci Eng 1(2):361–404. https://doi.org/10.3934/mbe.2004.1.361
Zhao H, Lu X, Deng Y, Tang Y, Lu J (2020) COVID-19: asymptomatic carrier transmission is an underestimated problem, Epidemiol Infection 148
Azhar ZI, Chen XW, Mohamad M, Saman MSA, Isa MR, Ismail N (2020) COVID-19 review: an epidemiological perspective and Malaysian scenario in handling the pandemic (January–May 2020). J Clin Health Sci 5(1):26–41
Donato V (2023) Managing the pandemic: the Italian strategy for fighting Covid-19 and the challenge of sharing administrative powers. Eur J Risk Regulat 14(1):113–140
Kumar A, Prasad N, Sirumban P, Anbalagan M, Durgambal K (1983) Community awareness about leprosy and participation in National Leprosy Control Programme. Leprosy India 55(4):701–711
Acknowledgements
The authors are grateful to the anonymous referees and Professor Jian-Qiao Sun (Editor-in-Chief) for their valuable comments and helpful suggestions, which have helped them to improve the presentation of this work significantly. The first author (Shimli Dutta) and the second author (Protyusha Dutta) are thankful to the Indian Institute of Engineering Science and Technology, Shibpur, India for providing fellowship.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All the authors have participated equally in all the aspects of this paper: conceptualization, methodology, investigation, formal analysis, writing-original draft preparation, writing-review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
With reference to the study, the authors claim that they do not have any conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, S., Dutta, P. & Samanta, G. Modelling disease transmission through asymptomatic carriers: a societal and environmental perspective. Int. J. Dynam. Control (2024). https://doi.org/10.1007/s40435-024-01387-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40435-024-01387-7