Abstract
The Coronavirus disease (Covid-19), caused by the SARS-CoV-2 virus, has produced significant social and economic disruptions in different countries. Current evidence suggests a strong correlation between the infection and the cohabitation of indoor spaces. International organizations and experts consider that the airborne transmission through aerosols can occur in specific conditions and that inadequate ventilation increases the risk of infection. As a result, the increase in ventilation rates and air filtration efficiencies are recommended for public buildings in the context of Covid-19, with significant impacts on energy consumption, and a paradigm shift in the design of ventilation systems is necessary for this new context. Therefore, this study has assessed the comparative performance of the displacement ventilation and the mixed ventilation mode on reducing the risk of long-range airborne infection for the Covid-19 in a small office application. A coupled multizone-CFD (Computational Fluid Dynamics) software developed by the National Institute of Standards and Technology was used in this study to assess the relative performance of several design solutions related to different ventilation modes, filter efficiencies, and outdoor air flow rates. The results demonstrate that the displacement ventilation technique produces a better overall performance in reducing the SARS-CoV-2 airborne infection risk than the conventional mixed ventilation for all the studied cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Covid-19 pandemic context
The Coronavirus disease (Covid-19), caused by the SARS-CoV-2 virus, has spread throughout a wide geographic area [1, 2], causing significant social and economic disruptions in different countries [1], as well as more than one million deaths to this date [2]. The restrictions on individual mobility, commercial activity, international travel, and school closures, related to the pandemics’ control, produced an unprecedented impact in the US stock market [3] as well as in the world economy [4].
1.2 Covid-19 airborne transmission in indoor spaces
The World Health Organization (WHO) [5] and the US Centers for Disease Control and Prevention (CDC) [6] suggest that the virus is mainly transmitted from person to person through respiratory droplets produced by expiratory activities. Some studies have demonstrated that the virus may remain viable after aerosolization in the indoor environment [7,8,9]. Qian et al. [10] evaluated 328 Covid-19 outbreaks involving three or more infected persons in 120 cities in China. The results show a strong correlation between the infection and the cohabitation of indoor spaces [10]. The recent outbreak cases at the Skagit Valley choral [11] in the USA, the call center in South Korea [12], and the bus travel episode in China [13], with a large number of infected subjects, have cast a light on the role of super spreaders and airborne propagation. Morawska and Milton [14] produced a letter, supported by 239 scientists, appealing to the medical community and to the relevant bodies to recognize the potential for airborne spread of Covid-19. The different vocabulary between the exposure science community and the medical infectious disease community regarding airborne transmission results in a communication difficulty that hampers collaborative efforts [15,16,17]. An arbitrary cutoff size of 5 μm is often used to separate the droplets’ direct transmission and the airborne transmission [16, 18, 19]. Particles much larger than 5 µm may be transported through air currents for a significant distance (when compared to a typical indoor room scale) [15, 16]. The cutoff size is affected by the droplet composition [18] and indoor airflow patterns [16,17,18], which introduces high uncertainty [18]. Small droplets (d ≤ 100 μm) could remain airborne for significant enough time (e.g., tens of seconds to several minutes) to be inhaled, depending on the indoor airflow patterns [17]. Specialized researchers on aerosol science and exposure science identify an urgent need to clarify the methodology to distinguish between aerosols and larger droplets (that leave the suspension in a low timescale due to gravity) using the threshold size of 100 µm, rather than the traditional size of 5 µm [16, 20]. This characteristic size is more effective to separate their aerodynamic behavior, inhalation capability, and efficacy of intervention measures [16, 20].
The American Society of Heating, Refrigeration and Air Conditioning Engineers (ASHRAE) suggests that the Transmission of SARS-CoV-2 through the air is sufficiently likely that airborne exposure to the virus should be controlled [21]. The WHO [5] and the CDC [6] consider that airborne transmission through aerosols can occur in specific conditions and that inadequate ventilation increases that risk.
1.3 Motivation, objectives, and justification of the present study
ASHRAE [22], the Federation of European Heating, Ventilation and Air-Conditioning Associations (REHVA) [23], the CDC [24], and the WHO [25] recommend measures related to the increase in ventilation rates and filtration efficiencies for public buildings in the context of Covid-19. MERV-13 filters, as rated by ANSI/ASHRAE 52.2 standard [26], are recommended by ASHRAE [22] and the CDC [24]. However, systems designed to meet the minimum requirements of a ventilation standard like ANSI/ASHRAE 62.1 standard [27] will have a MERV-8 filter [26], leading to a filter upgrade necessity in many cases. This filter upgrade may be hampered due to existing fan pressure constraints or may lead to an increase in energy consumption related to the higher filter pressure loss. Regarding ventilation, ASHRAE [22], REHVA [23], the CDC [24], and the WHO [25] recommend increasing outdoor airflow rate to the maximum allowable value (100% if possible). Providing 100% outdoor airflow rate to a commercial building produces an energy penalty that is seldom justified [28]. Therefore, the majority of the existing commercial buildings worldwide use the minimum affordable ventilation rate [28]. Increasing the ventilation rate in existing buildings may lead to indoor moisture control disruption in hot & humid climates and will increase energy consumption. A paradigm shift in the design of ventilation systems is necessary for this new context, with a focus on source control, advanced air distribution, and occupant-based microenvironment control [29]. ASHRAE suggests that research should address methods to quantify the relative airborne infection control performance and cost-effectiveness of specific engineering strategies [21]. Previous studies have demonstrated that the use of high values of ventilation rates has a limited efficiency on airborne contaminant control [30,31,32,33,34] and that an air distribution strategy that reduces the contaminant residence timescale at the room breathing zone provides better performance [32, 35,36,37]. Several studies have demonstrated that the displacement ventilation mode (DV) is a promising technique for enhancing the ventilation performance and reducing the risk of airborne infection in hospital settings [38] and commercial applications [39,40,41]. However, in all these studies, the contaminant concentration was modeled as null in the supply air, in a scenario that would resemble a 100% outdoor air ventilation system or a high-efficiency air filtration. Since recirculation and lower efficiency air filters are often applicable in commercial and office spaces [27], a complimentary evaluation of the DV ventilation performance with these features is necessary. Therefore, the objective of this study is to assess the comparative ventilation performance of the DV and the mixed ventilation (MV) system on reducing the risk of airborne infection for the Covid-19 in an office application for cases involving the use of air recirculation and different filter efficiencies. Given the current discussion by the scientific community regarding the appropriate particle size range for airborne transmission, this study has focused on controlling the fraction of the emitted respiratory droplets that may remain suspended in the air for a longstanding period, consistent with the long-range and long-time scales of airborne transmission in indoor settings. Exposure to the exhalation jet of an infector (short-range airborne or direct spray route) that occurs in close contact and leads to higher doses [18] was not considered in this study to focus on the ventilation role in limiting the long-range airborne transmission. This study is aligned with the ASHRAE proposition [21] and expands the evaluation of the DV technique on airborne infection control to commercial applications with air recirculation and different filtration efficiencies, contributing to a better understanding of the rational limits of ventilation control in the context of Covid-19 and other respiratory diseases.
2 Material and methods
2.1 General description of the method
Buonanno et al. [42, 43] proposed a method to estimate the rate of generation of SARS-CoV-2 infectious quanta that can be useful for assessing the long-range airborne infection risk of different ventilation configurations. In this approach, the quantum is the dose of respiratory droplets’ residues required to cause infection in 63% of the susceptible occupants of an enclosed space. The quanta emission rate is estimated by the method as a function of the viral load in the sputum, respiratory activity, and activity level of the infectious subject [42]. As many uncertainties persist at the moment regarding the new Coronavirus disease, the method presents many uncertainties and data extrapolation from the SARS-CoV-1 (also a coronavirus). Despite that, the authors sustain its relevance for allowing a quantitative assessment during the course of the pandemic, when limited data are available [42]. The method has been used to assess the efficiency of infection control measures related to ventilation and quarantine interventions [42, 43] and to assess the quanta emission rate related to specific outbreak cases in retrospective studies [11, 43].
Therefore, the method proposed by Buonanno et al. [42, 43] was used in this study in a coupled multizone-CFD analysis to estimate the long-range airborne infection risk of a cohabitant in a model of a small office configuration with two occupants. The quanta emission rate, estimated by the method of Buonanno et al. [42, 43], was used as a source input in a numerical model of the office’s ventilation system to predict the resultant quanta concentration at the breathing zone of the cohabitant (nb). CONTAM, a public domain software developed by the National Institute of Standards and Technology (NIST) [44], and its Computational Fluid Dynamics (CFD) module, developed by Srebric et al. [45], and improved by Wang et al. [46], were used in this study to model the office ventilation system and to assess the resultant quanta concentration of several design solutions related to ventilation modes, filter efficiencies, and outdoor air flow rates. As in the method of Buonanno et al. [42, 43], the resultant quanta concentration at the breathing zone of the cohabitant was integrated over time using the Wells–Riley equation [47] to determine the infection risk (R) of each scenario:
where IR is the inhalation rate [m3 h−1] and \(\tau\) is the exposure time. The infection risk of each occupant was assessed for each studied case, exchanging the position of the emitter and the susceptible. The following premises were adopted for the simulations: the mode of infection investigated was through the long-range airborne route only (direct and indirect contact risks were not investigated, to focus on ventilation performance); the simulation period was set as 8 h, related to a commercial shift of work; all occupants remain in the space during all the simulation period; inhalation rate was set as IR = 0.96 m3 h−1, consistent with an office activity [42]. “Appendix A” presents a flowchart that encompasses all the simulation steps. “Appendix B” presents the verification of the mathematical model of infection.
2.2 Description of the modeled office and its basic ventilation system
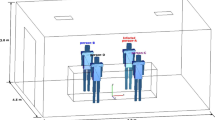
The configuration of the studied office is shown in Fig. 1 and is the same as the small office modeled by Chen et al. [39] for the ASHRAE research project RP-949. This setting was adopted given that: it resembles a typical configuration in the USA for small offices [39]; high-quality experimental data on airflow and contaminant distribution are available through the ASHRAE RP-949 benchmark [39] for CFD validation. The floor area is 18.8 m2 with two occupants (#1 and #2), and the total heat dissipation from people, light fixtures, and equipment is 634 W.
The air-conditioning system was modeled for the base case with the characteristics presented in Table 1, according to the ASHRAE RP-949 benchmark features [39] and the ASHRAE 62.1 standard [27] procedure for ventilation calculations regarding the outdoor air flow rate for offices. Two different ventilation modes were investigated with the same airflow and thermal load rates: MV (mixed) and DV (displacement). Figure 2a depicts the general arrangement of the air distribution solution modeled for the DV mode, while Fig. 2b depicts the arrangement modeled for the MV mode.
2.3 Estimative on quanta source strength for SARS-CoV-2
The approach proposed by Buonanno et al. [42, 43] is based on the hypothesis that the emitted droplets have the same viral load as the sputum of the infectious subject (aerosol source). The emitted viral load is therefore estimated by a mass balance, expressed in terms of quanta emission rate (ERq, quanta h−1), by the following equation [43]:
where \(c_{v} \) is the viral load in the sputum (RNA copies mL−1), \(c_{i} \) is the conversion factor (quanta RNA copies−1), \(\dot{V}_{d}\) is the droplets generation rate by the expiratory activity (mL h−1), and \(\emptyset_{d}\) is the droplet volume concentration at the exhaled flow (mL m−3).
2.3.1 Droplets generation rate
A wide range of droplets’ sizes is generated by the expiratory activity, from the sub-micrometer order to the millimeter order [18, 49]. Following their exhalation, dehydration due to liquid evaporation reduces the size of the droplets [18, 49]. The final dehydrated equilibrium sizes depend on droplet composition and surrounding air relative humidity [18] and range from 20% [49, 50] to 50% [51] of the initial sizes. Gravity acts on the deposition of the larger droplets, while the small droplets follow the airflow motion [18]. The threshold size between small and large droplets is understood to vary from 60 µm to 120 µm [18]. This study has focused on the fraction of the small droplets that may remain suspended in the air for a longstanding period, consistent with the time scales of indoor ventilation control. De Oliveira et al. [18] mention that recent studies [15, 17] suggest that values around 20 µm should be used for ventilation calculations and that the approach of using a single value for all applications is inappropriate because the particle cutoff size is flow dependent. Barzant and Bush [49] provide a method to assess the ‘critical particle size’ for long-range airborne transmission in a given scenario, based on the well-mixed sedimentation and ventilation timescales. For the cases assessed in this study, with an effective ventilation rate ranging from λc = 2.9 to λc = 4.2, the critical particle diameter according to Barzant and Bush [49] ranges from 9 µm to 12 µm (for a ‘well-mixed’ scenario). The real airflow field in an indoor setting presents several flow structures that modify the particle transport predictions by the ‘well-mixed’ scenario, as jets, thermal plumes, recirculation, entrainment, etc. The transient simulations performed by Vuorinen et al. [17] incorporated these flow features through CFD and demonstrated that the transport pattern of massless particles and 10-µm particles by the indoor airflow was relatively similar, while the transport of 20-µm particles was noticeable different and relatively limited, when compared to the former. This limited transport is especially highlighted if the analysis is focusing on a long-timescale transport as in the case of this study. Therefore, the use of respiratory droplets’ residues with a maximum diameter in the O(101)μm is a reasonable assumption for the current analysis. Different studies have produced estimates on \(\dot{V}_{d}\) with considerable discrepancies, given the uncertainty and complexity of the associated experimental measurements. A range from O(1) to O(10)nL h−1 for speaking was used in different studies [18, 52], based on past experimental data [53, 54]. Mikszewski et al. [55] suggest the value of 4900 nL h−1 based on the recent measurements of Stadnytskyi et al. [50] for loudly speaking. Prentiss et al. [56] mention that these discrepancies may be related to the sensitivity of each experimental method for the measurement of particles in the submicron and supermicron ranges and suggest that the estimate of Stadnytskyi et al. [50] is more appropriate for their study. Buonanno et al. [43] have adopted a range from 1000 to 2500 nL h−1 for breathing and 1000 to 4500 nL h−1 for speaking, consistent with Stadnytskyi et al. [50].
2.3.2 Viral load and infectious dose
The viral load ranges over many orders of magnitude across patients [43, 56], and for each patient, it changes during the disease progression [43, 56]. There is a risk of pre-symptomatic transmission of SARS-CoV-2, as well as transmission from asymptomatic individuals [57], and the higher viral loads are expected on or just prior to the day of symptom onset [57]. A survey on studies [58,59,60,61,62,63,64] that reported the viral load in the sputum throughout the infection is presented in Table 2. The reported values from these studies present the trend of higher values in the early days of the infection, with posterior reduction on the course of the disease.
Although high viral loads have been measured in these studies, there is still little experimental evidence of the actual amount of viable virus in aerosol samples from indoor air. The failure on the detection of viable SARS-CoV-2 from aerosol samples is often reported [9]. Lednicky et al. [9] consider that usual air sample methods may contribute to this failure due to their harsh collection process that may inactivate the virus. Using a new air sampling technology (VIVAS), Lednicky et al. [9] were able to identify viable virions from aerosol samples. The amount of airborne virus detected was small [9], and the authors [9] address technical issues that may have contributed to this result, related to the collection complexity and the high air exchange rates of the sample location (hospital). Further research on the topic is necessary [9], given the complexity and the uncertainties related to this new disease. Some studies [18, 42, 43, 49, 56] have presented estimates on the number of necessary virions to cause an infection on a susceptible person (N0, RNA copies quanta−1), based on epidemiological data. The associated \(c_{i } \left( { = 1/N_{0} } \right)\) from these studies [18, 42, 43, 49, 56] is assessed on the range from O(10−1) to O(10−3). Buonanno et al. propose the use of ci = 1.4 × 10−3 (quanta RNA copie−1) in their most recent research [55], improving the predictive estimation approach reported in previous works [42, 43].
2.3.3 Quanta generation rate
Buonanno et al. have provided estimates on the quanta emission rate using Eq. 2 and a Monte Carlo method to account for variations on input data [43, 55]. Probability density functions were considered using normal [43] and lognormal [55] distributions for the viral loads [43, 55], droplets emission rates [43], and infectious dose [43]. Table 3 presents the quanta generation rate estimated by these studies for two expiratory activities related to office work,.
The median values are related to the average viral load on the day of symptom onset reported in reference studies [58,59,60,61,62,63]. The average viral load produced a relatively low quanta emission rate spanning from O(10−1) to O(101), regardless of the expiratory activity. An emission rate on a higher-order (102) may be obtained for the higher percentiles (≥ 90th) estimates of the probability density functions in the case of vocalization. These resultant values are consistent with the emission rates of SARS-CoV-2 analyzed by other studies for oral breathing and speaking in light activities. Barzant and Bush [49] have inferred a value of 14, 15, and 45 quanta h−1 for the cases of the Ningbo tour bus [13], the Diamond Princess cruise ship [65], and the Wuhan city outbreak [10], respectively. Therefore, this study selected two orders of emission rates for evaluation, representing the boundaries of a possible range in an office application: 1 and 100 quanta h−1. The lower order was evaluated with the breathing aerosol size distribution, while the higher order was evaluated with the unmodulated vocalization aerosol size distribution, given the data in Table 3 and the former discussion.
2.4 The relative performance of higher filter efficiencies and ventilation scale-up on infection risk
Different filter efficiencies and outdoor air flow rates were also selected for evaluation with the MV and the DV modes to study the relative performance of different design arrangements on reducing the airborne infection risk of SARS-CoV-2 and to assess the quality of the numerical simulations. These different design arrangements were selected according to the recommendations of specialized organizations on filter and ventilation scale-up in the context of Covid-19 [22,23,24,25]. Therefore, the base cases presented in Table 1 were also evaluated with MERV-10 and MERV-13 filters (rated by ANSI/ASHRAE 52.2 standard [26]) and with 100% outdoor air exchange rate (no recirculation). It is expected that the infection risk that is predicted by the numerical simulations decreases with the filter efficiency upgrade and outdoor air flow rate improvement.
2.5 Numerical simulation
2.5.1 Coupled multizone-CFD simulation
A model of the office’s ventilation system was constructed in the coupled multizone- CFD software (CONTAM), as depicted in Fig. 3.
In the coupled simulation, one of the zones was modeled in detail through the CFD technique (office), while the others use the “perfect-mixing” assumption (air-handling unit and ducts). In this method, the multizone module provides the boundary conditions to the CFD module. After solving the detailed flow field, the CFD module returns the updated variables to the multizone model. An iterative solution is performed, where information of airflow rates, concentrations, and pressures at the boundaries is exchanged between the two codes in each step solution until a convergence criterion is reached. The paper from Wang and Chen [66] provides detailed information on this coupled method. “Appendix C” presents the grid refinement studies and the validation of the CONTAM CFD code.
2.5.2 CFD airflow simulation
The detailed indoor airflow simulations were performed through the CFD module of CONTAM that uses a RANS (Reynolds Averaged Navier–Stokes) formulation with the Chen and Xu [67] zero-equation turbulence model. This turbulence model uses data from DNS (direct numerical simulation) for indoor airflow simulations and was selected since it has already proved to produce useful results in indoor airflow simulations [46, 68]. The simulations were performed in non-isothermal steady-state conditions for 3-D incompressible flow (air). The heat output from occupants, lights, and equipment is the same as the benchmark by Chen et al. [39] for the small office case and was set in the CFD model accordingly. Given the coupled solution between the CONTAM and its CFD module, the numerical model of the office and its ventilation system, depicted in Fig. 3b, was set and calibrated to produce boundary conditions in the CFD zone that matches the operational conditions presented in Table 1 for the airflow rates and temperatures.
In this paper, equations’ residuals were used to assess convergence. The solutions were considered converged when the scaled residuals were less than 10−4.
2.5.3 Contaminant transport simulation
This research aims to study the airborne transport of pathogen-laden droplets’ residues, micrometer particles aerosolized from expiratory activities. For this class of particles, gravitational sedimentation and deposition may be neglected [69]. Some experimental studies [38, 70] have demonstrated that a tracer gas method using SF6 or N2O may be a suitable technique for studying the distribution of respiratory droplets’ residual particles indoors. Olander et al. [71] consider that the tracer gas technique is suitable to describe the behavior of suspended particles with an aerodynamic diameter up to 5 μm. The CFD simulations performed by Vuorinen et al. [17] demonstrated that the transport pattern of massless particles and 10-µm particles by the indoor airflow was sufficiently similar to enable the passive scalar modeling for this class of particles. So, given the focus of this study in modeling the respiratory droplets’ residues with a maximum diameter in the O(101)μm (as discussed in Sect. 2.3.1), the air contaminant distribution was modeled using the transport of a passive scalar (quantum). This passive scalar was defined with the same properties of a tracer gas (SF6) that has already been used to assess the transport of respiratory droplets’ residues in indoor settings [38]. This simplified assumption was selected to include the “quanta” infection control metric in the coupled multizone-CFD simulations given that this study’s purpose is to compare different scenarios’ performance rather than precisely predict the exposure dose.
Therefore, given the flow field characteristics and the quanta emission rate (source), the solution of the passive scalar transport equation predicts the resultant quanta concentration field. The CFD code uses the Reynolds’ Analogy to account for the turbulent contaminant diffusivity through the turbulent viscosity and the turbulent Schmidt number (Sct = 0.7).
In the tested scenarios, a quanta source was generated at the breathing region of the infectious subject. The source location was the same as the contaminant source of the ASHRAE RP-949 benchmark [39] for each infectious subject. The adopted CFD code does not provide resources to include a ‘sink’ term on the calculations, and therefore, the viral inactivation with time was not considered in this study. The relaxation time of droplets’ residual particles is negligible [72], and therefore, the quanta source approach was consistent with a source injected with no relative velocity to the room airflow. The following method was adopted to account for the difference in filter efficiency regarding particle size and the passive scalar quanta approach: the total-quanta emission source was sub-divided into different passive scalars sources, and artificial filtration efficiency was numerically imposed for each passive scalar in the CONTAM software, according to the representative respiratory droplets’ residual size and air-handling filter rating. The method of Buonanno et al. [42] uses an approximation for the small droplets size distribution at the exhaled flow. This approximation is based on the size distribution of respiratory droplets’ residues measured by Morawska et al. [53] for dehydrated particles up to 10 µm with four channels with midpoint diameters of D1 = 0.8 µm, D2 = 1.8 µm, D3 = 3.5 µm, and D4 = 5.5 µm. These midpoint diameters of each channel were used for the calculations in this study, as in the method of Buonanno et al. [42]. Morawska et al. [53] consider that the residence time of the respiratory droplets inside the probe used in their measurements is consistent with their estimated dehydration time, ensuring an equilibrium size for the particle distribution used in this study. Therefore, to account for the differences in filter efficiency regarding particle size, four passive scalars were included, with the same properties of a tracer gas (SF6) but with different filter penetration efficiencies (related to 0.8-, 1.8-, 3.5-, and 5.5-µm droplets’ residues size bins). The air-handling unit’s filter efficiency was numerically imposed for each passive scalar in the multizone software, given the MERV rating and data by Kowalski et al. [73] related to the filter efficiency for each particle size. Table 4 presents the numerical filter efficiency for each passive scalar. The quanta emission rate in each size bin \(\left( {ER_{i} } \right) \) is presented in Table 4 and was calculated based on the total emission rate \(\left( {ER_{q} } \right)\) and the relative volume of aerosols, by Eq. 14 and data from Morawska et al. [53]:
The resultant quanta concentration at the breathing zone of the cohabitant (nb) is the sum of the quanta concentration of each size bin. The solutions were considered converged when the scaled residuals were less than 10−6 for the passive scalar transport. Some differences in the exposure dose may arise for the cutoff at the 10 µm particle size. These differences are related to the possible exposure of larger droplets’ residues that belong to the same size order but have slightly higher sizes (e.g., 20 µm). The approach used in this study to model the transport of the infectious quanta (as a passive scalar rather than the droplets particles) reduces these differences, as long as the quanta emission rate is consistent with the long-range airborne transmission.
3 Results and discussion
The results of the predicted dose and infection risk of occupants #1 and #2 with different ventilation modes, filter efficiencies, and outdoor air flow rates are depicted in Fig. 4 (occupant #1) and 5 (occupant #2). Figures 4a, c, Fig. 5a, c present the results of the lower-order of emission rates (1 quanta h−1), while Figs. 4b, d, 5b, d present the results of the higher order (100 quanta h−1). The predicted doses and infection risks were lower for all the DV cases when compared to the same MV case. These results show a trend of reduction in the dose and infection risk according to the increase in filter efficiency and outdoor air flow rate, suggesting, in addition to the validation tests (“Appendix C”), consistent numerical simulation results. The results of Figs. 4 and 5 also imply that the infection risk is highly sensitive to the quanta generation rate, evidencing that a source control measure (e.g., the use of masks) may be a suitable and rational strategy for reducing the infection risk in a pandemic scenario. The higher-risk scenarios of this study were associated with an intensive vocalization expiratory activity, reinforcing that a social convention on the ‘vocalization etiquette’ would also be a rational strategy in indoor public spaces during the pandemic. The results also demonstrate that relatively low infection risk was predicted with the lower quanta generation rates, even for the base case with mixed ventilation (MERV-8 filters and outdoor air flow rates according to ASHRAE 62-1 standard). Given that the average value of the reported [60, 61, 63] viral loads produces a relatively low emission rate (≤ O (1) quanta h−1, refer to Table 3), the base air-conditioning and ventilation configuration according to the ASHRAE standards may be able to produce a low airborne infection risk of a large proportion of the predicted scenarios involving infectious subjects. Improving the filter efficiency produced little benefits in reducing the infection risk of these cases of lower quanta emission rates. The upgrade on filter class from MERV-8 to MERV-13 resulted in a net increase in the clean air delivery rate (CADR) of 42.6% (from 37 L s−1 to 53 L s−1) for these cases. The resultant dose for all the cases is much lower than one quantum (Figs. 4a and 5a). The combined effect of these features in the exponential dose–response model was a low variation on the infection risk with the upgrade on filter efficiency (Figs. 4c and 5c).
However, the probability of a high-viral load subject or a ‘superspreader’ that produces a high emission rate (≥ 100 quanta h−1) may not be neglected [42, 43]. For these cases, the accumulated dose for the MV mode is considerably higher than one quantum (Figs. 4b and 5b), reaching a plateau level where the infection risk is near 100%, regardless of the filter class (Figs. 4d and 5d). Increasing the filter efficiency presents limited effectiveness in reducing the infection risk of these cases due to the exponential characteristics of the dose–response infection model. Therefore, the upgrade to a higher-efficiency filter (MERV-13) provides little reduction in the long-range airborne infection risk with mixed ventilation (Figs. 4d and 5d) for the studied cases. The use of a displacement ventilation mode is more effective than the upgrade on filter efficiency and outdoor air flow rate in mixed ventilation mode for reducing that risk. The high ventilation effectiveness performance of the DV mode produced lower accumulated doses than the MV mode for all the cases. In the higher emission scenarios of 100 quanta h−1, the dose was sufficiently low with the DV mode (Figs. 4b and 5b) to enable a higher sensitivity of reduction on infection risk due to filter upgrade by the dose–response model (Figs. 4d and 5d).
Regarding the relative position between infector and susceptible, the accumulated dose and infection risk of occupant #2 was higher than for occupant #1 in all the studied cases (Figs. 4 and 5). Occupant #2 is located in a downstream position relative to occupant #1 and the supply airflow. The associated contaminant transport and distribution were affected by these relative positions. These results demonstrate that the airflow distribution provided by the MV mode did not comply with the ‘well-mixed’ assumption, emphasizing the benefits of a detailed airflow calculation through the CFD technique for improving the analysis of ventilation design solutions.
Increasing the outdoor air flow rate (from 32 to 100% air exchange rate) yields approximately the same performance as the use of MERV-13 filters for the studied cases with air recirculation. It is expected that the use of HEPA filters produces a similar result to the 100% outdoor air exchange rate case, given the high HEPA filter efficiency (> 99.97% for 0.3 µm particles). The increase in outdoor air flow rates may disturb the moisture control in hot & humid climates and will increase energy consumption. On the other hand, the filter upgrade may be accomplished in several existing buildings, with strategies like the use of a lower air face-velocity at the filter (resulting in lower pressure drop). This strategy must be analyzed in a ‘case-by-case’ method, geared to match the existing fan available pressure to the requirements of the new filter pressure loss. The filter upgrade will increase the energy consumption, but improving the outdoor air will also produce an energy penalty related to the pre-cooling or pre-heating necessity. A life-cycle cost analysis for each possible solution would provide the necessary consistency for selection. The simulations’ results suggest that the displacement ventilation technique produces a better overall performance on reducing the SARS-CoV-2 airborne infection risk than the conventional mixed ventilation mode.
The streamlines included in Fig. 6 demonstrate that the DV mode reduces the residence timescale of a contaminant generated by the occupants when compared to the MV mode. The typical residence time for contaminants was assessed as 4 min for the DV mode and 13 min for the MV mode. These residence timescales are considerably lower than the virus inactivation half-life timescale (order of 1 h [7]), which supports the validity of the results despite not including the virus inactivation process due to software constraints. The buoyant transport provided by the thermal plumes in DV mode (Fig. 6a) and the location of the exhaust grille at the ceiling reduce the contaminants’ residence time in the DV mode when compared to the MV mode. Therefore, the better overall performance on reducing the SARS-CoV-2 airborne infection risk of the DV mode in the studied cases is a result of its superior room air scavenging capability when compared with the MV mode. Figure 7 depicts the iso-surfaces of quanta concentration provided by the MV and DV mode for one of the studied cases.
The air distribution through a high-momentum jet above the occupied zone is characteristic of MV systems, and the related effects of entrainment, recirculation, and turbulence in air mixing result in the spread of the contaminant source throughout a larger proportion of the room (Fig. 7c and d) when compared to the DV mode (Fig. 7a and b). The use of the displacement ventilation mode produces the contaminant’s buoyant transport to the ceiling region and out of the breathing zone (Fig. 7a and b), increasing the ventilation effectiveness, and it is a characteristic feature of the displacement ventilation mode [74]. This technique has already been associated with the enhancement of thermal comfort [75] and energy use reduction [76]. It may be a relevant solution in this new post-pandemic era, where the design of new buildings demands features that address a better infection control solution in future pandemic or outbreak scenarios. Specific room configuration might impact the results, and a CFD analysis should be performed for each specific configuration. This study contributes to providing a basic framework that can be applied for these future analyses. An experimental study on the airborne transport of a real infectious agent by the room ventilation system presents some difficulties and limitations regarding safety concerns. The use of a numerical simulation overcomes these limitations, and this study demonstrates that this technique may provide valuable data for studying the relative performance of different ventilation solutions in the future challenges for this new post-pandemic scenario. In this research, the airborne infection risk was evaluated using the method of Buonanno et al. [42, 43] and the results of the contaminant concentration provided by the coupled multizone-CFD calculations. This approach links the capabilities of these two methods and expands their potential use for future analysis on ventilation performance and infection control.
4 Conclusions
This study has used a coupled multizone-CFD code and an infection risk model to assess the performance of different design arrangements on reducing the SARS-CoV-2 long-range airborne infection risk in a small office. The code was validated by the use of a high-quality benchmark. Comparing the results of the predicted dose and the infection risk of different solutions led to the following conclusions:
-
(a)
Relatively low infection risk was predicted for all the cases with a viral load of the infectious subject within the average values reported in specialized studies. This implies that a base air-conditioning and ventilation configuration according to the ASHRAE standards may be able to produce a low long-range airborne infection risk of a large proportion of the predicted scenarios involving infectious subjects in the cases assessed in this study (droplets’ residual size up to 10 µm).
-
(b)
Relatively high infection risk was predicted for all the cases with vocalization and a viral load of the infectious subject within the maximum values reported in specialized studies. The increase in outdoor air flow rates or filter efficiency in MV has little impact on reducing the risk of long-range airborne infection for these cases in this study (droplets’ residual size up to 10 µm). Additional infection control measures are necessary for this scenario.
-
(c)
The displacement ventilation technique produces a better overall performance on reducing the SARS-CoV-2 long-range airborne infection risk than the conventional mixed ventilation for all the studied cases in this study (droplets’ residual size up to 10 µm). The benefits of upgrading filter classes are highly dependent on the source term (quanta emission rate) and the method for modeling the infection risk. For the dose–response method used in this study, the use of a DV mode in the higher-emission cases produced a sufficiently low accumulated dose that enabled higher sensitivity of reduction in infection risk due to filter upgrade.
-
(d)
The results of coupled multizone-CFD calculations may be used with an infection risk model to assess the relative performance of different ventilation options to assist in a rational solution selection for future challenges in this new post-pandemic era. The framework presented in this study may contribute with this task.
This study has some limitations. Other factors affect the SARS-CoV-2 infection risk mitigation, as the hands’ hygiene, social distancing, the use of masks, and surface disinfection, among others. The main objective of this study was not to present a solution that mitigates the infection risk in an office but rather to promote a comparative study in the airborne infection control performance of different ventilation arrangements. The infection risk method presents many uncertainties, as many persist at the moment regarding the new Coronavirus disease. It uses data extrapolation from the SARS-CoV-1 (also a coronavirus), and high uncertainty is related to the infectious dose. The estimative on infectious droplets emission relies on a mass conservation criterion, and therefore, the droplets’ viral load is proportional to the droplets’ mass concentration. There are little data, at the time of this study, regarding the SARS-CoV-2 viral load distribution related to the droplets size distribution of commonly expiratory activities. However, there are available data on the relationship between viral load and droplets mass concentration for other respiratory viruses [77] that present this trend. The method uses experimental data of Morawska et al. [53] for an approximation regarding the size distribution of expiratory droplets’ residues. Morawska et al. [53] consider that a sufficient residence time inside the probe used in their measurements is consistent with an equilibrium size [53]. However, Stadnytskyi et al. [50] mention that droplet dehydration might have been incomplete due to high humidity in the experiment of Morawska et al. [53]. These experimental studies are inherently complex, and the result of this former possibility is that the droplets’ residues might reach smaller sizes than assessed in this study. However, these differences might not be substantial, given that the measurements of Morawska et al. [53] include some size reduction and that the evaporation time for the small droplets (the focus of this study) is considerably low. Some uncertainties arise from the method’s approach using the ‘quanta’ metric to study the transport of infectious droplets residues and the simplifications related to the ‘passive scalar’ model. The buoyant flow acceleration by thermal plumes, typical for DV systems, may improve the transport of droplets with other sizes that were not assessed in this study (e.g., > 10 µm). The fraction of these droplets that follow the airflow path will be transported to the unoccupied zone and scavenged by the exhaust. However, another fraction might become airborne for a sufficient time to be inhaled by the co-occupant. In this case, depending on the emitter–receptor transport timescale, the infection route may not fulfill the long-timescale airborne route criterion selected in this present study. It is a complex phenomenon, and the ‘passive scalar’ approach limits an appropriate analysis. Future work could include a particle-tracking approach to study this important matter. The viral inactivation with time was modeled in the method of Buonanno et al. [42] as an additional removal rate of λvi = 0.63 h−1 based on the virus airborne half-life assessment by van Doremalen et al. [7]. This approach may be suitable for ‘well-mixed’ calculations, but the extension to this study’s CFD detailed calculations may not apply, and therefore, the viral inactivation with time was not considered. However, the resultant contaminant residence timescales were considerably lower than the virus inactivation half-life timescale (order of 1 h [7]), which supports the validity of the results despite not including the virus inactivation process.
Despite these limitations, the study provides relevant information on the relative performance of different design solutions, allowing a quantitative assessment during the pandemic of a new disease, when limited data are available. Experimental tests on the performance of air-conditioning systems on infection control using infectious agents have proven to be difficult, and more research is necessary on this topic. On the other hand, the use of numerical simulations may be used for parametric tests and to study the relative performance of different solutions, and the framework presented in this study may contribute to this task. Future progress on the research of this new disease may demand the revision of some parameters of the model. The basic framework of this study is a practical application of a numerical tool already established that can be easily adapted to update these parameters. This study has focused on small office applications, and a specific CFD analysis using the framework of this study may be used to assess specific configurations. Future work will assess the performance of other applications.
Data availability
Not applicable.
Abbreviations
- c i :
-
Conversion factor (quanta RNA copies−1)
- c ν :
-
Viral load at sputum (RNA copies mL−1)
- D :
-
Diameter
- DV :
-
Displacement ventilation
- ER q :
-
Quanta emission rate (quanta h−1)
- IR :
-
Inhalation rate (m3 h−1)
- MERV :
-
Minimum efficiency reporting value
- MV :
-
Mixed ventilation
- N :
-
Droplets’ number concentration at the exhaled flow (m−3)
- n b :
-
Quanta concentration at the breathing zone of the susceptible person (quanta m−3)
- N 0 :
-
Infectious dose (RNA copies quanta−1)
- R :
-
Infection risk
- t :
-
Time (s, h)
- v :
-
Volume of a single droplet (m3)
- \(\dot{V}_{d}\) :
-
Droplets generation rate (mL h−1)
- λ c :
-
Effective air ventilation rate (h−1)
- λ vi :
-
Virus inactivation equivalent air ventilation rate (h−1)
- \(\tau\) :
-
Exposure time (h)
- \(\emptyset_{d}\) :
-
Droplets’ volume concentration at the exhaled flow (mL m−3
References
Iyanda AE, Adeleke R, Lu Y, Osayomi T, Adaralegbe A, Lasode M, Chima-Adaralegbe NJ, Osundina AM (2020) A retrospective cross-national examination of COVID-19 outbreak in 175 countries: a multiscale geographically weighted regression analysis (January 11–June 28, 2020). J Infect Public Health. https://doi.org/10.1016/j.jiph.2020.07.006
World Health Organization (2020) Novel Coronavirus (2019-nCoV) Weekly Epidemiological Updates October 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 24 Oct 2020
Baker SR, Bloom N, Davis SJ, Kost KJ, Sammon MC, Viratyosin T (2020) The unprecedented stock market impact of Covid-19. Natl Bureau Econom Res 26:945
United Nations (2020) World Economic Situation and Prospects as of mid-2020. https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/publication/WESP2020_MYU_Report.pdf. Accessed 01 Oct 2020
World Health Organization (2020) Q&A on coronaviruses (COVID-19), How does COVID-19 spread between people? https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted. Accessed 24 Oct 2020
Centers for Disease Control and Prevention (2020) Frequently Asked Questions on Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Spread. Accessed 24 Oct 2020
van Doremalen N, Morris DH, Holbrook MG et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567. https://doi.org/10.1056/NEJMc2004973
Fears AC, Klimstra WB, Duprex P et al (2020) Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis 26:2168–2171. https://doi.org/10.3201/eid2609.201806
Lednicky JA, Lauzardo M, Fan ZH et al (2020) Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 100:476–482. https://doi.org/10.1016/j.ijid.2020.09.025
Qian H, Miao T, Liu L et al (2020) Indoor transmission of SARS-CoV-2. Indoor Air 00:1–7
Miller SL, Nazaroff WW, Jimenez JL et al (2020) Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 00:1–10. https://doi.org/10.1111/ina.12751
Park SY, Kim YM, Yi S et al (2020) Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis 26:1666–1670. https://doi.org/10.3201/eid2608.201274
Shen Y, Li C, Dong H et al (2020) Community outbreak investigation of SARS-CoV-2 transmission among bus riders in Eastern China. JAMA Intern Med 180:1665–1671. https://doi.org/10.1001/jamainternmed.2020.5225
Morawska L, Milton DK (2020) It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis 71:2311–2313. https://doi.org/10.1093/cid/ciaa939
Milton DK (2020) A rosetta stone for understanding infectious drops and aerosols. J Pediatric Infect Dis Soc 9:413–415. https://doi.org/10.1093/jpids/piaa079
Tang JW, Bahnfleth WP, Bluyssen PM et al (2020) Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus (SARS-CoV-2). J Hosp Infect 110:89–96. https://doi.org/10.1016/j.jhin.2020.12.022
Vuorinen V, Aarnio M, Alava M et al (2020) Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf Sci 130:104866. https://doi.org/10.1016/j.ssci.2020.104866
de Oliveira PM, Mesquita LCC, Gkantonas S, Giusti A, Mastorakos E (2021) Evolution of spray and aerosol from respiratory releases: theoretical estimates for insight on viral transmission. Proc R Soc A 477:2020058. https://doi.org/10.1098/rspa.2020.0584
Atkinson J, Chartier Y, Lúcia Pessoa-Silva C, Jensen P, Li Y, Seto WH (2009) Natural ventilation for infection control in health-care settings-World Health Organization. Technical report
Prather KA, Marr LC, Schooley RT et al (2020) Airborne transmission of SARS-CoV-2. Science 370:303–304. https://doi.org/10.1126/science.abf0521
American Society of Heating, Refrigeration and Air Conditioning Engineers (2020) ASHRAE Position Document on Infectious Aerosols. https://www.ashrae.org/file%20library/about/position%20documents/pd_infectiousaerosols_2020.pdf. Accessed 01 Oct 2020
American Society of Heating, Refrigerating and Air-Conditioning Engineers (2020) ASHRAE Epidemic Task Force Resources on Building Guides: Commercial. https://www.ashrae.org/technical-resources/commercial. Accessed 01 Oct 2020.
Federation of European Heating, Ventilation and Air-Conditioning Associations (2020) How to operate HVAC and other building service systems to prevent the spread of the coronavirus (SARS-CoV-2) disease (COVID-19) in workplaces. https://www.rehva.eu/fileadmin/user_upload/REHVA_COVID-19_guidance_document_V3_03082020.pdf. Accessed 01 Oct 2020
Centers for Disease Control and Prevention (2020) Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-business-response.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fspecific-groups%2Fguidance-business-response.html. Accessed 01 Oct 2020
World Health Organization (2020) Q&A: Ventilation and air conditioning in public spaces and buildings and COVID-19. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-ventilation-and-air-conditioning-in-public-spaces-and-buildings-and-covid-19. Accessed 01 Oct 2020
American National Standards Institute and American Society of Heating, Refrigerating and Air-Conditioning Engineers (2017) ANSI/ASHRAE Standard 52.2-2017: Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size
American National Standards Institute and American Society of Heating, Refrigerating and Air-Conditioning Engineers (2019) ANSI/ASHRAE Standard 62.1-2019: Ventilation for Acceptable Indoor Air Quality
American Society of Heating, Refrigerating and Air-Conditioning Engineers (2019) Commercial and Public Buildings. In: ASHRAE Handbook of Applications, Chapter 3, Atlanta
Melikov AK (2020) COVID-19: Reduction of airborne transmission needs paradigm shift in ventilation. Build Environ 186:107336. https://doi.org/10.1016/j.buildenv.2020.107336
Memarzadeh F (2009) Effect of reducing ventilation rate on indoor air quality and energy cost in laboratories. J Chem Health Saf 16:20–26. https://doi.org/10.1016/j.jchas.2009.03.014
Pantelic J, Tham KW (2012) Assessment of the mixing air delivery system ability to protect occupants from the airborne infectious disease transmission using Wells-Riley approach. HVAC&R Res 18:562–574
Memarzadeh F, Xu W (2012) Role of air changes per hour (ACH) in possible transmission of airborne infections. Build Simul 5:15–28. https://doi.org/10.1007/s12273-011-0053-4
Faulkner WB, Memarzadeh F, Riskowski G, Hamilton K, Chang CZ, Chang JR (2013) Particulate concentrations within a reduced-scale room operated at various air exchanges rates. Build Environ 65:71–80. https://doi.org/10.1016/j.buildenv.2013.03.023
Chen C, Zhu J, Qu Z, Lin CH, Jiang Z, Chen Q (2014) Systematic study of person-to-person contaminant transport in mechanically ventilated spaces (RP-1458). HVAC&R Res 20:80–91. https://doi.org/10.1080/10789669.2013.834778
Bolashikov ZD, Melikov AK, Kierat W, Popiolek Z, Brand M (2012) Exposure of health care workers and occupants to coughed airborne pathogens in a double-bed hospital patient room with overhead mixing ventilation. HVAC&R Res 18:602–615. https://doi.org/10.1080/10789669.2012.682692
Grosskopf KR, Herstein KR (2012) The aerodynamic behavior of respiratory aerosols within a general patient room. HVAC&R Res 18:709–722. https://doi.org/10.1080/10789669.2011.587586
Pantelic J, Tham KW (2013) Adequacy of air change rate as the sole indicator of an air distribution system’s effectiveness to mitigate airborne infectious disease transmission caused by a cough release in the room with overhead mixing ventilation: a case study. HVAC&R Res 19:947–961. https://doi.org/10.1080/10789669.2013.842447
Yin Y, Xu W, Gupta JK, Guity A, Marmion P, Manning PA, Gulick B, Zhang X, Chen Q (2009) Experimental study on displacement and mixing ventilation systems for a patient ward. HVAC&R Res 15:1175–1191. https://doi.org/10.1080/10789669.2009.10390885
Chen Q, Glicksman L, Yuan X, Hu S, Hu Y, Yang X (1999) Performance Evaluation and Development of Design Guidelines for Displacement Ventilation. ASHRAE Research Project 949. Atlanta, American Society of Heating Refrigerating and Air-Conditioning Engineers, INC
Mui KW, Wong LT, Wu CL, Lai ACK (2009) Numerical modeling of exhaled droplet nuclei dispersion and mixing in indoor environments. J Hazard Mater 167:736–744
Xiaoping L, Jianlei N, Naiping N (2011) Spatial distribution of human respiratory droplet residuals and exposure risk fot the co-occupant under different ventilation methods. HVAC&R Res 17:432–445. https://doi.org/10.1080/10789669.2011.578699
Buonanno G, Stabile L, Morawska L (2020) Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ Int. https://doi.org/10.1016/j.envint.2020.105794
Buonanno G, Morawska L, Stabile L (2020) Quantitative assessment of the risk of airborne transmission of sars cov-2 infection: prospective and retrospective applications. Environ Int. https://doi.org/10.1016/j.envint.2020.106112
Walton GN, Dols WS (2013) CONTAM User Guide and Program Documentation. National Institute of Standard and Technology, Gaithersburg
Srebric J, Chen Q, Glicksman LR (1999) Validation of a zero-equation turbulence model for complex indoor airflows. ASHRAE Trans 105:414–427
Wang L, Dols WS, Chen Q (2010) Using CFD capabilities of CONTAM 3.0 for simulating airflow and contaminant transport in and around buildings. HVAC&R Res 16:749–763. https://doi.org/10.1080/10789669.2010.10390932
Riley C, Murphy G, Riley RL (1978) Airborne spread of measles in a suburban elementary school. Am J Epidemiol 107:421–443. https://doi.org/10.1093/oxfordjournals.aje.a112560
International Organization for Standardization (2016) ISO Standard 16.890-1/2016: Air Filters For General Ventilation-Part 1: Technical Specifications, Requirements and Classification System Based Upon Particulate Matter Efficiency (EPM)
Bazant MZ, Bush JWM (2021) Beyond six feet: a guideline to limit indoor airborne transmission of COVID-19. MedRxiv. https://doi.org/10.1101/2020.08.26.20182824
Stadnytskyi V, Bax CE, Bax A, Anfinrud P (2020) The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci 117:11875–11877. https://doi.org/10.1073/pnas.2006874117
Nicas M, Nazaroff WW, Hubbard A (2005) Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg 2:143–154. https://doi.org/10.1080/15459620590918466
Gao CX, Li Y, Wei J et al (2021) Multi-route respiratory infection: when a transmission route may dominate. Sci Total Environ 752:141856. https://doi.org/10.1016/j.scitotenv.2020.141856
Morawska L, Johnson GR, Ristovski ZD et al (2009) Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci 40:256–269. https://doi.org/10.1016/j.jaerosci.2008.11.002
Duguid JP (1946) The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond) 44(6):471–479
Mikszewski A, Stabile L, Buonanno G, Morawska L (2021) the airborne contagiousness of respiratory viruses: a comparative analysis and implications for mitigation. MedRxiv. https://doi.org/10.1101/2021.01.26.21250580
Prentiss M, Chu A, Berggren KK (2020) Superspreading events without superspreaders: using high attack rate events to estimate no for airborne transmission of COVID-19. MedRxiv. https://doi.org/10.1101/2020.10.21.20216895
World Health Organization (2020) Advice on the use of masks in the context of COVID-19, Interim Guidance. https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak. Accessed 15 Jul 2020
Pan Y, Zhang D, Yang P, Poon LLM, Wang Q (2020) Viral load of SARS-CoV-2 in clinical samples. The Lancet 20:411–412. https://doi.org/10.1016/S1473-3099(20)30113-4
Liu WD, Chang SY, Wang JT et al (2020) Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect 81:329–331. https://doi.org/10.1016/j.jinf.2020.03.063
To KK-W, Tsang OT-Y, Leung W-S et al (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet 20:565–574. https://doi.org/10.1016/S1473-3099(20)30196-1
Wölfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. https://doi.org/10.1038/s41586-020-2196-x
Xu T, Chen C, Zhu Z et al (2020) Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis 94:68–71. https://doi.org/10.1016/j.ijid.2020.03.022
To KK-W, Tsang OT-Y, Yip CC-Y et al (2020) Consistent detection of 2019 novel coronavirus in Saliva. Clin Infect Dis 71:841–843. https://doi.org/10.1093/cid/ciaa149
Fajnzylber J, Regan J, Coxen K et al (2020) SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 11:5493. https://doi.org/10.1038/s41467-020-19057-5
Kakimoto K, Kamiya H, Yamagishi T et al (2020) Initial investigation of transmission of COVID-19 among crew members during quarantine of a cruise ship, Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep 69:100
Wang L, Chen Q (2007) Theoretical and numerical studies of coupling multizone and CFD models for building air distribution simulations. Indoor Air 17:348–361. https://doi.org/10.1111/j.1600-0668.2007.00481.x
Chen Q, Xu W (1998) A zero-equation turbulence model for indoor airflow simulation. Energy Build 28:137–144. https://doi.org/10.1016/S0378-7788(98)00020-6
Wang L, Chen Q (2008) Applications of a coupled multizone-cfd model to calculate airflow and contaminant dispersion in built environments for emergency management. HVAC&R Res 14:925–939. https://doi.org/10.1080/10789669.2008.10391047
Wang W, Lin CH, Chen Q (2012) Advanced turbulence models for predicting particle transport in enclosed environments. Build Environ 47:40–49. https://doi.org/10.1016/j.buildenv.2011.05.018
Bivolarova M, Ondráček J, Melikov A, Ždmíal V (2017) A comparison between tracer gas and aerosol particles distribution indoors: the impact of ventilation rate, interaction of airflows, and presence of objects. Indoor Air 27:1201–1212. https://doi.org/10.1111/ina.12388
Olander L, Welling I, Fletcher B (2001) Local ventilation: evaluation of local ventilation systems. In: Goodfellow H, Tähti E (eds) Industrial ventilation design guidebook. Academic Press, San Diego. https://doi.org/10.1016/b978-0-12-289676-7.x5000-0
Morawska L (2006) Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 16:335–347. https://doi.org/10.1111/j.1600-0668.2006.00432.x
Kowalski W, Bahnfleth WP, Whittam T (1999) Filtration of airborne microorganisms: modeling and prediction. ASHRAE Trans 105:4–17
Schiavon S, Bauman F, Tully B, Rimmer BJ (2011) Air change effectiveness in laboratory tests of combined chilled ceiling and displacement ventilation. UC Berkeley: Center for the Built Environment. https://escholarship.org/uc/item/7f26s1xb. Accessed 04 Dec 2020
Pantelic J, Rysanek A, Miller C et al (2018) Comparing the indoor environmental quality of a displacement ventilation and passive chilled beam application to conventional air-conditioning in the Tropics. Build Environ 130:128–142. https://doi.org/10.1016/j.buildenv.2017.11.026
Parameshwaran R, Karunakaran R, Muthumariappan S, Bipasha S (2010) An energy efficient air conditioning system using displacement ventilation and chilled ceiling for modern office buildings. Int J Vent 9:25–44. https://doi.org/10.1080/14733315.2010.11683864
Alonso C, Raynor PC, Davies PR, Torremorell M (2015) Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS ONE. https://doi.org/10.1371/journal.pone.0135675
Wang H, Zhai Z (2012) Application of coarse-grid computational fluid dynamics on indoor environment modeling: optimizing the trade-off between grid resolution and simulation accuracy. HVAC&R Res 18:915–933. https://doi.org/10.1080/10789669.2012.688012
Liu S, Novoselac A (2014) Lagrangian particle modeling in the indoor environment: a comparison of RANS and LES turbulence methods (RP-1512). HVAC&R Res 20:480–495. https://doi.org/10.1080/10789669.2014.884380
Zhang Z, Zhang W, Zhai ZJ, Chen Q (2007) Evaluation of various turbulence models in predicting airflow and turbulence in enclosed environments by CFD: part 2-comparison with experimental data from literature. HVAC&R Res 13:871–886. https://doi.org/10.1080/10789669.2007.10391460
Zhai ZJ, Zhang Z, Zhang W, Chen Q (2007) Evaluation of various turbulence models in predicting airflow and turbulence in enclosed environments by CFD: part 1-summary of prevalent turbulence models. HVAC&R Res 13:853–870. https://doi.org/10.1080/10789669.2007.10391459
Wang M, Chen Q (2009) Assessment of various turbulence models for transitional flows in enclosed environment (RP-1271). HVAC&R Res 15:1099–1119. https://doi.org/10.1080/10789669.2009.10390881
Acknowledgements
The first author would like to thank the LTTC laboratory for providing the computational resources involved in this research’s simulation.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Code availability
Not applicable.
Additional information
Technical Editor: Monica Carvalho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Appendix A: Method summarization
Figure
8 presents a flowchart that encompasses all the simulation steps.
Appendix B: Verification of the mathematical model of infection risk
The mathematical implementation of the Buonanno et al. [42] infection risk model was verified by the reproduction of the Skagit Valley choral and Chinese restaurant scenarios studied by Buonanno et al. [43]. Figure
Results of infection risk of the scenarios studied by Buonanno et al. [37] a The Skagit Valley choral b The Chinese restaurant
9 depicts the results of the analytical solution of the mathematical infection risk of the cases, which are the same as those predicted by Buonanno et al. [43].
Appendix C: Grid refinement tests and validation of the CFD code
Three sets of grids were tested, with representative element sizes of 0.2 m (coarse), 0.1 m (intermediate), and 0.05 m (fine), and progressive refinement. The simulation results for the SF6 concentration were compared with the ASHRAE RP-949 [39] benchmark’s experimental data for the measurements at the nine poles. The simulations used structured hexagonal grid meshes, manually built via the software graphical interface. The results of the grid refinement study are depicted in Fig.
Results of the grid refinement tests a Plan view of the small office with the location of the measurement poles, heat sources and SF6 source, according to the benchmark of Chen et al. [39]. Simulation results of SF6 concentration for three sets of grids at poles 3 (b), 4 (c), 7 (d), and 8 (e)
10. The finer grid, with a representative element size of 0.05 m, was chosen due to little improvements from the intermediate mesh and an overall better agreement with the experimental data. This grid size (102 × 74 × 48) is close to the grid independent size (123 × 86 × 54) used by Wang and Zhai [78] in their simulations for the same case.
The same benchmark was also simulated with another CFD code (Fluent) that has been widely used in indoor airflow simulations [34, 79, 80] to provide a comparison parameter and a complimentary assessment on the quality of the results obtained by the CONTAM CFD code. The Fluent code was used with the RNG-κε turbulence model, given the superior performance among other eddy viscosity models for flow prediction in similar problems [80]. A non-structured mesh comprising tetrahedral elements with a representative element size of 0.05 m was built and used for the Fluent simulation. The results were also compared with the simulations provided by Chen et al. [39] for the same benchmark.
Figure
Results of the validation test with the CONTAM and Fluent codes and comparison with the simulations provided by Chen et al. [39] for the same benchmark (a) Plan view of the small office with the location of the measurement poles, heat sources and SF6 source. Simulation results of dimensionless velocity for poles 2 (b) and 3 (c). Simulation results of dimensionless SF6 concentration for poles 2 (d), 3(e), 4 (f), 6 (g), and 8 (h)
11 depicts the results of dimensionless velocity and SF6 profiles predictions at selected poles and a comparison with the experimental data from the ASHRAE RP 949 benchmark and the simulations provided by Chen et al. [39] for the same case.
The CFD simulations performed in this study for the indoor flow field prediction involve inherent complexity, given the asymmetric three-dimensional geometry and the specific characteristics of a non-isothermal transitional indoor airflow [81, 82]. The Archimedes Number (Ar, relative magnitude of buoyancy forces to the inertial forces) for the simulated cases is Ar = 70, which increases the simulation complexity and reduces the accuracy of the simulated results. Some differences between the experimental and predicted data arise for the validation case (Fig. 11), due to: modeling simplifications; boundary conditions uncertainties; heat transfer and turbulence model limitations; discretization errors and numerical approximations. However, The CONTAM CFD simulation of the validation case reproduces the vertical contaminant stratification trend measured by Chen et al. [39] (Fig. 11), which is a characteristic feature of the displacement ventilation mode. It also produced a reasonable prediction on contaminant concentration at the breathing height and a similar performance when compared to the Fluent results and the simulations performed by Chen et al. [39] for the same case (Fig. 11). Therefore, the CONTAM CFD modeling was considered to provide acceptable predictions on ventilation performance for the office application, despite the inherent modeling complexity.
Rights and permissions
About this article
Cite this article
Barbosa, B.P.P., de Carvalho Lobo Brum, N. Ventilation mode performance against airborne respiratory infections in small office spaces: limits and rational improvements for Covid-19. J Braz. Soc. Mech. Sci. Eng. 43, 316 (2021). https://doi.org/10.1007/s40430-021-03029-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-021-03029-x