Abstract
We here provide, first, a general introduction into the woody angiosperm family Meliaceae, including updated numbers of the genera and species found in different parts of the globe, paying attention to geographic centres of diversity and patterns of endemism. Second, and more specifically, we review the latest literature concerning land connections (i) between Eurasia and North America, (ii) between North America and South America, as well as (iii) dispersal paths between Africa and South America that have existed since the proposed evolutionary origin of modern Meliaceae, i.e. from the Upper Cretaceous onwards (ca. 100 Million years ago). Comparing geological evidence with the fossil record as well as biogeographic studies, there is indication that the nowadays pantropically distributed family has made use of all these three routes. Five out of the eight modern Neotropical genera have a fossil record, namely Carapa Aubl., Cedrela P. Browne, Guarea F. Allam., Swietenia Jacq., and Trichilia P. Browne. Carapa and Trichilia have a modern transatlantic disjunction (distribution in Africa, Central and South America), and a fossil record in Africa and North/Central America (Trichilia), or Africa and Eurasia (Carapoxylon). Cedrela has a rich fossil record in Eurasia and the Americas. The global decrease in temperatures and a lack of Cedrela fossils in North America from the Late Miocene onwards suggest the genus had gone extinct there by that time, leading to its modern distribution in Central and South America. Oligocene to Pliocene fossils of Guarea, Swietenia and Trichilia in Central American key regions support biotic interchange between North and South America at various times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The mahogany family, Meliaceae, comprises woody plants widely distributed throughout the tropics and subtropics, occurring occasionally in temperate zones. With ca. 740 species in 58 genera (Table 1 and references therein), Meliaceae is a medium-sized family in Sapindales. The Indo-Malesian region is the geographic centre of diversity, harbouring ca. 220–223 species in 28 genera (Table 1). Africa-Madagascar is almost as diverse as Indo-Malesia with ca. 205 species in 26 genera, followed by Australasia (ca. 151-152 spp in 22 genera). Interestingly, only eight genera are present in the Neotropics, but they are as diverse as the Africa-Malagasy region as for the number of species (202). The two species-rich Neotropical genera Trichilia P. Browne and Guarea F. Allam. constitute two recent radiations that have been identified in Meliaceae (Koenen et al. 2015). Concerning endemic genera, six are present in the Neotropics: Cabralea A. Juss., Cedrela P. Browne, Guarea F. Allam., Ruagea H. Karst., Schmardaea H. Karst., and Swietenia Jacq. Africa-Madagascar harbours the highest number of endemics (20 out of 26 genera; Table 1). In addition, 13 out of these 20 genera are small, having four species or less, such as Ekebergia Sparrm. and Lepidotrichilia (Harms) T.D. Penn. & Styles, with a few of them being even monospecific (e.g. Neoguarea (Harms) E.J.M. Koenen & J.J.de Wilde, Nymania Lindb., Quivisianthe Baill.). In some cases, such as for Ekebergia, Neobeguea, Nymania and Quivisianthe, the low number of species might indicate substantial extinction, as they are relatively old genera (older than 30 Million years of age, Myr; Koenen et al. 2015). Indo-Malesia harbours seven endemic genera (Chukrasia A. Juss., Cipadessa Blume, Heynea Roxb., Lansium Corrêa, Munronia Wight, Sphaerosacme Wall. ex M.Roem., and Soymida A.Juss.), and Australasia has the lowest number with only two genera being endemic (Owenia F. Muell. and Synoum A. Juss.).
During the last two decades, enormous progress has been achieved towards resolving the phylogenetic relationships of Meliaceae and several genera within it. The first molecular phylogenetic study of the family (Muellner et al. 2003) provided support for the recognition of only two subfamilies (Melioideae and Cedreloideae; the latter previously known by the invalid name Swietenoideae (see Thorne 2007)), instead of four, as formerly recognized by Pennington and Styles (1975). Monophyly of the tribes Aglaieae, Sandoriceae and Melieae, as circumscribed by Pennington and Styles (1975), was demonstrated by Muellner et al. (2008a, b). At the same time, Guareeae was found to be paraphyletic and a complex evolutionary history of Turraeeae, Trichilieae and Vavaeeae was revealed by incongruencies found between plastid and nuclear DNA datasets (Muellner et al. 2008a, b), which was in accordance with their problematic circumscription based on morphology (Pennington and Styles 1975). Koenen et al. (2015) investigated the evolution of rainforest hyperdiversity using Meliaceae as a case study and provided the latest and most comprehensive phylogenetic analysis of Meliaceae to date, mainly based on datasets of phylogenetic studies by Muellner et al. (2003, 2005, 2006, 2008a, b, 2009a, b, 2010, 2011), Köcke et al. (2013), and Grudinski et al. (2014a, b), but also adding new data. Apart from these, further studies have contributed to disentangle the phylogenetic relationships within several genera of Meliaceae, such as Aglaia (Muellner et al. 2005; Grudinski et al. 2014a, b; Pannell et al. 2020), Dysoxylum (Holzmeyer et al. 2021), Guarea (Pennington and Clarkson 2013), Ruagea (Rojas-Andrés et al. in prep), Trichilia (Clarkson et al. 2016), Cedrela (Muellner et al. 2009a, b), Carapa (Kenfack 2011; Duminil et al. 2012), and Toona (Lin et al. 2018), among others.
Ecologically, the species of Meliaceae, being trees and shrubs characterized by their compound leaves (simple in a few genera), grow in a wide variety of habitats, from rain forests to semi-deserts and mangrove swamps. In the Neotropics, most of the species are evergreen (ca. 80%), while others are deciduous, and occur from the sea level up to 3400–3500 m. They are common in lowland rain forests (e.g. Cabralea, Carapa, Guarea, Trichilia), as well as in montane rainforests (e.g. Cabralea, Carapa, Cedrela, Ruagea), cloud forests of the Andes (e.g. Ruagea, Schmardaea), and tropical deciduous forests (Cedrela, Swietenia, Trichilia). Species of some genera also occur in gallery forests (Cabralea, Carapa, Swietenia), riparian woodlands (Carapa), open dry pastures (Schmardaea) and rough scrub or rocky hillsides (Swietenia) (Pennington et al. 1981, 2021; Pennington and Muellner‐Riehl 2010; Kenfack 2011; Pennington and Clarkson 2013; Pennington 2016).
Dispersal is a crucial ecological process that allows species to expand their range. In Meliaceae, dispersal is achieved by several mechanisms, and all of these are present in the Neotropical Meliaceae. The winged seeds of Cedreloideae are wind-dispersed, while the unwinged seeds of Carapa with corky testa are capable of hydrochory and zoochory (Mabberley 2011). In the remaining genera, the arillate or fleshy seeds (e.g. Guarea, Ruagea, Trichilia) are dispersed by birds or mammals (Pennington and Styles 1975).
With the aim of shedding light on the current knowledge about the biogeography of Neotropical Meliaceae, in the following, we first review the latest knowledge concerning land connections and thus potential dispersal paths that existed since the origin of Meliaceae in the Upper Cretaceous (ca. 100 Million years ago, Ma). Then, we review the rich fossil record of those genera nowadays occurring in the Neotropics, as well as the evolutionary and biogeographic studies that have been performed on Meliaceae during the last ca. 20 years. Based on all these lines of evidence, we shed light on how modern Neotropical taxa may have reached South America from other parts of the world.
2 Land connections and dispersal paths
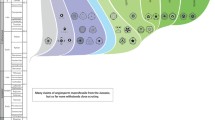
The Neotropical flora is composed of indigenous and immigrant lineages. The first ones were already present in South America when it started separating from Africa about 135–130 Ma (McLoughlin 2001). The immigrant species reached South America from other continents by two main ways: (1) via long-distance dispersal by means of migratory birds, wind, or in natural rafts of soil and vegetation (Renner 2004; Van Duzer and Munz 2004), and (2) through dispersal paths (compare Graham 2018) in the form of continuous land bridges or stepping-stone island chains, such as those that allowed the expansion of the boreotropical flora during the Upper Cretaceous and early Paleogene (Morley 2003; Pennington and Dick 2004). In the following, we will provide an updated review of the literature concerning the geological connections between South America and adjacent regions that have existed since the Upper Cretaceous and that might have been relevant as dispersal paths for megathermal angiosperms to reach South America (Fig. 1).
World map showing the land connections and dispersal paths that Meliaceae may have used since the Upper Cretaceous, as well as the fossil findings of those Meliaceae genera which have a modern distribution in the Neotropics (see Tables 2, 3, 4, 5, 6 and 7 for details). E, Early; M, Middle; L, Late. Coloured circles above or below the route names indicate the epochs when the routes were available. For details see main text. Dark red: Upper Cretaceous; red: Paleocene; orange: Eocene; dark yellow: Oligocene; green: Miocene; blue: Pliocene; purple: Pleistocene, pink: Holocene. The map uses Equal Earth projection. It was created with R packages ‘rnaturalearth’ v. 0.1.0 (South 2017) and ‘sp’ v.1.4–5 (Pebesma and Bivand 2005; Bivand et al. 2013), and modified manually
Connections between Eurasia and North America
– During the Upper Cretaceous (100–66 Ma), the Northern Hemisphere was divided into two floristic provinces according to pollen types. The Normapolles province in eastern North America and Europe, and the Aquilapollenites province in western North America and Asia (Wolfe 1975). Both provinces were isolated by epicontinental seaways; the Turgai Strait, in the area currently occupied by the Ural Mountains, separated Europe and Siberia; the Mid-Continental seaway, were the High Plains of North America are nowadays, separated western and eastern North America (Tiffney 1985). Towards the latest Cretaceous—early Paleocene, the epicontinental seaways separating the Normapolles and Aquilapollenites provinces started to retreat (Sanmartín et al. 2001; Brikiatis 2014). This, together with the existence of land connections between North America and Eurasia allowed the spread of taxa throughout the Northern Hemisphere giving rise to the “boreotropical flora” (Wolfe 1975; Tiffney 1985; Brikiatis 2014).
The Beringia dispersal route. Beringia, defined as the region extending from the Lena River in Russia to the Mackenzie River in Canada, has long been recognized by biogeographers as an important route for biotic exchange (e.g. Hultén 1937; Hopkins 1967; Szalay and McKenna 1971; Sanmartín et al. 2001). Connecting North East Asia and northwestern North America (Fig. 1), the Bering area served as a dispersal path since its formation in the Upper Cretaceous (ca. 100 Ma), with periods of complete land exposure alternating with those of marine connection between the Arctic and Pacific oceans (Sanmartín et al. 2001; Brikiatis 2014). Thus, plant dispersal was possible until the late Pliocene (3.5 Ma) through a continuous or a discontinuous land bridge (Sanmartín et al. 2001). The Bering route was mostly used by deciduous and temperate plants, while dispersal of megathermal elements of the boreotropical flora was probably more restricted. Winter light was probably a primary limitation for evergreen angiosperms in this area, which was located further north (ca. 75° N) during the K/Pg boundary than it is at present (Tiffney 1985; Morley 2003; Manchester et al. 2009; Brikiatis 2014). On the other hand, the terranes currently forming the southern edge of Alaska have arrived at their current position at different times, with two major events of collision taking place during the Mesozoic (Wrangellia composite terrane) and the Cenozoic (Yakutat terrane) (Trop and Ridgway 2007; Enkelmann et al. 2017). Before accreting, these terranes might have formed a stepping-stone island chain connecting North America with Asia through island chains associated with the Aleutian arc, thus acting as a southern dispersal route for megathermal plants in the Beringian area (Tiffney 1985). Post-Eocene climatic cooling finally restricted the passage of broadleaved evergreen taxa across the Bering land bridge (Tiffney 2000).
The North Atlantic land bridges: the De Geer and the Thulean routes. The De Geer and the Thulean routes constituted two different temporal and geographic land bridges connecting eastern North America and Europe via Greenland (Brikiatis 2014; Fig. 1). The De Geer route was a northerly path joining Northern Scandinavia to North America via a subaerial Barents Shelf, northern Greenland and Queen Elisabeth Islands (Tiffney 1985; Brikiatis 2014). It was terrestrially exposed from the late Maastrichtian to the early Palaeocene (around 71–63 Ma; Brikiatis 2014) and during the Eocene (56–34 Ma), until the areas involved rifted apart in the late Eocene—early Oligocene (Tiffney 1985; Sanmartín et al. 2001). The Thulean route offered a southerly connection to North America from France and the British Isles via the Faroes, Iceland, southern Greenland and Baffin Island (Tiffney 1985; Brikiatis 2014). This area formed a continuous land bridge during two time frames in the late Paleocene (ca. 57 Ma and ca. 56 Ma; Brikiatis 2014) until it was broken in the early Eocene (Tiffney and Manchester 2001). While a continuous land connection between North America and Europe was interrupted in the late Eocene at the latest, some degree of connectivity, probably through island chains, might have allowed floristic exchange of temperate taxa during the Oligocene and Miocene (Tiffney and Manchester 2001). The De Geer route was probably less relevant than the Thulean route for evergreen taxa of the boreotropical flora, in part because of winter light limitation. This assumption is supported by the presence of only deciduous plants in the early Eocene flora of Ellesmere Island (Tiffney 1985). In addition, the Danish-Polish Through isolated Fennoscandia from the rest of Europe from the early Albian (ca. 113 Ma) to the early Oligocene (ca. 34 Ma) (Rögl 1998; Lehmann et al. 2013). The southern position of the Thulean route and its temporal coincidence with the late Paleocene/early Eocene Thermal Maximum clearly allowed the dispersal of megathermal plant taxa through this route (Morley 2003).
Connections between North America and South America
– North and South America started to diverge from each other in the Upper Triassic-Lower Jurassic when Gondwana and Laurasia started to rift apart (Pitman et al. 1993). Effective joining of both continents through a continuous land bridge was not complete until the closure of the Isthmus of Panama, at around 3 Ma (O’Dea et al. 2016). However, impermanent connections occurred at different times since the Upper Cretaceous, allowing floristic interchange (Wolfe 1975; Pennington and Dick 2004).
Proto-Greater Antilles route. During the late Cretaceous (Campanian; ca. 80 Ma) the leading edge of the Caribbean Plate, which corresponds to the present Greater Antilles and Aves Ridge, formed an island arc connecting Yucatan with South America (Fig. 1). This route, which was at a convergent margin, was subjected to tectonic stress, motion and sea level fluctuations, probably changing from a corridor to a filter to an impasse (Pitman et al. 1993). Vertebrate and pollen fossils provide evidence for the existence of a connection during the late Cretaceous and Paleocene (Morley 2003). This route was interrupted around the middle Eocene (49–39 Ma) when the Caribbean Plate further drifted eastwards (Morley 2003).
GAARlandia. Near the Eocene–Oligocene boundary (ca. 33–35 Ma) the leading edge of the Caribbean plate would have formed a large peninsula extending from South America until central Cuba (Iturralde-Vinent and MacPhee 1999). This landspan, called GAARlandia (Greater Antilles + Aves Ridge; Fig. 1), would have been continuous or discontinuous, with some narrow water gaps between islands. On its western edge, it would have been separated from Central America only by two narrow straits, the Havana-Matanzas and the Yucatan channels. The GAARlandia hypothesis has been used to explain the dispersal of plants such as Styrax (Styracaceae; Fritsch 2003), Croton (Euphorbiaceae; van Ee et al. 2008), and Ficus (Moraceae; Pederneiras et al. 2018); and animals such as sloths (MacPhee et al. 2000; Dávalos 2004), rodents (MacPhee et al. 2003; Dávalos 2004), toads (Alonso et al. 2012), and frogs (Moen and Wiens 2009). While the role of GAARlandia in explaining Caribbean biogeography of non-flying terrestrial animals remains controversial (Ali 2012), this landspan might have well functioned as a filter connection through an island chain allowing plant dispersal in a stepping-stone manner (Pennington and Dick 2004).
Panama land bridge. The most important interchange between the Americas occurred at ca. 3 Ma resulting in an increased dispersal of terrestrial mammals in both directions. This wave of migration, known as the Great American Biotic Interchange (GABI; Simpson 1980; Webb 2006), followed the formation of the Isthmus of Panama (Fig. 1). However, the timing of formation of the Panama Isthmus has been the subject of recent debate (e.g. Stone 2013; Erkens 2015; O’Dea et al. 2016; Jaramillo et al. 2017; Molnar 2017). The classic scenario consists of a relatively rapid rise of the isthmus with a final closure at 4–3 Ma (Coates and Stallard 2013; O’Dea et al. 2016), while other studies found evidence for an earlier formation at around 15–13 Ma or even 25 Ma (e.g. Farris et al. 2011; Montes et al. 2012, 2015; Bacon et al. 2015). According to the model of Montes et al. (2015), marine connections probably occurred through shallow and transient channels, west of where the Panama Canal is nowadays, which would have allowed some degree of biotic interchange. A recent study provides a revised kinematic reconstruction of the Central American Seaway (CAS) region, reconciling alternative models about the time of the CAS closure (McGirr et al. 2021). According to it, the Isthmus of Panama would have suffered fluctuations in dynamic uplift or subsidence and intermittent shallow-water connections would have existed until the CAS was completely closed at ca. 3 Ma. This is consistent with an earlier availability of the Panama land-bridge for many terrestrial organisms, including plants, for which it has been shown that many lineages already dispersed across the Isthmus of Panama prior to the entire closure of the CAS (Cody et al. 2010).
Connections between Africa and South America
– Separation of Africa and South America began at about 135–130 Ma with sea-floor spreading in the South Atlantic. At lower latitudes, both continents remained connected along the area of the Benue Trough (in west equatorial Africa) and the North Brazilian Coast until 119–105 Ma (McLoughlin 2001). In spite of an opening Atlantic, numerous plant dispersals between Africa and South America have been documented until the Maastrichtian (ca. 72 Ma) based on the simultaneous appearance of novel pollen types in both continents (Morley 2003). The frequency of plant dispersals decreased gradually after this time, enhancing provincialism, with crossings taking place even until the Miocene (Morley 2003). Studies on Annonaceae and Asteraceae have invoked this dispersal route to explain current distribution patterns of these plant families (Richardson et al. 2004; Katinas et al. 2013). While the latest dispersal events might have been achieved via sweepstake dispersal, the high frequency of crossings during the Upper Cretaceous and Paleogene suggests the existence of a dispersal route involving the area of the Walvis Ridge/Rio Grande Rise and Sierra Leone Ridges (Fig. 1). According to paleogeographic reconstructions, the currently submerged Walvis Ridge and Rio Grande Rise (between 20° and 30° S) constituted a series of islands and shallow waters in the South Atlantic until the Eocene (40–50 Ma), after continuous land connection had been severed in the early-mid Cretaceous (Parrish 1987; Lawver and Gahagan 2003; Markwick and Valdes 2004; de Oliveira et al. 2009). This route would have allowed plant dispersal in an island-hopping mode.
3 Fossil record of Meliaceae in Europe, the Americas, and Africa
Apart from the geological conditions, outlined in detail above, which can potentially favour the arrival of megathermal angiosperms, such as Meliaceae, in the Neotropics, the fossil record provides valuable evidence for actual presence in key regions. First, fossils found in those areas that may have acted as dispersal corridors, such as (former) land connections, provide evidence that a specific route was used at a certain time. Second, gradients of fossil age found e.g. at different latitudes can provide information about dispersal directions (shifts in distribution due to climate change). Third, fossils found in Central and South America provide information when certain taxa were already present in the Neotropics themselves. For Meliaceae with their relatively rich fossil record, there is evidence for all three examples, further detailed in the following.
Out of the eight genera occurring in the Neotropics nowadays (Table 1), five have a known fossil record, namely (in alphabetical order) Carapa, Cedrela, Guarea, Swietenia, and Trichilia (Tables 2, 3, 4, 5, 6, and 7; due to the large number of fossil findings in Meliaceae, these lists cannot possibly claim to be complete). Out of these genera, hydro- and zoochorous Carapa and zoochorous Trichilia have a transatlantic disjunction, with a modern distribution in both Africa and South America. Each of them could therefore potentially be used to test biogeographic explanations for this transatlantic disjunction. For both genera, phylogeny-based biogeographic studies are currently underway (Trichilia: Kannan et al. in preparation; Carapa: Kenfack et al. in preparation).
Although fossils that exhibit only characteristics of Carapa have not been identified to date, wood fossils exhibiting characters shared among several genera of extant Meliaceae, including Carapa, have been found in several sites in Eurasia and Africa (Carapoxylon, Table 2). The oldest fossil record of Carapa´s presumable ancestor, Carapoxylon, exhibiting characters shared among modern Carapa, Xylocarpus and Entandrophragma, dates back to the Eocene, Oligocene and Miocene of Africa and Europe (Mädel 1960; Lakhanpal and Prakash 1970; Prakash 1976; Selmeier 1989), pointing towards an Old World origin of the genus.
Extensive paratropical evergreen Carapoxylon forests are known from the mid Miocene of Germany (Böhme et al. 2007). The earliest African records of Carapoxylon date back to the Oligocene of Algeria (Louvet 1963; Prakash 1976) and to the Miocene of the Congo and Burundi (Lakhanpal and Prakash 1970; Fairon-Demaret et al. 1981; Dupéron-Laudoueneix and Dupéron 1995). Based on the fossil record of Carapa´s ancestral lineage, which is confined to the Old World, one may assume that it is more likely that modern Neotropical Carapa is derived from an old world stock. Since no fossils of Carapoxylon have been found in North America, there is no supporting evidence for the use of Beringia or the De Geer and the Thulean routes (see Sect. 2.1), and thus the use of a boreotropical route to finally arrive in the Neotropics. Long-distance dispersal may thus be viewed as a viable explanation for Carapa´s transatlantic disjunction and modern distribution on both continents, Africa, and South America. This is supported by yet unpublished phylogenetic work on the genus (Kenfack et al. in preparation). Until new data are available, it remains speculative whether lineages would have been old enough to also make use of the Walvis Ridge/Rio Grande Rise and Sierra Leone Ridges which would have allowed plant dispersal in an island-hopping mode until the Eocene (see Sect. 2.3).
For the second genus having a transatlantic disjunction, Trichilia, the putatively oldest fossil record is from the Eocene–Oligocene boundary of Florissant, Colorado (MacGinitie 1953; Table 7). Interestingly, these fossil leaflets match those of the living Trichilia havanensis Jacquin (“the correspondences are exact and leave no doubt as to the correct assignment of the fossil”, MacGinitie 1953, p. 132), a species which in the genus has an isolated phylogenetic position outside the core clade(s) of Trichilia (Clarkson et al. 2016, supported by Kannan et al. in preparation) and thus—also based on independent genetic evidence—may be postulated to constitute an early representative of this evolutionary lineage (“living fossil”). Pollen fossils (with putative association to Trichilia) are known from the Oligocene of Cameroon (Salard-Cheboldaeff 1978). Several fossil flowers were found in Dominican amber deposits, the latter being of controversial age (Chambers et al. 2011; Chambers and Poinar 2012a, b). The youngest proposed age is 20–15 Ma (Miocene), based on foraminifera (Iturralde-Vinent and MacPhee 1996), while the oldest proposed age is 45–30 Ma (Eocene–Oligocene), based on coccoliths (Cêpek in Schlee 1990). There is no fossil evidence supporting a boreotropical route through Eurasia. Phylogenetic work currently underway (Kannan et al. in preparation) will provide important clues for putting the fossil record into a bigger biogeographic picture. For example, as for Carapa, long-distance dispersal may be invoked as a viable explanation for Trichilia´s transatlantic disjunction and modern distribution in both Africa and South America. The fossil record from the Eocene in North America and the Oligocene–Miocene in key regions of Central America further suggests that the genus made use of geological connections provided by GAARlandia, existing near the Eocene–Oligocene boundary (ca. 33–35 Ma; Sect. 2.2), probably in a stepping-stone manner.
A different case is presented by the example of Cedrela, for which ample evidence supports the use of a Northern Hemisphere, boreotropical dispersal route (Tables 3 and 4). The genus has a modern distribution in both Central and South America and has been subject to detailed biogeographic reconstruction and investigation of its ecological niche evolution, drawing together independent evidence from both extant species and the fossil record (Muellner et al. 2010; Koecke et al. 2013). These investigations have been facilitated by the fact that—compared to the other genera in Meliaceae—Cedrela has an exceptionally rich fossil record, including findings in biogeographic key regions (Tables 3 and 4). The latter include: Eocene fossil findings in Alaska, supporting a potential use of the Bering land bridge; Eocene, Oligocene, and Miocene findings both in different European countries (incl. the British isles) and North America, in line with a potential use of both North Atlantic land bridges (the De Geer and the Thulean routes; see Sect. 2.1); and Miocene, Pliocene and Pleistocene fossil findings across Central America (Mexico, El Salvador, Panama), providing evidence for using the Panama land bridge as dispersal route between North and South America (see Sect. 2.2). The global decrease in temperatures and a lack of Cedrela fossils in North America (north of Mexico) from the late Miocene onwards suggests the genus had gone extinct there by that time. The fossil record of Cedrela suggests a major biome shift from paratropical conditions into warm-temperate seasonal climates in the early Oligocene of western North America (Koecke et al. 2013). Besides Northern Hemisphere extinctions, the fossil record indicates niche tracking into more southern areas, finally leading to its present distribution restricted to Central and South America. For example, Cedrela species were present in La Quinta (southeastern Mexico, Table 3) and Gatún (Panamanian isthmus, Table 3) by the Miocene and Pliocene, respectively. These Central American fossils occurred in subtropical habitats of wet and seasonal conditions, respectively. The ancestral niche reconstructions and comparison with the fossil record by Koecke et al. (2013) revealed that climatic tolerances of species are less conserved in one clade than in the other. The increase in climatic disparity within one clade follows the major Andean uplift and the Miocene cooling 10–7 Ma (Hoorn et al. 2010). Fossil evidence shows that Cedrela was present in South America in the Miocene (fossil from Salto de Tequendama in Colombia, Hooghiemstra et al. 2006), which is in agreement with molecular dating analysis (Koecke et al. 2013; see Sect. 3). The initial Andean uplift (23–10 Ma; Hoorn et al. 2010) provided habitats comparable to those north of the Panamanian isthmus. Furthermore, as outlined further above (Sect. 2.2), the Panamanian isthmus may have already closed much earlier (early Miocene; Farris et al. 2011; Montes et al. 2012) than previously suggested (late Pliocene to early Pleistocene; Bartoli et al. 2005), providing opportunities for the wind-dispersed Cedrela to disperse into South America (Koecke et al. 2013).
Evidence of the importance of both, the Proto-Greater Antilles/GAARlandia as well as Panamanian isthmus routes, for biotic interchange between North and South America at various times, is further provided by Oligocene to Pliocene fossils of Guarea, Swietenia and Trichilia in Central American key regions (Tables 5, 6 and 7). Flower fossils of Trichilia and Swietenia are known from the late Oligocene or early Miocene tropical forests of Hispaniola (Chambers et al. 2011, Chamber and Poinar 2012b; Tables 6 and 7). Guarea is known from several fossil sites, ranging from the mid Oligocene of northern Puerto Rico, to the early to late Miocene fossils of Mexico, as well as Pliocene fossils from Panama and Colombia (Table 5).
4 Biogeographic studies on modern Meliaceae
The first global biogeographic study of Meliaceae, based on a generic-level phylogenetic framework and using information from fossils and extant distribution of diversity/endemism, was performed by Muellner et al. (2006). This study indicated that Meliaceae likely were of Gondwanan origin, that dispersal played an important role for Meliaceae to achieve their modern distribution, that the direction of dispersal might have been an “out-of-Africa” scenario with important dispersal routes across Eurasia and between Eurasia and North America provided by Beringia and the North Atlantic land bridge(s), and North America and South America via island chains and/or direct land connections. Although at that time still based on a limited consideration of the fossil record, Muellner et al. (2006) suggested that populations in North America, Europe, and East Asia were presumably eliminated as tropical climates disappeared from these areas during the Miocene, and were forced to move southwards into more favourable climates, which later was corroborated by more in-depth studies on single genera with a particularly good fossil record, even allowing for the investigation of fossil niche evolution through time, such as Cedrela (Muellner et al. 2010; Koecke et al. 2013; see also previous section). The work by Muellner et al. (2006) thus supported the idea that the entry of megathermal (frost-intolerant) angiosperms into southern continents from Oligocene to Pliocene must be considered as an important means of establishing modern pantropical distribution patterns. It is worth to note here that indeed the currently oldest known fossil of Meliaceae is an exceptionally well-preserved fruit from the Upper Cretaceous (79–72 Ma, Campanian) of North America (Atkinson 2020). A family-scale macroevolutionary study by Koenen et al. (2015), focusing on temporal dynamics of evolution of rainforest clades within Meliaceae, suggested that these rainforest clades diversified from the Late Oligocene or Early Miocene onwards, and that most species-level diversity of Meliaceae in rainforests was rather recent.
Building upon the insights of the family-level study by Muellner et al. (2006), which had hinted at a particularly rich fossil record spanning three continents for Cedrela, Muellner et al. (2010) and Koecke et al. (2013) investigated the biogeographic history and evolution of Cedreleae in more detail. Based on molecular clock dating, crown group diversification in Cedrela started in the Oligocene/Early Miocene and intensified in the late Miocene and early Pliocene. Interestingly, modern Central American Cedrela species do not form a clade, implying re-entry from South America into Central America after the closure of the Panamanian isthmus (Muellner et al. 2010), which is in agreement with the fossil evidence (see Sect. 3). Muellner et al. (2010) mentioned that, while modern Cedrela was distributed in both dry and humid habitats, morphological features might suggest an origin in dry forest under seasonal climates, fitting with Miocene and Pliocene Cedrela fossils from deciduous forests. This topic was picked up again by Koecke et al. (2013), who investigated also the pre-Miocene fossil record in more detail (see also previous Sect. 3), while at the same time employing an independent niche modelling approach based on data from extant species of Cedrela. In brief, Koecke et al. (2013) found that Cedrela experienced a major biome shift from paratropical conditions into warm-temperate seasonal climates already in the early Oligocene of western North America (see Sect. 3). By the Miocene, Cedrela extended from North America to northern South America (Fig. 1, Table 3). Diversification in the early evolutionary history was mainly driven by changes in precipitation. Temperature had an increasing impact on ecological diversification of Cedrela from the Miocene onwards. Sister-species comparisons revealed that recent speciation events may be related to divergence of climatic tolerances. Koecke et al. (2013) concluded that these results highlight the complexity of climatic niche dynamics, and show how ecological niche conservatism and evolution have acted on different temporal scales and climatic parameters in Cedrela.
Apart from the larger-scale geographic analyses mentioned above, several studies on selected single modern Neotropical Meliaceae species, or their complexes, have been conducted in the past two decades. Among these are the works by Cavers et al. (2003, 2013) on Cedrela odorata L., by Mangaravite et al. (2019) on C. fissilis Vell., and by Scotti-Saintagne et al. (2013) on a species complex in Carapa. Cavers et al. (2003) studied populations of Cedrela odorata in Central America, phylogenetically grouped into three lineages (northern, central and southern), and spatial analysis confirming a deviation from a pattern of isolation by distance. The authors attributed this finding to a likely repeated colonization of Central America from South American source populations. Repeated colonization of Central America, both from the North and South, is in line with the fossil record as well as the phylogeny of the genus (see further above; Muellner et al. 2010; Koecke et al. 2013; Table 3). What was unknown at the time of publication of the work by Cavers et al. (2003), however, was that “Cedrela odorata”, until then believed to be one of the two widespread species in the genus (apart from C. fissilis), has multiple independent origins, with at least three species hiding under its name (Muellner et al. 2009a, b, 2010; Pennington and Muellner 2010), rendering the “species” polyphyletic, as delimited back then. This calls for caution when conducting population genetic, phylogeographic work, on extremely widespread tropical species, as they might, in fact, constitute different evolutionary lineages, hard to differentiate morphologically (“cryptic species”). The type of C. odorata is from Jamaica, and a specimen from the West Indies fell in a clade with the sequences from El Salvador and Belize, indicating that the Central American accessions represented C. odorata in the strict sense (Muellner et al. 2010). Cavers et al. (2013) found evidence of four morphologically cryptic species within C. odorata, based on an expanded dataset. These new data supported diversification of Cedrela in South America with subsequent recolonization into Central America prior to the formation of the Isthmus of Panama. Cavers et al. (2013) also found within-species phylogeographical divergence across the Andes and within Central America, the latter suggestive of Pleistocene climatic influence. The impact of Quaternary climatic fluctuations on Cedrela populations was investigated in more detail by Mangaravite et al. (2019), who combined analyses of genetic diversity, phylogeographic patterns, and past geographic distributions with a particular focus on highland populations of C. fissilis. Different phylogeographic scenarios have been proposed for plants in Neotropical cloud forests during the Last Glacial Maximum based on paleoecological data: the dry refugia hypothesis and the moist forest hypothesis (Ramírez-Barahona and Eguiarte 2013). The habitat suitability projections over the past 140,000 years by Mangaravite et al. (2019) showed less fragmentation relative to the present, indicating a higher connectivity and gene flow. Overall, the results provided support for both the moist forest as well as the dry refugia hypothesis, suggesting a mixture of these processes has acted through space and time. In their work on Neotropical representatives of Carapa, Scotti-Saintagne et al. (2013) suggested an Amazonian centre of origin and diversification, with subsequent migration to the Pacific coast of South America and Central America. Their results pointed towards gene flow occurring among species, with introgression supported by the absence of complete lineage sorting between nuclear and chloroplast genomes. Scotti-Saintagne et al. (2013) argued that the lack of phylogeographical structure may be a result of the ineffectiveness of geographical barriers among populations and of reproductive isolation mechanisms among incipient and cryptic species.
5 Future perspectives
A careful revision of the entire fossil record of Meliaceae will be an important step into a more comprehensive understanding of the biogeographic history of this nowadays pantropically distributed family, which may serve as a prime example for understanding the evolution of rainforest (and related) taxa on a global scale. Another aim will be to arrive at a complete sampling of the ca. 740 modern species currently recognized in the family for phylogenetic investigations, accompanied by molecular clock dating making use of the rich fossil record. This needs to be complemented by biogeographic reconstructions making use of both, the distribution of modern taxa, as well as the fossil record. Detailed niche reconstructions using the fossil record, so far only accomplished for Cedrela (Köcke et al. 2013), need to be expanded to other genera, to understand the relative importance of both, niche conservatism, and niche evolution, for diversification in the family through time until the present, with possible general implications for the future fate of woody tropical lineages. All this will likely only be achievable by a scientific community effort, bringing together researchers from different disciplines, who have a keen interest in Meliaceae evolution. The vision of this longer-term research agenda would be to establish this—also economically very important—family as a model group for a better understanding of angiosperm evolution.
References
Ali JR (2012) Colonizing the Caribbean: is the GAARlandia land-bridge hypothesis gaining a foothold? J Biogeogr 39:431–433. https://doi.org/10.1111/j.1365-2699.2011.02674.x
Alonso R, Crawford AJ, Bermingham E (2012) Molecular phylogeny of an endemic radiation of Cuban toads (Bufonidae: Peltophryne) based on mitochondrial and nuclear genes. J Biogeogr 39:434–451. https://doi.org/10.1111/j.1365-2699.2011.02594.x
Atkinson BA (2020) Fossil evidence for a Cretaceous rise of the mahogany family. Am J Bot 107:139–147. https://doi.org/10.1002/ajb2.1416
Axelrod DI (1985) Miocene floras from the Middlegate Basin, west-central Nevada. University of California Press, Berkeley
Axelrod DI (1991) The early Miocene Buffalo Canyon flora of western Nevada. University of California Press, Berkeley
Axelrod DI (1995) The Miocene Purple Mountain Flora of western Nevada. University of California Press, Berkeley
Axelrod DI (2000) A Miocene (10–12 Ma) evergreen laurel-oak forest from Carmel Valley. University of California Press, Berkeley
Bacon CD, Silvestro D, Jaramillo C et al (2015) Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc Natl Acad Sci 112:6110–6115. https://doi.org/10.1073/pnas.1423853112
Bartoli G, Sarnthein M, Weinelt M, Erlenkeuser H, Garbeschönberg D (2005) Final closure of Panama and the onset of northern hemisphere glaciation. Earth Planet Sci Lett 237:33–44
Bestland EA, Forbes MS, Krull ES, Fremd T (2008) Stratigraphy, paleopedology, and geochemistry of the middle Miocene Mascall Formation (type area, central Oregon, USA). PaleoBios 28:41–61
Bivand RS, Pebesma E, Gómez-Rubio V (2013) Applied spatial data analysis with R. Springer, New York
Böhme M, Bruch AA, Selmeier A (2007) The reconstruction of Early and Middle Miocene climate and vegetation in Southern Germany as determined from the fossil wood flora. Palaeogeogr Palaeoclimatol Palaeoecol 253:91–114
Brikiatis L (2014) The de Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography. J Biogeogr 41:1036–1054. https://doi.org/10.1111/jbi.12310
Brown RW (1937) Further additions to some fossil floras of the western United States. J Wash Acad Sci 27:506–517
Castaneda-Posadas C, Cevallos-Ferriz SRS (2007) Swietenia (Meliaceae) flower in Late Oligocene: early Miocene amber from Simojovel de Allende, Chiapas, Mexico. Am J Bot 94:1821–1827. https://doi.org/10.3732/ajb.94.11.1821
Cavers S, Navarro C, Lowe AJ (2003) Chloroplast DNA phylogeography reveals colonization history of a Neotropical tree, Cedrela odorata L., in Mesoamerica. Mol Ecol 12:1451–1460
Cavers S, Telford A, Arenal Cruz F, Castañeda AJP, Valencia R, Navarro C, Buonamici A, Lowe AJ, Vendramin GG (2013) Cryptic species and phylogeographical structure in the tree Cedrela odorata L. thoughout the Neotropics. J Biogeogr 40:732–746
Chambers KL, Poinar GO (2012a) A Mid-Tertiary fossil flower of Swietenia (Meliaceae) in Dominican amber. J Bot Res Inst Tex 6:123–127
Chambers KL, Poinar GO (2012b) Additional fossils in Dominican amber give evidence of anther abortion in Mid-Tertiary Trichilia (Meliaceae). J Bot Res Inst Tex 6:561–565
Chambers KL, Poinar GO, Brown A (2011) Two fossil flowers of Trichilia (Meliaceae) in Dominican amber. J Bot Res Inst Tex 5:463–468
Chandler MEJ (1964) The lower Tertiary floras of southern England. Bull Br Mus Nat Hist Geol 12:1–151
Clarkson JJ, Pennington TD, Chase MW et al (2016) Phylogenetic relationships in Trichilia (Meliaceae) based on ribosomal ITS sequences. Phytotaxa 259:6–17. https://doi.org/10.11646/phytotaxa.259.1.4
Coates AG, Stallard RF (2013) How old is the Isthmus of Panama? Bull Mar Sci 89:801–813. https://doi.org/10.5343/bms.2012.1076
Cody S, Richardson JE, Rull V et al (2010) The Great American Biotic Interchange revisited. Ecography (cop) 33:326–332. https://doi.org/10.1111/j.1600-0587.2010.06327.x
Cuong NT, Hoan DT, Mabberley DJ (2014) Munronia petiolata (Meliaceae), a new species from Vietnam. Blumea J Plant Taxon Plant Geogr 59:139–141. https://doi.org/10.3767/000651914X685834
Dávalos LM (2004) Phylogeny and biogeography of Caribbean mammals. Biol J Linn Soc 81:373–394. https://doi.org/10.1111/j.1095-8312.2003.00302.x
de Oliveira FB, Molina EC, Marroig G (2009) Paleogeography of the South Atlantic: a route for primates and rodents into the New World? In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American Primates. Springer, New York, pp 55–68
de Wilde JJFE (2007) Revision of the African genus Heckeldora (Meliaceae). Blumea 52:179–199. https://doi.org/10.3767/000651907X612436
Duminil J, Kenfack D, Viscosi V et al (2012) Testing species delimitation in sympatric species complexes: the case of an African tropical tree, Carapa spp. (Meliaceae). Mol Phylogenet Evol 62:275–285. https://doi.org/10.1016/j.ympev.2011.09.020
Dupéron-Laudoueneix M, Dupéron J (1995) Inventory of Mesozoic and Cenozoic woods from equatorial and north equatorial Africa. Rev Palaeobot Palynol 84:439–480
Enkelmann E, Piestrzeniewicz A, Falkowski S et al (2017) Thermochronology in southeast Alaska and southwest Yukon: implications for North American Plate response to terrane accretion. Earth Planet Sci Lett 457:348–358. https://doi.org/10.1016/j.epsl.2016.10.032
Erdei B, Hably L, Kázmér M et al (2007) Neogene flora and vegetation development of the Pannonian domain in relation to palaeoclimate and palaeogeography. Palaeogeogr Palaeoclimatol Palaeoecol 253:115–140
Erkens RHJ (2015) The less-splendid isolation of the South American continent. Front Biogeogr. https://doi.org/10.21425/f57328193
Fairon-Demaret M, Dreesen R, Reekmans M (1981) A propos de la découverte de bois fossiles de la fin du Tertiaire: début du Quaternaire près de Cibitoke (vallée de la Haute Rusizi, Burundi). Ann Soc Géol Belg 104:115–125
Farris DW, Jaramillo C, Bayona G et al (2011) Fracturing of the Panamanian Isthmus during initial collision with South America. Geology 39:1007–1010. https://doi.org/10.1130/G32237.1
Fischer E, Killmann D, Leh B, Janssens SB (2021) Carapa wohllebenii (Meliaceae), a new tree species from montane forests in the Democratic Republic of Congo, Rwanda, and Burundi. Phytotaxa 511:20–36. https://doi.org/10.11646/phytotaxa.511.1.2
Fritsch PW (2003) Multiple geographic origins of Antillean Styrax. Syst Bot 28:421–430. https://doi.org/10.1043/0363-6445-28.2.421
Graham A (1976) Studies in Neotropical paleobotany. II. The Miocene communities of Veracruz, Mexico. Ann Missouri Bot Gard 63:787–842
Graham A (1991a) Studies in Neotropical paleobotany. IX. The Pliocene communities of Panama-Angiosperms (Dicots). Ann Missouri Bot Gard 78:201–223
Graham A (1991b) Studies in Neotropical paleobotany. X. The Pliocene communities of Panama-composition, numerical representations, and paleocommunity paleoenvironmental reconstructions. Ann Missouri Bot Gard 78:465–475
Graham A (1993) History of the vegetation: Cretaceous (Maastrichtian)-Tertiary. Flora N Am 1:57–70
Graham A (1999) Studies in Neotropical paleobotany. XIII. An Oligo-Miocene palynoflora from Simojovel (Chiapas, Mexico). Am J Bot 86:17–31
Graham A (2018) The role of land bridges, ancient environments, and migrations in the assembly of the North American flora. J Syst Evol 56(5):405–429. https://doi.org/10.1111/jse.12302
Graham A, Jarzen DM (1969) Studies in Neotropical paleobotany. I. The Oligocene communities of Puerto Rico. Ann Missouri Bot Gard 56:308–357
Grudinski M, Pannell CM, Chase MW et al (2014a) An evaluation of taxonomic concepts of the widespread plant genus Aglaia and its allies across Wallace’s Line (tribe Aglaieae, Meliaceae). Mol Phylogenet Evol 73:65–76. https://doi.org/10.1016/j.ympev.2014.01.025
Grudinski M, Wanntorp L, Pannell CM, Muellner-Riehl AN (2014b) West to east dispersal in a widespread animal-dispersed woody angiosperm genus (Aglaia, Meliaceae) across the Indo-Australian Archipelago. J Biogeogr 41:1149–1159
Hably L (2006) Catalogue of the Hungarian Cenozoic leaf, fruit and seed floras from 1856 to 2005. Studia Bot Hung 37:41–129
Hickey LJ, Hodges RW (1975) Lepidopteran leaf mine from the early Eocene Wind River Formation of northwestern Wyoming. Science 189:718–720
Holzmeyer L, Hauenschild F, Mabberley DJ, Muellner-Riehl AN (2021) Confirmed polyphyly, generic recircumscription and typification of Dysoxylum Blume ex Raspail (Meliaceae), with revised disposition of currently accepted species. Taxon. https://doi.org/10.1002/tax.12591
Hooghiemstra H, Wijninga VM, Cleef AM (2006) The paleobotanical record of Colombia: implications for biogeography and biodiversity. Ann Missouri Bot Gard 93:297–325. https://doi.org/10.3417/0026-6493(2006)93[297:TPROCI]2.0.CO;2
Hoorn C, Wesselingh FP, Ter Steege H, Bermudez MA, Mora A, Sevink J, Sanmartin I, Sanchez-Meseguer A, Anderson CL, Figueiredo JP, Jaramillo C, Riff D, Negri FR, Hooghiemstra H, Lundberg J, Stadler T, Sarkinen T, Antonelli A (2010) Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330:927–931
Hopkins DM (1967) The Cenozoic history of Beringia: a synthesis. In: Hopkins DM (ed) The Bering land bridge. Stanford Univ. Press, Stanford, pp 451–484
Hultén E (1937) Outline of the history of arctic and boreal biota during the Quaternary period. Lehre J Cramer, New York
Iturralde-Vinent MA, MacPhee RDE (1996) Age and Paleogeographical origin of dominican amber. Science 273:1850–1852
Iturralde-Vinent MA, MacPhee RDE (1999) Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bull Am Museum Nat Hist 238:1–72
Jaramillo C, Montes C, Cardona A et al (2017) Comment (1) on “Formation of the Isthmus of Panama” by O’Dea et al. Sci Adv 3:e1602321. https://doi.org/10.1126/sciadv.1602321
Katinas L, Crisci JV, Hoch P et al (2013) Trans-oceanic dispersal and evolution of early composites (Asteraceae). Perspect Plant Ecol Evol Syst 15:269–280. https://doi.org/10.1016/j.ppees.2013.07.003
Kenfack D (2011) Resurrection in Carapa (Meliaceae): a reassessment of morphological variation and species boundaries using multivariate methods in a phylogenetic context. Bot J Linn Soc 165:186–221. https://doi.org/10.1111/j.1095-8339.2010.01104.x
Köcke AV, Muellner-Riehl AN, Cáceres O, Pennington TD (2015) Cedrela ngobe (Meliaceae), a new species from Panama and Costa Rica. Edinburgh J Bot 72:225–233. https://doi.org/10.1017/S0960428615000098
Koecke AV, Muellner-Riehl AN, Pennington TD et al (2013) Niche evolution through time and across continents: the story of Neotropical Cedrela (Meliaceae). Am J Bot 100:1800–1810
Koenen EJM, de Wilde JJFE (2012) A taxonomic revision of the reinstated genus Leplaea and the newly recognized genus Neoguarea (Meliaceae, Sapindales): the exclusion of Guarea from Africa. Plant Ecol Evol 145:209–241. https://doi.org/10.5091/plecevo.2012.656
Koenen EJM, Clarkson JJ, Pennington TD, Chatrou LW (2015) Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytol 207:327–339. https://doi.org/10.1111/nph.13490
Lakhanpal RN, Prakash U (1970) Cenozoic plants from Congo. I. Fossil woods from the Miocene of Lake Albert. Ann K Mus Voor Midden-Afr 64:1–20
Lawver LA, Gahagan LM (2003) Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr Palaeoclimatol Palaeoecol 198:11–37. https://doi.org/10.1016/S0031-0182(03)00392-4
Legrand P (2003) Inventaire de la macroflore du Miocene superieur de la diatomite de Murat (Cantal, Masif Central, france). Ann Soc Géol Du Nord 2:25–55
Lehmann J, Owen HG, Beckert W (2013) A new ammonite fauna from NE Germany: evidence for an Early Albian cooling and the initial transgression in the Danish-Polish Trough. Neues Jahrb Geol Palaontol Abh 268:199–235. https://doi.org/10.1127/0077-7749/2013/0327
Lin N, Moore MJ, Deng T et al (2018) Complete plastome sequencing from Toona (Meliaceae) and phylogenomic analyses within Sapindales. Appl Plant Sci 6:1–11. https://doi.org/10.1002/aps3.1040
Lötschert W, Mädler K (1975) Die plio-pleistozäne Flora aus dem Sisimico-Tal, El Salvador. Schweizerbart, Stuttgart, Germany
Louvet P (1963) Sur un Acajou fossile du Tertiare d'Algerie: Entandrophragmoxylon boureaui n. gen., n. sp. Congr Nat Soc Savantes (88th), Sect Sci, C R 2:493–504
Mabberley DJ (1979) The species of Chisocheton (Meliaceae). Bull Br Mus (Nat Hist) Bot Ser 6:301–386
Mabberley D (2003) New species of, and other notes on, Chisocheton and Walsura (Meliaceae). Gard Bull (Singapore) 55:189–200
Mabberley DJ (2011) Meliaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Springer, Berlin Heidelberg, pp 185–211
Mabberley DJ (2017) Mabberley’s Plant-book. Cambridge University Press, Cambridge
Mabberley DJ, Pannell CM, Sing AM (1995) Meliaceae. In: Flora Malesiana series I. Rijksherbarium/Hortus Botanicus, Leiden University, Leiden, Netherlands
MacGinitie HD (1941) A middle Eocene flora from the Central Sierra Nevada. Carnegie Inst Washington Publ 534:1–94
MacGinitie HD (1953) Fossil plants of the Florissant beds, Colorado. Contributions to paleontology (Carnegie Institution of Washington); Carnegie Institution of Washington publication, 599. Washington [Carnegie Institution of Washington]
MacPhee R, White J, Woods CA (2000) New megalonychid sloths (Phyllophaga, Xenarthra) from the Quaternary of Hispaniola. Am Mus Novit 3303:1–32. https://doi.org/10.1206/0003-0082(2000)3303%3c0001:nmspxf%3e2.0.co;2
MacPhee RDE, Iturralde-Vinent MA, Gaffney ES (2003) Domo de Zaza, an early Miocene vertebrate locality in South-Central Cuba, with notes on the tectonic evolution of Puerto Rico and the Mona Passage 1. Am Museum Novit 3394:1–42. https://doi.org/10.1206/0003-0082(2003)394%3c0001:ddzaem%3e2.0.co;2
Madagascar Catalogue (2021) Catalogue of the vascular plants of Madagascar. Missouri Botanical Garden, St. Louis, U.S.A. & Antananarivo, Madagascar. http://www.efloras.org/madagascar Accessed 15 May 2021
Mädel E (1960) Mahagonihölzer der Gattung Carapoxylon n.g. (Meliaceae) aus dem europäischen Tertiär. Senck Leth 41:393–421
Manchester SR (2001) Update on the megafossil flora of Florissant, Colorado. In Evanof E, Gregory-Wodzicki KM, Johnson KR (eds) Fossil flora and stratigraphy of the Florissant Formation, Colorado. Proceedings of the Denver Museum of nature & science. Series 4, No. 1:137–161. Denver Museum of Nature & Science, Denver, Colorado, USA
Manchester SR, McIntosh WC (2007) Late Eocene silicified fruits and seeds from the John Day formation near Post, Oregon. PaleoBios 27:7–17
Manchester SR, Chen ZD, Lu AM, Uemura K (2009) Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J Syst Evol 47:1–42. https://doi.org/10.1111/j.1759-6831.2009.00001.x
Mangaravite É, da Silveira TC, Huamán-Mera A, de Oliveira LO, Muellner-Riehl AN, Schnitzler J (2019) Genetic diversity of Cedrela fissilis (Meliaceae) in the Brazilian Atlantic Forest reveals a complex phylogeographic history driven by Quaternary climatic fluctuations. J Syst Evol 6:655–669
Markwick PJ, Valdes PJ (2004) Palaeo-digital elevation models for use as boundary conditions in coupled ocean–atmosphere GCM experiments: a Maastrichtian (late Cretaceous) example. Palaeogeogr Palaeoclimatol Palaeoecol 213:37–63. https://doi.org/10.1016/j.palaeo.2004.06.015
McGirr R, Seton M, Williams S (2021) Kinematic and geodynamic evolution of the Isthmus of Panama region: implications for Central American Seaway closure. Bull Geol Soc Am 133:867–884. https://doi.org/10.1130/B35595.1
McLoughlin S (2001) The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Aust J Bot 49:271–300. https://doi.org/10.1071/BT00023
Meyer HW, Manchester SR (1997) The Oligocene Bridge Creek flora of the John Day Formation, Oregon. University of California Press, Berkeley
Moen DS, Wiens JJ (2009) Phylogenetic evidence for competitively driven divergence: body-size evolution in Caribbean treefrogs (Hylidae: Osteopilus). Evolution (N Y) 63:195–214. https://doi.org/10.1111/j.1558-5646.2008.00538.x
Molnar P (2017) Comment (2) on “Formation of the Isthmus of Panama” by O’Dea et al. Sci Adv 3:e1602320. https://doi.org/10.1126/sciadv.1602320
Montes C, Cardona A, McFadden R et al (2012) Evidence for middle Eocene and younger land emergence in central Panama: implications for Isthmus closure. Bull Geol Soc Am 124:780–799. https://doi.org/10.1130/B30528.1
Montes C, Cardona A, Jaramillo C et al (2015) Middle Miocene closure of the Central American Seaway. Science 348:226–229. https://doi.org/10.1126/science.aaa2815
Morley RJ (2003) Interplate dispersal paths for megathermal angiosperms. Perspect Plant Ecol Evol Syst 6:5–20. https://doi.org/10.1078/1433-8319-00039
Muellner AN, Samuel R, Johnson SA et al (2003) Molecular phylogenetics of Meliaceae (Sapindales) based on nuclear and plastid DNA sequences. Am J Bot 90:471–480. https://doi.org/10.3732/ajb.90.3.471
Muellner AN, Samuel R, Chase MW et al (2005) Aglaia (Meliaceae): an evaluation of taxonomic concepts based on DNA data and secondary metabolites. Am J Bot 92:534–543. https://doi.org/10.3732/ajb.92.3.534
Muellner AN, Savolainen V, Samuel R, Chase MW (2006) The mahogany family “out-of-Africa”: divergence time estimation, global biogeographic patterns inferred from plastid rbcL DNA sequences, extant, and fossil distribution of diversity. Mol Phylog Evol 40:236–250
Muellner AN, Pannell CM, Coleman A, Chase MW (2008a) The origin and evolution of Indomalesian, Australasian and Pacific island biotas: insights from Aglaieae (Meliaceae, Sapindales). J Biogeogr 35:1769–1789
Muellner AN, Samuel R, Chase MW et al (2008b) An evaluation of tribes and generic relationships in Melioideae (Meliaceae) based on nuclear ITS ribosomal DNA. Taxon 57:98–108. https://doi.org/10.2307/25065951
Muellner AN, Greger H, Pannell CM (2009a) Genetic diversity and geographic structure in Aglaia elaeagnoidea (Meliaceae, Sapindales), a morphologically complex tree species, near the two extremes of its distribution. Blumea 54:207–216
Muellner AN, Pennington TD, Chase MW (2009b) Molecular phylogenetics of Neotropical Cedreleae (mahogany family, Meliaceae) based on nuclear and plastid DNA sequences reveal multiple origins of “Cedrela odorata”. Mol Phylogenet Evol 52:461–469. https://doi.org/10.1016/j.ympev.2009.03.025
Muellner AN, Pennington TD, Koecke AV, Renner SS (2010) Biogeography of Cedrela (Meliaceae, Sapindales) in Central and South America. Am J Bot 97:511–518
Muellner AN, Schaefer H, Lahaye R (2011) Evaluation of DNA barcodes for economically important timber species of the mahogany family (Meliaceae). Mol Ecol Res 11:450–460
O’Dea A, Lessios HA, Coates AG et al (2016) Formation of the Isthmus of Panama. Sci Adv 2:1–12. https://doi.org/10.1126/sciadv.1600883
Palacios W (2012) Four new tree species from Ecuador. Caldasia 34:75–85
Palacios WA (2016) Una especie nueva de Guarea (Meliaceae) del suroriente de Ecuador. Neotrop Biodivers 2:69–71. https://doi.org/10.1080/23766808.2016.1171019
Palacios WA, Santiana J, Iglesias J (2019) A new species of Cedrela (Meliaceae) from the eastern flanks of Ecuador. Phytotaxa 393:84. https://doi.org/10.11646/phytotaxa.393.1.8
Pannell CM (1992) A taxonomic monograph of the genus Aglaia Lour. (Meliaceae). Kew Bull Additional Series XVI. HMSO, London, UK
Pannell CM (1997) A new, cassowary-dispersed, species of Aglaia (Meliaceae, section Amoora) from Papua New Guinea. Kew Bull 52:715–717
Pannell CM (2004) Three new species, two new subspecies and five new combinations at the subspecific level in Aglaia Lour. (Meliaceae). Kew Bull 59:87–94. https://doi.org/10.2307/4111078
Pannell CM (2005) Aglaia soepadmoi (Meliaceae), a new species for Borneo. Gard Bull (Singapore) 57:183–185
Pannell CM (2020) Aglaia. In: Flora Aust. Aust. Biol. Resour. Study, Dep. Agric. Water Environ. Canberra. https://profiles.ala.org.au/opus/foa/profile/Aglaia
Pannell CM, Schnitzler J, Muellner-Riehl AN (2020) Two new species and a new species record of Aglaia (Meliaceae) from Indonesia. PhytoKeys 155:33–51. https://doi.org/10.3897/PHYTOKEYS.155.53833
Parrish JT (1987) Global palaeogeography and palaeoclimate of the Late Cretaceous and Early Tertiary. In: Friis EM, Chalone WG, Crane PR (eds) The origins of angiosperms and their biological consequences. Cambridge University Press, Cambridge, pp 51–73
Pebesma EJ, Bivand RS (2005) Classes and methods for spatial data in R. R News 5 (2) https://cran.r-project.org/doc/Rnews/
Pederneiras LC, Gaglioti AL, Romaniuc-Neto S, Mansano VDF (2018) The role of biogeographical barriers and bridges in determining divergent lineages in Ficus (Moraceae). Bot J Linn Soc 187:594–613. https://doi.org/10.1093/botlinnean/boy036
Pennington TD (2016) Systematic treatment of American Trichilia (Meliaceae). Phytotaxa 259:18–162. https://doi.org/10.11646/phytotaxa.259.1.5
Pennington TD, Clarkson JJ (2013) A revision of Guarea (Meliaceae). Edinburgh J Bot 70:179–362. https://doi.org/10.1017/S0960428613000036
Pennington RT, Dick CW (2004) The role of immigrants in the assembly of the South American rainforest tree flora. Philos Trans R Soc B Biol Sci 359:1611–1622. https://doi.org/10.1098/rstb.2004.1532
Pennington TD, Muellner-Riehl AN (2010) A monograph of Cedrela (Meliaceae). Dh Books, Milborne Port
Pennington TD, Styles BT (1975) A generic monograph of the Meliaceae. Blumea 22:419–540
Pennington TD, Styles BT, Taylor DAH (1981) Meliaceae, with accounts of Swietenioideae and chemotaxonomy. Flora Neotrop 28:1–470
Pennington TD, Barker A, Rojas-Andrés BM (2021) A revision of the genus Ruagea (Meliaceae: Melioideae). Kew Bull. https://doi.org/10.1007/s12225-021-09957-0
Petrescu I (1978) Studiul lemnelor fosile din oligocenul din nord-vestul Transilvaniei. [Study of fossil wood from Oligocene of NW Transylvania.] Mémoires (IGR). XXVII:113–184 [in Romanian]
Petrescu I, Nicorici E, Biţoianu C, et al (1987) Geologia zăcămintelor de cărbuni. 2. Zăcăminte din Romania, 2. Editura Tehnică, Bucharest
Pitman WC, Cande S, LaBrecque J, Pindell J (1993) Fragmentation of Gondwana: the separation of Africa from South America. In: Goldblatt P (ed) Biological relationships between Africa and South America. Yale University Press, New Haven; London, pp 15–34
Pons D, Franceschi DD (2007) Neogene woods from western Peruvian Amazon and palaeoenvironmental interpretation. Bull Geosci 82:343–354
POWO (2019) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. http://www.plantsoftheworldonline.org. Accessed 15 May 2021
Prakash U (1976) Fossil woods resembling Dichrostachys and Entandrophragma from the Tertiary of the Middle East. Abhandlungen des Zentralen Geologischen Institutes, Berlin 26: 499–507
Ramírez-Barahona S, Eguiarte LE (2013) The role of glacial cycles in promoting genetic diversity in the Neotropics: the case of cloud forests during the Last Glacial Maximum. Ecol Evol 3:725–738
Reid EM, Chandler MEJ (1933) The London Clay flora. British Museum (Natural History), London
Renner S (2004) Plant dispersal across the tropical Atlantic by wind and sea currents. Int J Plant Sci 165:S23–S33
Richardson JE, Chatrou LW, Mols JB et al (2004) Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos Trans R Soc B Biol Sci 359:1495–1508. https://doi.org/10.1098/rstb.2004.1537
Rögl VF (1998) Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene). Ann Naturhist Mus Wien 99A:279–310
Roiron P (1991) La macroflore d’age Miocene superieur des Diatomites de Murat (Cantal, France): Implications Paleoclimatiques. Paleontographica Abt B 223:169–203
Rueangruea S, Tagane S, Suddee S et al (2015) Toona calcicola, a new species and Reinwardtiodendron humile, a new record to Thailand. Thai for Bull 43:79–86
Salard-Cheboldaeff M (1978) Sur la palynoflore Maestrichtienne et Tertiaire du basin sédimentaire littoral du Cameroun. Pollen Spores 20:215–260
Sanmartín I, Enghoff H, Ronquist F (2001) Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biol J Linn Soc 73:345–390. https://doi.org/10.1006/bijl.2001.0542
Schlee D (1990) Das Bernstein-Kabinett. Stuttgarter Beitr Naturk Ser C 28
Scotti-Saintagne C, Dick CW, Caron H, Vendramin GG, Guichoux E, Buonamici A, Duret C, Sire P, Valencia R, Lemes MR, Gribel R, Scotti I (2013) Phylogeography of a species complex of lowland Neotropical rainforest trees (Carapa, Meliaceae). J Biogeogr 40:676–692
Selmeier A (1989) Ein verkieselter Mahagonistamm (Meliaceae) aus dem Ortenburger Schotter (Niederbayern). Nat Z Niederbayern 31:81–106
Simpson GG (1980) Splendid isolation: the curious history of South American mammals. Yale University Press, New Haven
Sivaraj I, Nithaniyal S, Bhooma V et al (2018) Species delimitation of Melia dubia Cav. from Melia azedarach L. complex based on DNA barcoding. Botany 96:329–336. https://doi.org/10.1139/cjb-2017-0148
South A (2017) rnaturalearth: World Map Data from Natural Earth. R package version 0.1.0. https://CRAN.R-project.org/package=rnaturalearth
Stone R (2013) Battle for the Americas. Science 341:230–233. https://doi.org/10.1126/science.341.6143.230
Szalay FS, McKenna MC (1971) Beginning of the age of mammals in Asia: the late Paleocene Gashato fauna, Mongolia. Bull Am Mus Nat Hist 144:269–318
Takeuchi W (2000) A floristic and ethnobotanical account of the Josephstaal forest management agreement area, Papua New Guinea. SIDA, Contrib Bot 19:1–63
Takeuchi W (2009) Occurrence records in Papuasian Aglaia (Meliaceae): A. pannelliana and A. puberulanthera from the southern karst of Papua New Guinea. Harv Pap Bot 14:31–38. https://doi.org/10.3100/025.014.0106
Thorne RF, Reveal JL (2007) An updated classification of the class Magnoliopsida (“Angiospermae”). Bot Rev 73:67–181. https://doi.org/10.1663/0006-8101(2007)73[67:AUCOTC]2.0.CO;2
Tiffney BH (1985) The Eocene North Atlantic land bridge: its importance in Tertiary and modern phytogeography of the Northern Hemisphere. J Arnold Arboretum 66:243–273. https://doi.org/10.5962/bhl.part.13183
Tiffney BH (2000) Geographic and climatic influences on the Cretaceous and Tertiary history of Euramerican floristic similarity. Acta Univ Carol Geol 44:5–16
Tiffney BH, Manchester SR (2001) The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int J Plant Sci 162:S3–S17. https://doi.org/10.1086/323880
Trop JM, Ridgway KD (2007) Mesozoic and Cenozoic tectonic growth of southern Alaska: a sedimentary basin perspective. Spec Pap Geol Soc Am 431:55–94. https://doi.org/10.1130/2007.2431(04)
Van Duzer CA, Munz GC (2004) Floating Islands: a global bibliography: with an edition and translation of G.C. Munz’s "Exercitatio academica de insulis natantibus" (1711). Cantor Press, Los Altos Hills
Van Ee BW, Berry PE, Riina R, Gutiérrez Amaro JE (2008) Molecular phylogenetics and biogeography of the Caribbean-centered Croton subgenus Moacroton (Euphorbiaceae s.s.). Bot Rev 74:132–165. https://doi.org/10.1007/s12229-008-9003-y
Webb SD (2006) The great American biotic interchange: patterns and processes. Ann Missouri Bot Gard 93:245–257
Wolfe JA (1977) Paleogene floras from the Gulf of Alaska region, U.S. Govt. Print. Off., Washington, 1–99
Wolfe JA (1975) Some aspects of plant geography of the Northern Hemisphere during the late Cretaceous and Tertiary. Ann Missouri Bot Gard 62:264. https://doi.org/10.2307/2395198
Wongprasert T, Phengklai C, Boonthavikoon T (2011) A synoptic account of the Meliaceae of Thailand. Thai For Bull Bot 4:210–266
Zayas-Ocelotl L, Castañeda-Posadas C, Estrada-Ruiz E, Andrés-Hernández AR (2014) Hojas de angiospermas de San Esteban Tizatlán (Mioceno), Tlaxcala, México. Rev Bras Paleontol 17:327–342
Zhang RJ, Chen HF, Xing FW, Ye YS (2009) Munronia yinggelingensis sp. nov. (Meliaceae) from Hainan, China. Nord J Bot 27:376–378. https://doi.org/10.1111/j.1756-1051.2009.00491.x
Acknowledgements
Funding was provided by Leipzig University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
ANMR conceived the idea for this work; both ANMR and BRA performed the literature search and analysis, and wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muellner-Riehl, A.N., Rojas-Andrés, B.M. Biogeography of Neotropical Meliaceae: geological connections, fossil and molecular evidence revisited. Braz. J. Bot 45, 527–543 (2022). https://doi.org/10.1007/s40415-021-00770-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-021-00770-4