Abstract
To best sustain endurance activity, two systems must be effectively coordinated: ventilation and locomotion. Evidence has long suggested that these two mammalian rhythms are linked, yet determinants and implications of locomotor–respiratory coupling (LRC) continue to be investigated. Two general areas explaining the potential mechanisms underlying LRC are (1) neural interactions between central and peripheral controllers of locomotion and respiration, and (2) mechanical interactions between locomotor dynamics and respiratory mechanics. Additional suggested explanations for/consequence of the existence of LRC in mammals include an improved energetic cost of locomotion and a reduced sensation of breathlessness. As such, any perturbation to LRC, via alterations in breathing or kinematic patterns, could have negative performance implications to both athlete and patient populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evidence has long suggested that ventilatory and locomotor rhythms are linked, yet determinants and implications of locomotor–respiratory coupling (LRC) continue to be investigated. This review will focus on literature related to ventilation and locomotion in terrestrial vertebrates, as well as the evidence for, and modulators, mechanisms, and implications of entrainment of these two rhythms. Before discussing the coupling of locomotor and respiratory rhythms, this review will first delve more deeply into the two rhythms, independently. The review is presented according to the following general areas of focus: (1) the how and why of ventilation, (2) mechanics and energetics of animal locomotion, (3) LRC in non-human vertebrates, (4) LRC in humans, (5) proposed mechanisms underlying LRC, (6) modulators of LRC, (7) implications of LRC, and (8) potential perturbations to LRC.
Ventilation: Why is it Necessary and How is it Accomplished?
Ventilation refers to the movement of air into and out of the pulmonary system, which includes the nose, mouth, trachea, and lungs. The terms breathing and respiration are also often used synonymously to refer to this process of ventilation; though to be clear, respiration is actually the process of gas exchange at the cellular level rather than the movement of air within the system. The total volume of air transported into and out of the lungs in 1 min is referred to as the minute ventilation (V E) and is simply the product of the average volume of each breath (tidal volume; V T) and the frequency of breathing (f B) [159]. Ventilation serves four main purposes: (1) exchange of oxygen (O2) and (2) carbon dioxide (CO2), (3) control of blood pH, and (4) oral communication [30]. In other words, ventilation is necessary to sustain life, and the central nervous system’s (CNS) control of ventilation is closely regulated, accordingly. The level of ventilation is controlled via the so-called “respiratory control center” located the brainstem, specifically the pontomedullary (pons, medulla oblongata) region. This neural center, in healthy individuals, maintains a general rhythmicity of inspiration and expiration. Inputs to the control center include neural and chemical factors that act alone and jointly to determine the frequency and intensity of output from the CNS, while output from the respiratory control center is largely to the phrenic and intercostal nerves, which supply the muscles of respiration.
Mechanics of Ventilation
The respiratory muscles can be divided into those that predominantly achieve inspiration (e.g., diaphragm, external intercostal muscles), expiration (e.g., abdominal muscles, internal intercostal muscles), and the accessory muscles. The primary muscle of inspiration is the diaphragm; it contributes to approximately 70 % of inspiratory tidal volume in a healthy individual at rest [18]. Because of the elastic properties of the lung and chest wall, the muscles of expiration are essentially quiescent at rest, as the lung and chest wall return to functional residual capacity (FRC) after being actively expanded. During heavy exercise or voluntary hyperventilation, additional inspiratory muscles (e.g., accessory) are recruited, and the expiratory muscles become much more active.
Changes in thoracic and/or abdominal volumes via contraction of respiratory muscles result in changes in intrapulmonary pressure [30]. When the volume of the thorax increases with contraction of the diaphragm and external intercostal muscles, intrapulmonary pressure briefly decreases causing atmospheric air to move into the pulmonary system, i.e., inspiration. Similarly, when the inspiratory muscles relax and the diaphragm recoils reflexively toward FRC, intrapulmonary pressure increases and air is forced out, i.e., expiration. During hyperpnea and exercise, more muscles become involved in the ventilatory process, producing larger intrapulmonary pressure swings, and subsequently larger increases in bulk air flow. Additionally, increased stimulation to the respiratory control center causes an increase in the frequency of breathing, primarily accomplished via decreases in both inspiratory and expiratory times (T I and T E, respectively). However, T E decreases relatively more than T I; correspondingly, peak expiratory flow rates and pressures must increase more than inspiratory flows—a phenomenon which may end up being important with regard to limitations of the ventilatory system in athletes. Ultimately, the transport of air into and out of the pulmonary system allows partial pressures of oxygen and carbon dioxide in the alveoli to remain close to atmospheric levels, generally even during exercise. This process enhances the diffusion gradient between venous blood entering the pulmonary system and alveolar gases, as well as the gradient between arterial blood gases and body tissues.
Stimuli to Ventilation
In the most general schematic, there are three basic components to the respiratory control system: a central controller, sensors, and effectors [159]. Effectors encompass the respiratory muscles, discussed previously, that must work in a coordinated fashion to accomplish ventilation. This crucial task falls to the respiratory control center in the pontomedullary region of the brainstem, which coordinates receptor inputs with appropriate impulses to respiratory muscles. Within the control center are groups of neurons with various roles; together, these neurons regulate the cyclic nature of inspiration and expiration. Other areas of the brain can alter breathing patterns as well; for example, the cortex controls voluntary changes in breathing, while the limbic system and hypothalamus affect breathing patterns in response to emotions.
A variety of receptors exist to recognize deviations from homeostasis and signal the appropriate controller. Two important regulated factors in humans are blood pH and arterial partial pressures of oxygen and carbon dioxide (PaO2 and PaCO2, respectively). Central and peripheral chemoreceptors provide input to the brain regarding the chemical composition of blood and body fluids, while impulses from the CNS to the respiratory muscles cause the appropriate ventilatory response. An overview of ventilatory stimuli can be seen in Fig. 1. The role of afferent feedback within the complex process of ventilation warrants further discussion. The term afferent signifies a direction of flow toward the CNS (as opposed to efferent—indicating away from the CNS). Studies have demonstrated that contraction-induced mechanical and chemical stimuli activate afferent nerve fibers in working skeletal muscles; specifically, Group III (small, fast-conducting, and myelinated) and Group IV (small, slow-conducting, and nonmyelinated) afferents provide the neural feedback that mediates ventilatory responses. The earliest work showed that stimulation of limb movement in anesthetized cats resulted in a reflexive increase in ventilation [109, 114]. This “peripheral neurogenesis” is likewise evident when looking at the converse: a pharmacological blockade or physical severance of these muscle afferents attenuates the rise in ventilation during limb movement [4, 92]. Not only do Group III and IV afferents exist in and provide signals from locomotor muscles, but they also have nerve endings in the respiratory muscles that mediate respiratory-related reflexes, as well [66]. While chemical and mechanical stimulation of locomotor muscle Group III/IV afferents elevate ventilation, the overall impact of respiratory muscle afferent stimulation is less clear. Phrenic afferent activation appears to depress phrenic efferent drive, but because of the myriad of other inputs to ventilatory response and poor selectivity of afferent stimulation, its actual physiological effects on ventilation are inconclusive [5, 52, 66, 127, 128].
Overview of inputs to and impulses from the respiratory control center. Adapted from [39]
Limitations of the Respiratory System
While historically the respiratory system was not thought to limit healthy individuals, during exercise or otherwise, over the past two decades, research has consistently shown that, in fact, the respiratory system can be a limiting factor in the performance of healthy individuals. In most instances, the respiratory system affords effective maintenance of blood gases and tissue oxygenation, even during vigorous exercise. In the well-trained individuals, exercise ventilation has the potential to increase 30-fold over resting values—a much greater relative increase than is seen in—for example, cardiac output during exercise. However, in highly fit individuals in particular, the capacity of the ventilatory system often becomes unable to match the sizeable metabolic demands of heavy exercise.
A recent review expounded on three primary limitations of the healthy respiratory system to whole-body endurance exercise performance: (1) exercise-induced arterial hypoxemia (EIAH), (2) intrathoracic pressure effects on cardiac output, and (3) exercise-induced respiratory muscle work and the associated metaboreflex [3]. Of particular importance to this review, high levels of ventilation during intense exercise result in considerable increases in the work of breathing. The respiratory muscles use energy, just as any other skeletal muscle, and so as additional inspiratory and expiratory muscles are recruited to augment ventilation, the associated energetic cost rises. This relationship between ventilation and the cost of breathing is not a linear one; rather, research indicates an exponential increase in metabolic cost with increases in minute ventilation [1, 6, 91, 108]. Estimates for the oxygen cost of breathing as a percent of whole-body VO2 range from <1 % at rest up to 15 % during maximal exercise in some individuals [1, 78]. Not only does a greater work of breathing likely lead to more rapid respiratory muscle fatigue (RMF) [7], but it also compromises locomotor muscle blood flow—certainly an adverse consequence for successful endurance performance. In an elegant study by Harms and colleagues [77], competitive cyclists completed exercise bouts at VO2max, while inspiratory muscle work was either increased (via graded resistive loads) or decreased (via a proportional assist ventilator). The authors found that with increases in respiratory muscle work, leg muscle perfusion and VO2 (as a percent of total oxygen consumption) were decreased from control conditions. Likewise, a decrease in work of breathing allowed increased locomotor muscle blood flow. The likely cause is a metabolite-mediated activation of group IV phrenic afferents that increases sympathetic vasoconstrictor activity to the exercising limbs [82, 144]. Essentially, it appears that in the so-called “hierarchy” of physiological processes, the work of the respiratory muscles takes precedence over limb locomotor muscle activity.
RMF is determined to be present when respiratory muscle strength, or maximal pressure generation, is decreased [60]. The phenomenon has been observed in healthy individuals after resistive breathing against an inspiratory load [17, 118], mimicking a high level of ventilation for a prolonged period of time [8], and performing an isocapnic maximal voluntary ventilation maneuver for 2 min [76]. However, breathing against resistive loads (for example, by adding mesh screens to the inspiratory line) and/or at rest can impose different muscle recruitment patterns, flow rates, and elastic recoil properties than those seen during exercise hyperpnea. Studies thus sought to determine if RMF in healthy individuals could arise from whole-body exercise (e.g., running, swimming, rowing, cycling), with varying results [46, 47, 50, 100]. Johnson et al. [90] ascribed the inconsistent findings to the use of indirect/volitional techniques to assess RMF. Using bilateral phrenic nerve stimulation (BPNS) as a more objective measure to assess fatigue, the John Rankin Laboratory researchers found that exercising at 85 and 95 % VO2max until exhaustion (31 ± 8 and 14 ± 3 min, respectively) caused decreases in Pdi at multiple starting lung volumes. The stimulated Pdi measurements remained at least partially reduced for an average of 70 min after completion of exercise [90]. Subsequent work by the same laboratory found that simply mimicking these exercise ventilatory parameters while at rest was not sufficient to induce RMF [1, 7]. When the effects of whole-body exercise at 86–93 % VO2max to exhaustion were compared with voluntary increases in ventilatory parameters (Pdi, tidal volume, and breathing frequency) to match those seen during exercise, subjects displayed significant diaphragmatic fatigue during the former but not the latter [7]. Likewise, when duration was not held constant, the time to task failure for mimicking maximal exercise ventilatory parameters at rest was 3–10 times greater than the length of the maximal exercise bout [1]. These results suggest that the RMF induced by heavy exercise is not due to high levels of ventilation, per se, but rather high levels of ventilation in conjunction with some phenomena of exercise itself. More specifically, the competition for blood flow between exercising locomotor muscles and the respiratory musculature, described previously, intensifies the progression of RMF during exercise.
RMF does not necessarily imply task failure—even when fatigued, individuals are still able to breathe! However, the way in which this ventilation is achieved appears to be different than in non-fatigued conditions. There is consistent evidence for an increase in overall ventilation during exercise (or chemically stimulated V E) following induction of global respiratory or inspiratory muscle fatigue [103, 104, 142, 143, 153]. The increase in V E is achieved via significant increases in breathing frequency, which is sometimes coupled with decreases in tidal volume. Mador and Acevedo found that inspiratory muscle fatigue, induced through a resistive breathing protocol, caused increased ventilation/frequency of breathing during bouts of cycling exercise at 50–100 % of maximal workload (but not at rest or 25 % of maximum). The increased ventilatory rates obviously required a decrease in inspiratory and expiratory times, and as such, a concomitant increase in flow rates [103]. A subsequent study saw a similar result during an endurance cycling trial at 90 % of VO2max. At equivalent time points, V E was 12 % greater, and breathing frequency/flow rates were increased during the inspiratory muscle fatigue trial compared to a control condition. Furthermore, time to task failure was 23 % lower with inspiratory muscle fatigue [104]. Spengler et al. [143] compared the subsequent ventilatory responses during exercise after (1) exercise to fatigue at 78 % VO2max, (2) resting voluntary hyperpnea mimicking exercise ventilatory parameters, and 3) resting voluntary hyperpnea at a ventilation 20 % greater than that of the exercise bout (“supermimic”) to control measures. As would be expected based upon the work by Babcock et al. [7], the resting exercise ventilation mimic had no effect on subsequent exercise ventilation. On the other hand, the supermimic and the exercise to fatigue resulted in similar increases in ventilation, increases in frequency of breathing, and decreases in tidal volume compared to control [143].
Animal Locomotion
Locomotion, i.e., the energy-consuming act of self-propulsion, serves multiple purposes, which at least from the perspective of evolution, is necessary for survival. Nearly, all species, save, perhaps, the average modern human, rely on movement to catch/scavenge for food and to escape predators. However, one simply needs to observe a variety of moving animals to see that the variability in gaits is enormous, even within-species. To appreciate these kinematic differences more thoroughly, it is important to understand those factors that control movement, the mechanics of the gaits themselves, and the energetic cost of locomotion (potentially a modulating factor in the “choice” of a particular gait).
Neural Control of Locomotion
The motor system consists of all the body’s muscles and the neurons that control them [13]. During movement, muscles can shorten, stay the same length, and/or be stretched to accomplish a given action [148]. Remarkably, the neural organization underlying locomotion is quite similar across vertebrate species. This control and coordination (phase-dependent pattern) of locomotion can be divided into two sections: (1) the brain command and control of motor programs in the spinal cord, and (2) the spinal cord command and control of coordinated muscle contraction via motor programs.
CNS command can be broken down further to essentially three levels of function. First, the “higher thinking” areas of the forebrain’s neocortex and basal ganglia are concerned with strategy, i.e., the goal of movement and the movement strategy that best achieves the goal. Second, the motor cortex and cerebellum coordinate the sequence of muscle contractions required to smoothly achieve a particular movement. Finally, the brain stem can be grouped with the spinal cord in an effort to execute and fine-tune the goal-directed movement [13]. While the motor neurons in the spinal cord are tasked with achieving actual movement, (typically) the brain must communicate with the spinal cord in some manner. As such, axons from different regions of the brain descend the spinal cord in two branches: (1) lateral pathways, which control higher function and voluntary movement, and (2) ventromedial pathways, which control posture and locomotion. For the purposes of this review, the focus will remain primarily on ventromedial pathways and the lower levels (brainstem/spinal cord) of motor control hierarchy.
In the late 1960s, specific brainstem regions dedicated to the initiation and control of locomotion were identified: the diencephalic locomotor region (DLR) and the mesencephalic locomotor region (MLR) [73, 139]. These regions receive inputs from the basal ganglia and hypothalamus, via mechanisms that are yet to be entirely elucidated, and activate reticulospinal (RS) neurons that in turn activate spinal cord locomotor networks. The RS cells also receive peripheral inputs that are integrated with the central inputs to generate a coherent motor command [99]. When the MLR (or DLR, though it has not been as extensively studied) is activated through electrical or chemical stimulation, motor output is elicited in a variety of vertebrates. Stronger stimulation results in faster locomotor frequency, with very high activations causing additional recruitment from hindbrain neurons to further boost locomotor output. Interestingly, even when the MLR is stimulated on only one side, locomotor output remains bilaterally symmetrical; although not completely understood, the level of this phenomenon appears to be at the MLR inputs to the RS neurons [29].
At the level of the spinal cord, it is now generally agreed upon that networks of interneurons, considered central pattern generators (CPGs), are responsible for the generation of basic rhythmic muscle activity. Within these neuronal circuits, individual neurons whose membrane properties allow them to act as pacemakers set the primary intrinsic rhythm. The pacemaker neurons are embedded within CPGs whose synaptic connections and cross-extensor reflex activity (a type of reciprocal inhibition that generates simultaneous activation of extension/inhibition of flexion, and vice versa, of appropriate muscles) produce coordinated locomotor patterns. CPGs also receive afferent feedback from locomotor muscle that is beneficial in controlling phase transitions and refining ongoing activity [151]. Specifically, during the stance phase of the step, lower limb loading is detected by Group I extensor muscle afferents and Group II cutaneous afferents; these sensory neurons activate the extensor portion of the CPG, enhancing extensor muscle activity during the beginning of the stance phase. Conversely, at the end of the stance phase (i.e., just before initiation of the swing phase), Group Ia afferents of the flexor muscle excite the flexor portion of the CPG. Within the central pattern generator, the flexor and extensor “half-centers” are mutually inhibitory; thus, excitation of one causes inhibition of the other, and vice versa. In addition to afferent feedback mechanisms, brainstem neurons that descend the spinal cord can act to modulate central pattern generators.
Because of the intricate network of circuits in the spinal cord, spinal control of movement in the absence of both higher inputs and afferent feedback is possible. Classic experiments in the early 20th century first suggested the concept of a spinal locomotor center. In these studies, cats with transected spinal cords and cut dorsal roots still displayed rhythmic alternating contractions in ankle flexors and extensors [32, 33]. Numerous studies since have confirmed the ability of spinal centers to provide normal locomotor output after transection of dorsal roots. Additional evidence for the inherent rhythmicity of the spinal interneurons comes from a phenomenon referred to as “fictive locomotion,” whereby muscular movement itself is blocked (via pharmacological or physical means), but non-rhythmic stimulation of the nerve cord still results in rhythmic periods of activity (organized between agonists and antagonists) of the efferent nerves at the ventral root [107]. However, it appears that the stability and maintenance of the locomotor pattern may be slightly compromised without intact afferent feedback [57].
The majority of findings on the underlying structure and mechanisms of central pattern generators in the spinal cord are as a result of non-human, animal research. Clearly, assessments of decerebrate locomotion, fictive movements, and in vitro study of the spinal cord are not easily accomplished in humans. Nevertheless, there does appear to be evidence for innate locomotion-generating networks in primates, including humans. In 1905, research found that a monkey with a transected spinal cord showed alternating movements of the hindlimbs 1 month post-lesion [124]. There are even cases of a decerebrate, spinalized (transected spinal cord) marmoset monkey and a spinalized squirrel monkey that exhibited fictive locomotion and rhythmic stepping movements [87, 154]. Similarities in the locomotor circuitry between spinalized cats and humans provide further evidence supporting the human CPG. Human patients, like spinalized felines, display afferent flexor reflexes that respond to footfall by inducing transition from flexion to foot placement, presumably through inhibition of the CPG flexor half-center. Stimulation of spinal cord sites in patients with spinal cord injury (SCI) elicits stepping movements with organized electromyogram of symmetric muscles [70]. Additionally, SCI patients display rhythmicity of their involuntary and stepping movements, alternating flexor/extensor activity. This rhythm is elicited by considerable locomotor training (e.g., harnessed treadmill walking with variable body weight support), and disappears soon after training is ceased, reinforcing the importance of the afferent feedback loop. It remains unclear whether the rhythmically generated locomotor patterns (CPGs) in the SCI scenario are the same as those used during normal walking [57, 151].
Healthy humans provide evidence for the existence of CPGs, as well. Sleep-related periodic leg movements have been observed in both older, healthy individuals and SCI patients; these lower limb movements occur during sleep and are periodic and repetitive [23, 162]. Moreover, locomotor-like movements during weightless suspension can be produced simply by exogenous muscle vibrations in relaxing, healthy individuals [135]. Finally, it is well established that newborn infants display primitive step-like movements that are similar to those seen earlier in the prenatal phase [49, 65, 149]. Despite the evidence for human CPGs, it does appear that in primates, at least compared to quadrupeds, the spinal circuitry for locomotion is suppressed by cortical input; this may be in order to free the movements of the upper limbs from locomotor movements of the hindlimbs [57].
Mechanics of Locomotion
Locomotion is essentially moving the body from one location to another, and can be explained in terms of kinematics, i.e., how far, fast, and consistently a body moves, and kinetics, i.e., the forces that cause such movement [79]. When considering terrestrial locomotion, the total distance covered is determined by the distance of each step/stride [step/stride length (SL)] and the number of steps/strides taken [step/stride frequency (SF)]. Likewise, the average speed at which an animal moves is dependent upon these same two factors. The terms stride and step are not used consistently in the literature (and even less so in the clinical or athletic community), so it is important to distinguish between the two basic units of locomotion. A stride is measured from the ground contact of one foot to the next touchdown of the same foot (ipsilateral footstrike to ipsilateral footstrike), whereas a step is simply a half stride, or that from ipsilateral footstrike to contralateral footstrike [152]. Across the animal kingdom, SL appears to be at least partially dependent on anatomical considerations such as leg length, while SF is limited by the ability to move limbs through the air and time limitations to producing forward impulse [80, 152].
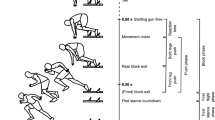
A gait is defined as a particular manner or carriage of forward progression in walking or running. While the instinct may be to consider bipedal running as a natural extension of walking, in actuality, the two gaits are quite different (Fig. 2) (similarly, quadrupeds are able to locomote using a number of distinct gaits [130]). The kinematics of walking can be described as an inverted pendulum, in which the body vaults up and over each stiff leg in an arc motion. The kinetic energy from the first half of the stance (support) phase is transformed into gravitational potential energy, which is partially recovered as the body falls forward and downward during the second half of the stance phase. Walking is characterized by a double support phase, during which both feet are in contact with the ground. Running, on the other hand, is comparable to bouncing and even involves a “flight” phase, where neither leg is in contact with the ground [37, 152]. As the leg strikes the ground during running, the muscles eccentrically contract, and kinetic energy and potential energy are temporarily stored as elastic energy in muscles, tendons, and ligaments. Nearly, all the energy is recovered during the propulsive (concentric contraction) second half of the stance phase. This distinct pattern of movement can be represented as a spring-mass model, where the spring is the behavior of the support leg and the mass is the total body mass (Fig. 3) [2, 41, 51, 53, 81]. The elastic behavior of a spring is dependent upon its stiffness and its deformation. In terms of the lower limb as a spring, the stiffness of the muscles and/or tendons and the forces exerted on the ground determine the energy recoil. As Roberts and Azizi argue in a recent commentary, the elastic mechanism in vertebrate movement allows the locomotor system to function beyond the limits of the muscle motor [129].
The spring-mass model in running. Thick lines represent the eccentric phase of motion. z max, z strike, and z min are the maximum, footstrike, and minimum vertical positions of the center of mass. Adapted from [51]
Energy Cost of Locomotion
Movement, whether it be running an ultramarathon or lifting a television remote, requires energy to produce the necessary work. The amount of energy expended can be determined via direct or indirect methods. The former determination, proposed by Lavoisier around 1780, is achieved by measuring the amount of heat lost, while the indirect method relies on changes in the composition of inspired and expired gases. The indirect method is just as accurate, while being far simpler and less expensive than direct calorimetry; as such, it is commonly employed in today’s physiological experiments. At its core is the fact that oxygen consumed and carbon dioxide produced by the breakdown of fuel substrates are the direct index of energy expended. Haldane and Douglas are credited with the development of this indirect method of quantifying metabolism [40]. Efficiency, in general, describes the ratio of work done to the energy expended for that work. However, muscular/gross efficiency has components that have not yet been determined, including changes in physiological maintenance during work, fraction of mechanical work stored elastically, transferred and reutilized, and differences in positive, “zero,” and negative work. Further complicating the use of the term efficiency is another subset of the word: muscle efficiency, or the process of converting substrate into muscular tension, which encompasses phosphorylation coupling and contraction coupling. Conversely, the term economy is both “conceptually clear and practically useful for evaluation of performance in endurance activities” [42]. Economy of locomotion is defined as the energy demand for a given submaximal velocity, measured via steady-state oxygen consumption, with lower VO2 at a given velocity indicating greater economy [42, 132]. Remarkably, despite distinct differences in gait (i.e., bipedalism vs. quadrupedalism), humans and other mammals display relatively similar mass-independent energetics of locomotion [75].
While sprinting is concerned with speed and thus requires large power output without regard to energetic cost, endurance activities appear to favor economical motion. Certainly, different movement patterns and speeds affect submaximal VO2, and as such, energetic cost often determines the most appropriate gait during endurance exercise. For example, in horses, who can walk, trot, or gallop, the transitions between gaits occur at the speeds that maximize metabolic economy [72]. In other words, when the cost of walking at a particular speed becomes greater than the cost of trotting at that same speed, the transition will occur (and likewise for the trot–gallop transition) (Fig. 4). Humans exhibit a similar phenomenon; at approximately 150 m min−1 (2.5 m s−1), the oxygen cost of walking becomes greater than that of running, and the typical human will switch from a walking gait to a run [31].
Oxygen cost to move a given distance for horses walking, trotting, and galloping on treadmill. Histogram shows gaits when one horse was allowed to select her own speed while running overground—she chose three speeds coinciding with energetically optimal speed for each gait. Adapted from [86]
As mentioned previously, achieving a particular running speed depends on the combination of SL and SF. For any given speed, humans tend to choose a combination of SL and SF that minimizes their metabolic cost [43, 45], and forced variations from the preferred gait result in increased VO2. This tendency holds true even during a fatiguing 1-h high-intensity run—throughout the run, VO2 increased and SF decreased, but the “optimal” (metabolically speaking) SF decreased as well. In other words, runners were able to self-select a preferred SF that matched the most economic SF at both the beginning and end of the run [88]. As speed increases, either SL, SF, or both need to increase, and evidence suggests that SL increases preferentially over SF. In recreational male distance runners running at five speeds from 3.15 to 4.12 m s−1, SL was lengthened by 28 %, while SF rose only 4 % [43]. The authors then investigated/manipulated anthropometric variables and found little correlation between these variables and SL and SF. They suggested that economy is the most important governor of the SL and SF components of gait; and more specifically, there is a single (or small range), most-economical stride rate for all running speeds. Of note, at higher running velocities, SL plateaus and further increases in speed must be achieved via increases in SF. While generally these higher velocities are not seen during sustained aerobic exercise, highly trained athletes may reach submaximal speeds where increases in SL are not feasible—to the author’s knowledge, the metabolic impact of this transition (from primarily increasing SL to primarily increasing SF to achieve faster velocities) in these athletes is yet unknown.
Other variables, besides preferred SL and SF, can impact the cost of locomotion. Overall, the relationship between running mechanics and economy appears to be quite complex. Some of the earliest research comparing the biomechanical characteristics of elite and non-elite runners showed that elite runners had better running economy (RE), less vertical oscillation, shorter absolute and relative SL, and better body symmetry while running at steady speeds between 4.96 and 6.44 m s−1 [44]. However, the authors acknowledged that their study did not answer the question of whether “efficient” running is a function of good style, sub-cellular biochemistry, or a weighing of both, as well as what other factors are important, and they suggested using the technique of multiple regression analysis to identify which of the many variables are important for efficient running. One decade later, Williams and Cavanagh [161] performed such a multiple regression to investigate the relationship between distance running mechanics, RE, and performance, and again found that more economical runners tend to have identifiable patterns in their running mechanics. Vertical ground reaction forces were lower, and shank, trunk, and plantar flexion angles were greater, and minimum knee velocity was lower in the most-economical group of runners. A number of other variables had consistent, but not significant, trends between groups separated on the basis of economy, including less arm movement, less vertical oscillation, and a tendency toward a “rearfoot” strike. Overall, of the variables submitted to the multiple regression analysis of biomechanical variables on submaximal VO2, three (shank angle at footstrike, maximal plantar flexion angle, and net positive power) were retained to give an overall R 2 = 0.54. The authors concluded that no single variable can explain differences in economy but rather economy is related to a weighted combination of the influences of many variables.
The cost of locomotion will also be dependent upon the amount of force exerted on the ground (or, likewise, air, and water), and the degree to which the force-generating apparatus (muscles, tendons, etc.) can effectively utilize stored energy. Elite distance runners often exhibit shorter ground contact time than their non-elite counterparts, accomplished primarily by increasing ground reaction forces [34, 160]. Unfortunately, generating greater ground reaction forces comes at the price of increased metabolic energy expenditure and the potential for premature fatigue. Kram and Taylor [96] reported an inverse relationship between the rate of energy used while running and the time the foot applies force to the ground during a single stride. They measured steady-state VO2 and average foot contact time over a range of speeds in kangaroo rats (32 g), ground squirrels (210 g), spring hares (3 kg), dogs (26 kg), and ponies (141 kg), hypothesizing that larger animals with longer legs and step lengths would have lower costs of locomotion. The results showed that the cost of running, regardless of speed, was primarily dependent on the cost of supporting the animal’s weight and the time course of generating this force. However, all of the animals tested in this study were quadrupeds, so it was unknown whether the results would be applicable to human locomotion. Hoyt et al. [85] developed an electronic foot contact monitor that would allow estimation of metabolic energy expenditure during locomotion in humans. The investigators compared data from the ambulatory foot contact monitor with measures of energy expenditure calculated via indirect calorimetry and found a strong correlation (r 2 = 0.93) during both walking and running.
Other research has shown that an increase in stiffness of the lower extremity is associated with improved RE [35, 51, 71, 112], as increased stiffness appears to allow greater use of temporarily stored elastic energy. More specifically, the elastic energy stored when stretching contracted muscles (i.e., the eccentric portion of the support phase during running) can be used as additional energy during the shortening of active muscles (i.e., the concentric portion of the support phase). Early research by Cavagna et al. [41] concluded, after a number of calculations, that (1) efficiency in running is about 40–50 %, (2) such a high value requires a substantial contribution of energy delivered at a low cost, (3) the low-cost energy appears to be elastic recoil energy, and (4) the elastic work contributes roughly half of the total mechanical work performed in running.
As described previously, the running motion can be regarded as a bouncing pattern in which the leg acts similarly to a linear spring. The so-called mass-spring model has allowed researchers to understand kinematic relationships, such as the proportional one between stiffness characteristics and SF. Using this model where human running movements are regarded as an oscillating system consisting of a spring (the leg) and a mass (the body mass), the energy costs of running and leg stiffness have been found to be highly inversely correlated [51, 81]. Similarly, acute manipulation of vertical spring stiffness affects VO2 [112]. When subjects run at a given speed with progressively exaggerated knee flexion, a corresponding increase in ground contact time is seen. Running in this manner reduces the effective vertical stiffness, while also increases the rate of oxygen consumption by up to 50 %. Such a large increase in VO2 is likely attributable to the “novelty” of the motion. As has been consistently shown, animals tend to naturally select the most metabolically efficient gait, and acute changes in preferred biomechanics cause increases in VO2 [45, 88, 115]). Regardless, the results conflict with the idea that shortening ground contact time increases metabolic energy expenditure, and the relative contributions of greater stiffness and lower ground contact time have not been determined. Taken as a whole, the literature on biomechanical determinants of economical motion suggests the following: (1) mechanics that can be considered “superfluous” increase energetic cost, (2) kinematics related to the elastic storage and return of energy influence economy, and (3) trained individuals tend to “self-optimize” their kinematic characteristics in order to be as economical as possible.
Locomotor–Respiratory Coupling
In the early 20th century, the medical doctor Irwin Hance wrote an essay on rhythmical breathing (1919). In this published work, he espoused that much energy was wasted when breathing was not coordinated with muscular motion, stating that “the greatest human efficiency can be secured by rhythmical breathing…gauged by the demands made upon the body: it must always be rhythmical, however.” Since that time, research has shown evidence of locomotor–respiratory coupling (LRC), also referred to as ‘entrainment,’ in many animals, including humans. Entrainment, in general, is defined as frequency and phase locking between two periodic systems [119]. In terms of LRC, this means that limb movements and breathing efforts, which each exhibiting cyclic patterns, are not isolated actions; rather, the outcome of one exerts some influence on the outcome of the other. Despite strong evidence of its existence, the mechanisms underlying and consequences resulting from locomotor–respiratory (un)coupling are still not well understood.
LRC in Non-Human Vertebrates
LRC has been observed in several species; from reptiles to rabbits, flying geese to hopping marsupials, and wild antelope to domesticated felines; many animals exhibit this rhythmicity of movement and breathing. Using animals as models to study, LRC has also allowed researchers to begin to better understand the neuromechanical basis of how these patterns may become coordinated. Research performed as early as 1912 showed that coordination exists between rates of respiration and pectoral fin movement in fish [125, 157]. These studies concluded that synchronization of the two rhythms has multiple advantages, including minimizing the energy requirement of pulling water into the pharynx, increasing the backward speed of exhaled water, and potentially stabilizing flow by reducing drag [131]. Evolutionarily, the coordination between respiration and fin movement in fish certainly makes sense; in early vertebrates, several respiratory muscles developed for purposes that were not primarily aimed at respiration. Vertebrates, such as birds and reptiles, also (akin to fish) lack a diaphragm muscle; in these animals, intercostal and abdominal muscles, which were originally developed for posture and locomotion, are now required to accomplish ventilation. The dual purposes of these muscles result in many instances of locomotor–respiratory coordination.
The breathing of lizards and other reptiles is quite constrained by locomotion because of their “sprawling” limb posture. To accelerate forward, reptiles must laterally bend their axial skeleton back-and-forth. With each stride, one side of the thorax is compressed, while the other is expanded. Because of this mechanical constraint, reptiles generally cannot increase tidal volume during exercise and exhibit increased levels of ventilation post-exertion [38]. In the case of reptiles, there is no LRC (ratio), per se, but the two rhythms are essentially required to demonstrate some degree of coordination. On the other hand, ventilation in birds, which is also governed by muscles assisting with locomotion, is able to occur during the movements themselves. Avian breathing depends on movements of the sternum, a bone which is attached to the muscles for flight. Studies on a variety of bird species have consistently shown coupling between wing beats and respiratory frequencies. The most common ratio observed during flight is three wing beats to one breath (3:1), though relationships of 6:1, 5:1, 5:2, 2:1, and 1:1 have all also been seen [19, 26, 36]. Additionally, there appears to be a strong phase locking between the two rhythms in birds, in which the transitions from expiration to inspiration, and vice versa, occur at consistent points in the wing stroke [36]. Specifically, the transition from expiration to inspiration occurs during the upstroke, while expiration is coordinated with the downstroke of the wing beat. Because the upstroke is associated with expansive effects on avian air sac pressures, and downstroke relates to compressive actions, the phasic relationship of wing beats and breaths in birds seems to assure more “assistance” than “interference” of locomotion to respiration [24]. Similarly, many hopping, running, and galloping mammals breathe at specific points during the locomotor cycle (as will be described in more detail below).
Animals with a diaphragm muscle are able to accomplish ventilation “free” from the specific constraints of dual-purpose abdominal/intercostal muscles; the diaphragm’s singular purpose is to achieve ventilation. Alligators have a diaphragmatic muscle, and as such, when alligators walk, they are able to increase tidal volume and ventilation markedly (despite the same sprawling limb posture of other reptiles) [63]. However, diaphragm-boasting animals still exhibit a coupling between locomotion and respiration. Hopping animals, such as rabbits and wallabies, generally phase lock LRC at a simple ratio of 1:1, with inspiration occurring during liftoff from the ground [12, 141]. While at slower speeds there is a good deal of flexibility in the phase relationship, the phase locking is particularly strong at faster hopping speeds. Similarly, in mammals like dogs and horses, LRC is almost exclusively at a ratio of 1:1 at speeds beyond the trot–gallop transition, but variable during walking/trotting [24].
One of the more “popular” animals in which to study LRC is horses; and the findings from this research apply to other cursorial quadrupeds. Horses have three distinct gaits: walking, trotting, and galloping (four, if one includes the “canter” or slow gallop). Over distances from 200 yards to one mile, the fundamental coupling ratio appears to be 1:1 in the trot and gallop, with substantial locomotor–respiratory coupling seen particularly during the (canter and) gallop [28, 98, 164]. Slow motion analysis indicates that mechanical constraints influence the coordination of gait and breathing in the horse. Specifically, inhalation coincides with the launch of the lead forelimb, and exhalation occurs forcefully at the point of peak thoracic loading. As the speed of locomotion increases, the respiratory and gait cycles also increase, continuing to be in-phase, but with a reduction in the end-inspiratory “pause” and decrease in tidal volume [28]. These changes in ventilation with increasing levels of physical work differ from those seen with chemical stimulation of ventilation at rest. For example, increases in VE at rest are accomplished primarily by increases in tidal volume, whereas throughout exercise, the major contribution is from shifts in breathing frequency [98]. At the trot–gallop transition, however, both breathing and stride frequencies are near maximal. As breathing frequency increases at faster speeds in horses (i.e., from slow trot → fast trot → canter), the frequency coupling becomes even tighter, as evidenced by consistently lower coefficients of variation at gaits with 1:1 LRC compared to those without [98]. As for what happens to LRC at still faster velocities, when breathing frequency would presumably plateau, there has been little dedicated research.
LRC in Human Locomotion
In the mid-20th century, physiologists began to suggest that respiratory frequency in humans is dependent on movement frequency. The noted locomotor–respiratory relationship was not the primary question of the earliest studies, but its existence has fueled a series of investigations since. Perhaps, the first study to observe these coordinated rhythms was one done by Bannister et al., which explored the cause of exercise hyperpnea by having healthy subjects run at approximately 6 mph under various workload (treadmill grades from 0 to 6 %) and inspired gas conditions [9]. The authors attempted to keep subjects’ stride rates constant during any given bout by instructing them to keep time with a metronome. Purely by observation, they noted that in all subjects (granted, n = 4), the respiratory rate was a submultiple of the stride rate. Alternatively, while seeking to understand the effects of added dead space on pulmonary ventilation during cycling exercise, Kelman and Watson [94] noticed that there was no relationship between pedaling rate and breathing frequency. In this study, pedaling rate was kept constant (similar to Bannister et al.’s constant stride rate); though, perhaps important to note, this was accomplished through a speedometer and without the use of a metronome. Subsequent investigations have continued to both confirm and refute the existence of LRC in humans during a variety of activities.
Because of differences in the mode of exercise used to investigate LRC in those earliest studies, Kay et al. [93] compared ventilatory parameters during various pedaling rates and workloads while cycling and different treadmill speeds and grades during brisk walking in five healthy young men. The result was the same regardless of the exercise condition: no clear relationship was seen between pedal/stride rate and breathing frequency. The authors concluded that ventilatory patterns during exercise are simply determined by metabolic demand, and not by movement patterns as had been suggested in “textbooks and reviews” with “few accessible data.” However, a subsequent study by Bechbache et al. [15] found evidence for at least some degree of entrainment during walking, running, and cycling. Groups of healthy men and women (n = 15, per group) were recruited to perform a variety of exercise protocols: (a) cycle at a moderate workload two times, once with a metronome set to 50 rpm and again at the same rate with a speedometer but no metronome; (b) cycle at a moderate (but slightly higher than the first condition) workload two times, once with a metronome set to 70 rpm and again at the same rate with a speedometer but no metronome; and (c) exercise on a treadmill at a moderate workload two times, once at a comfortable running speed and again at a comfortable walking speed (with an increased gradient to match total load). In the conditions sans metronome, subjects listened to classical music via headphones. Using a technique of cross-correlation, the authors then classified individual exercise bouts as having strongly entrained, weakly entrained, or not entrained locomotor–respiratory rhythms. Subjects with strong and weak entrainments were all considered “entrained,” and were thus combined to give the percent of subjects entrained for a given condition. The various protocols resulted in a range of subject entrainment from 20 % (50 rpm cycling with no metronome) to 80 % (treadmill running). Cycling with the metronome and at faster rates increased the percent entrainment (53 and 60 %, respectively). Interestingly, across both exercise bouts with the metronome, entrainment was less prevalent at the end of the test compared to the beginning, suggesting a familiarization with the sound and/or an increase in other factors affecting ventilation. A later study by the same authors, examining the effects of a variety of inspirates and stressors on breathing patterns, found a similar percent entrainment for non-metronome-mediated cycling [14].
A landmark publication in Nature [28] re-emphasized the existence of LRC in a variety of animals, including humans. Looking at LRC through a more comparative physiological perspective, the authors discussed commonalities and differences in entrainment between species. The evolution of the bipedal gait has reduced the mechanical constraints on ventilation, and during human running, there are certainly fewer/lesser compressive forces acting on the thoracic cavity compared to during quadrupedal movement. Yet, the investigators found that runners exhibited entrainment as tightly coupled as that seen in quadrupeds. Specifically, experienced runners (typically marathoners) were able to phase lock LRC within only a few strides at the beginning of the run and maintain coupling for distances greater than one mile, though inexperienced runners, even those with high fitness, showed little tendency to entrain locomotor and respiratory rhythms at all. In contrast to quadrupeds, however, human runners use a range of coupling patterns. Rabbits, horses, dogs, etc. largely are confined to a constant 1:1 ratio of strides per breath, whereas humans typically employ a phase-locked pattern of 2:1, with ratios of 4:1, 3:1, 5:2, and 3:2 all also utilized. Shifts from one coupling ratio to another occur smoothly in experienced runners and are the result of changes in breathing patterns as opposed to gait, which generally remains fairly constant during running. This comparative flexibility in frequency coupling likely stems from the decreased mechanical constraints on breathing with bipedal gait. A comparison of LRC characteristics between humans and non-human vertebrates can be seen in Table 1.
Modulators of LRC in Humans
As evidenced above, in human movement, LRC is certainly more transient than in other animals; however, certain factors have been implicated in its manifestation, including training, exercise mode, exercise intensity, and external stimulation. Individuals who are highly trained in a particular mode of exercise tend to exhibit a greater degree of LRC than less- or untrained individuals. Bramble and Carrier [28] noted a continuum, whereby highly trained runners coordinated movement and breaths within a few strides of beginning treadmill exercise, less-trained runners took more time to exhibit the entrainment, and untrained runners were unlikely to exhibit entrainment at all. Importantly, the less- and untrained subjects were not necessarily less fit—rather, they engaged in cycling, swimming, and other types of exercise—indicating that it is not fitness, but rather deliberate practice (© Dr. K. Anders Ericsson), which results in LRC. Similarly, in a study looking at LRC during various cycling workloads, 70–100 % of competitive cyclists were shown to couple (freely chosen) pedaling and breathing rates, while only 25–63 % of untrained cyclists showed entrainment of these rhythms [95]. In the sport of rowing, elite oarswomen show significantly more consistent coupling ratios than collegiate or untrained rowers [106] over the course of an incremental rowing ergometer test. The differences in LRC between trained and untrained individuals do not appear to be a cross-sectional phenomenon, but rather an actual training effect. Over the course of an eight-month training season, there was a significant increase in the number of oarswomen on a novice collegiate team who exhibited LRC at a workload corresponding to VO2max [105]. The influence of training on LRC can be seen in the clinical setting, as well. A case study on a 38-year-old man with incomplete cervical SCI found that 12 weeks of body weight supported treadmill training (three 45-min sessions per week) not only lowered submaximal exercise ventilation, but also resulted in LRC that was not present before training [138]. The authors suggest that training to invoke LRC could be quite important in this population by allowing patients to combine ventilatory and postural functions of the respiratory muscles—lowering the overall metabolic cost of both tasks and allowing greater blood flow to the working limb muscles.
Another apparent modulator of LRC in humans is the type of rhythmic exercise being performed. A sport like rowing, with its repeated spinal flexion and extension (resulting in thoracic expansion and compression), requires coordination of breathing and movement in order to favorably complete both tasks. In much the way that some animals use intercostal/abdominal muscles for dual purposes, the human respiratory musculature during rowing must both assist the propulsive force generation (to move) and be the effector of ventilation [145]. At the stroke “catch” and/or “finish,” intra-abdominal pressure is high, impairing ventilation, while during the drive phase consisting of knee and hip extension, ventilation is actually assisted [62, 140]. The frequency coupling ratios seen in rowing are typically 1:1, 2:1, or 3:1 stroke frequency to breathing frequency, which in general is more variable than the steady 1:1 seen in galloping mammals, but less than the range of ratios seen in running humans.
While rowing appears to result in the greatest mechanical constraints on/coordinated locomotion with ventilation, other human activities display varying levels of LRC. For example, comparative research indicates that LRC is more stable during running than walking or cycling at the same relative intensities [15, 21, 22]. Initial explanations for this difference related to the involvement of the upper body in the movement [15]; however, subsequent research controlling arm and thorax movement during running still found that LRC prevalence was greater in running compared to cycling [21]. Healthy subjects not trained in any particular mode of exercise are generally more familiar with running than with cycling as a means of locomotion. Certainly evolutionarily, running is a more ingrained action than cycling. However, in a study of eight triathletes who exhibited LRC during both running and cycling, entrainment was actually greater during cycling than running, suggesting again the importance of specific training in LRC [25]. Disparities in entrainment patterns between cycling and running may also stem from a more mechanical basis. Running kinematics involve more “bounce” than cycling movements—as a result, muscles of the diaphragm and abdominal wall are more active in both maintaining posture and, possibly, stabilizing abdominal viscera with each footfall.
External stimuli also appear to affect LRC. Specifically, audible and visual rhythms tend to cause coordination of other physiological rhythms. In bimanual coordination, for example, the anchoring effect describes how external stimuli (generally, sound) can stabilize coupling of the two hands in both the in-phase and anti-phase configurations [64]. A periodic auditory stimulus can also postpone the anti-phase (more stable) to in-phase (less stable) transition in hand coordination, as well as cause respiratory rhythms to match finger tapping rhythms [74]. This same phenomena is seen in LRC, as has been mentioned briefly previously [15], where LRC is more prevalent when subjects exercised with a metronome. Research since has clearly indicated that an external sound cue, i.e., metronome, does, in fact, influence LRC [21, 25, 84, 150]. Instances where a metronome does not assist coordinated rhythms are rare; however, Jasinskas et al. [89] found substantial LRC during cycling at low and high workloads, regardless of whether or not a metronome was used. In fact, while pedaling at their preferred cycling rates, 100 % of the 16 subjects exhibited a high or moderate degree of entrainment. The authors suggest a couple of explanations for the difference from the preceding study where a metronome was used. First, the subjects breathing rate was determined via a trace from chest wall movement as opposed to a mouthpiece. It is possible, certainly in healthy subjects not accustomed to laboratory tests, that the heightened awareness of breathing could impact the subject breathing patterns. Second, the freely chosen pedaling frequency further enhanced the “natural” environment for the subject, and allowed him or her to dissociate from any regulatory cues (e.g., speedometer, etc.). With the metronome being set to the subject’s preferred rate, its influence on the subject may have been minimized compared to an arbitrary frequency. Simply put, ensuring that the running/cycling laboratory environment is not substantially different from the subjects’ typical exercise, training may be important for observing measures of LRC.
A final proposed determinant of LRC in humans is the exercise intensity, with some studies suggesting greater entrainment at higher workloads [15, 20, 21, 126]. For example, increasing cycling rates from 50 rpm to 70 rpm in healthy, untrained volunteers increased the incidence of entrainment by approximately 10 % [15]. Similarly, increasing running speeds from 50 to 80 % of the anaerobic threshold increased entrainment in both untrained individuals and triathletes [20]. Faster walking speeds (i.e., from 1.0 to 1.8 m s−1) also increased incidence of LRC by nearly 20 % [126]. However, this relationship is not consistent across studies, with some research showing no effect of intensity on LRC during cycling and [89, 120], respectively) and one showing the opposite response during increasing running speeds and cycling loads in triathletes [25].
If, as has been suggested, afferent feedback from working limbs influences ventilatory rhythms, the possibility exists that increases in rhythmic limb movement at higher workloads (via faster speeds) could increase incidence of entrainment. Accordingly, the enhanced LRC seen at the higher workloads described above has emanated primarily from increased pedal rates/treadmill speeds, and not higher ergometer loads/running gradients [15, 20, 126]. In fact, Rabler and Kohl specifically examined the effects of altering treadmill speeds and slopes independently and found that only changes in walking speed affected LRC (within the same group of healthy subjects). At faster speeds, a concomitant shortening of phases within both rhythms could make distinguishing a difference between the patterns more difficult using certain quantification techniques, and may partially explain inconsistencies between studies.
Measurement and Determination of LRC
Discrepancies in the results of previous LRC studies may be due, in part, to differences in the actual measurement and determination of LRC (Table 2). The most straightforward method of quantifying entrainment is to simply determine the quotient between the 1-min step rate and the breathing frequency [9, 25, 94, 122]. A simple determination of frequency coupling ratios and patterns can also be done through visual inspection of the ventilation and steps/revolutions oscilloscopes [28]. Limits of ±0.05 of the step-to-breath ratio are often used as the boundaries for a particular frequency coupling. A slightly more rigorous determination of step-to-breath ratios involves their calculation via power spectral analysis of the measured breathing and gait signal frequencies (e.g., fast Fourier transformation) [22, 89, 120, 121].
Entrainment is not simply a frequency phenomenon, though a pattern must also exist within the phase relationship of breaths and steps. The phase locking of LRC has been examined in a couple of ways: (a) comparing the time interval between a reference point in the stride cycle (e.g., step onset) and a reference point in the respiratory cycle (e.g., onset of inspiration or expiration) [126, 146, 150], or (b) counting the number of inspirations (or expirations) beginning in the same phase of the stride cycle (which is first divided into a number of equal parts) and expressing it as a percentage of the total number of breaths recorded during a given exercise bout [21]. Another method involves determining discrete relative phase for each right heel strike within a breath cycle by calculating a quotient of the time lag from the beginning of the stride in which end-inspiration occurred to the subsequent end-inspiration and the time duration of the stride in which end-inspiration occurred [111]. Expanding on this concept, Hoffmann et al. [84] used a rigorous sine-circle map model, wherein locomotion and ventilation are considered as non-linear coupled oscillators. In this model, the interaction of the two oscillators results in an attraction to a certain frequency ratio, dependent upon the individual oscillators’ eigenfrequencies and the strength of their coupling. The stability of this frequency ratio can be assessed through its hierarchical position in the Farey tree.
Because LRC can be transient and variable, particularly in humans, the degree of LRC is often also quantified. A common way in which to report the degree of coupling is to compute the percentage of breaths (or, alternatively, percent of steps or amount of time) that occur at a distinct step-to-breath ratio. Furthermore, researchers acknowledge that not all subjects exhibit any degree of LRC, and thus also report the percent of subjects within a given experiment, who show entrainment. The degree of entrainment (in terms of percentage of breaths, steps, or time that is entrained) depends on the modulators described previously, and can vary anywhere from 25 to 90 %. However, there is currently no minimal percentage of steps (or breaths or time) for demarcating the presence/absence of coupling. As such, a customary approach is to make a statistical comparison between the degree of coupling that occurs in the exercise bout and that which would be expected to occur by chance.
Proposed Mechanisms Underlying LRC
There are two general areas explaining the potential mechanisms underlying LRC: (1) mechanical interactions between locomotor dynamics and respiratory mechanics (within which three primary mechanisms have been proposed), and (2) neural interactions between central and peripheral controllers of locomotion and respiration. The degree to which of these interactions predominates and how the two areas themselves are related is not completely understood. It does appear, however, that certain species and movements are more constrained by mechanical interactions than others, as indicated above.
Biomechanically, three possible hypotheses have been postulated to explain LRC: (1) the movement of the internal organs with locomotion physically affects the movement of the diaphragm (i.e., the “visceral piston” theory), (2) pressure and/or volume changes within the thoracic cavity as a result of the concussive forces related to ground contact, and 3) pressure and/or volume changes within the thoracic cavity as a result of lumbosacral flexion and extension [24, 28, 54]. Likely, it is a combination of these proposed mechanisms that underlies the mechanical constraint on ventilation. Research on the mechanical basis of LRC has been conducted primarily with regard to galloping quadrupeds, such as horses and canines. The structural components of this biomechanical model include the cranio-cervical unit, the thoracic unit, the lumbo-pelvic unit, and the visceral piston (represented chiefly by the liver, which is directly attached to the diaphragm) (Fig. 5).
Simulated breath signal (sinusoid) and right heel strike signal (pulse). EI end-inspiration, HS heel strike, T time between ipsilateral heel strikes, t time from end-inspiration to preceding right heel strike. Adapted from [111]
Inspiration begins near ground take-off of the forelimbs, when the animal is in a stage of suspension. At this point, the cranio-cervical region is flexed, lowering the head/neck and increasing rib cage volume by drawing it forward. Additionally, the thoracic and lumbo-pelvic units are extended as the forelimbs stretch forward, which increases the abdominal cavity volume and lowers intra-abdominal pressure. Finally, because the body is accelerating forward at this point, there is a corresponding rearward displacement of the visceral piston. Ultimately, these increased thoracic/abdominal volumes and decreased pressures effectively produce inspiratory movements. In a similar manner, the pressure/volume changes and viscera movement associated with ground contact impact expiration. Exhalation in galloping mammals begins shortly after forelimb impact with the ground. The resultant external loading on the thorax compresses the rib cage, decreasing thoracic volume and increasing intrathoracic and pulmonary pressures. At the same time, the forward swing/flexion of the lumbo-pelvic region decreases the volume of the abdominal cavity. In terms of the visceral piston, the braking forces on the truck cause the viscera (and attached diaphragm) to be anteriorly displaced, further reducing thoracic volume and aiding expiration [27]. As discussed previously, the temporal relationship between breaths and wingbeats in birds is also consistent with a mechanical constraint on ventilation (Figs. 6, 7) [36].
Noted structural/functional components of mammalian locomotor–respiratory coupling based on large cursorial species. The axial system is composed of three major areas: cranio-cervical, thoracic, and lumbo-pelvic units. Adapted from [27]
Schematic of the multiple neural inputs to pontomedullary respiratory control center that may play a role in, or impact the strength of, locomotor–respiratory coupling. Adapted from [24]
While LRC in humans during certain activities (e.g., rowing, hand rim wheelchair exercise, swimming, etc.) is at least partially mechanically driven [61, 105], overall there does not appear to be much of a mechanical impact of locomotion on ventilation in bipedal humans. In fact, Banzett et al. [10] quantified the airflow changes related to a human running stride and found its contribution to be only 1–2 % of tidal volume. Healthy men (n = 5) performed five maneuvers during walking at 5.3 km h−1 and again while running at 10.6 km h−1: (1) spontaneous breathing, (2) breathing entrained with stepping, as per instruction, (3) breathing paced to prevent entrainment, via use of a metronome, (4) apnea with closed glottis, and (5) apnea with open glottis. Flows at the mouth, pleural pressure, and vertical acceleration were measured under each condition and compared to discern differences in ventilation due to coordinated/uncoordinated steps. The average step-related flow changes were 0.36–1.31 l min−1 making the volume displaced 15–31 ml, or 1–2 % of the corresponding tidal volume. While the movement velocities (and exercise tidal volumes) at which these airflow changes were measured were quite pedestrian, i.e., speeds at which LRC tends to be observed less frequently, the finding is consistent with the idea that entrainment in humans is predominately driven by neurophysiological, as opposed to mechanical, factors.
Multiple neural inputs to the respiratory control center likely contribute to the coordination of locomotor and respiratory rhythms, including both central ‘feedforward’ and peripheral ‘feedback’ influences. In terms of feedforward mechanisms, central pattern generators (CPGs) are neural networks in the brain and spinal cord that gives rise to any rhythmic motor activity [13, 55]. As discussed previously, both respiration and locomotion exhibit general rhythmicities that are determined by these types of neural networks. Traditionally, CPGs were assumed to be independent circuits; however, evidence now supports the concept that many CPG neurons are a part of multiple actions/behaviors [55]. In other words, the coordination of various activities occurs via interactions and overlap between competing and/or cooperating CPGs. The coordination of these neural outputs is essential for matching actions like swallowing and breathing, but it is also apparent in tasks where coupling is not entirely necessary (e.g., locomotion and respiration).
Upon the initiation of exercise, there is an immediate increase in pulmonary ventilation, with the magnitude of the increase being closely related to the rate of the movement. The immediacy of this coordination, and subsequent swiftness with which frequency and phase coupling is displayed [28], indicate that central feedforward signals are largely responsible for the entrainment. This likely mechanism was recognized as early as 1913, when Krogh and Lindhard [97] suggested that the increase in ventilation at the beginning of work was due to impulses from the motor cortex (i.e., to the working limbs) concurrently diverging to the respiratory control center. Empirical evidence for feedforward mechanisms comes from studies showing that stimulation of subthalamic locomotor regions results in an increase in ventilation, even in paralyzed animals [59, 117]. Eldridge and coworkers performed a series of studies comparing ventilatory and locomotor responses in unanesthetized decorticate and anesthetized brain-intact cats [58, 59, 113]. The decorticate animals walked/ran on a treadmill spontaneously or by electrical stimulation of the subthalamic locomotor region. Regardless of the way in which locomotion was generated, the decorticate cats displayed increases in ventilation preceding the onset of locomotion, and throughout the exercise ventilation increased in parallel with treadmill speed. Because the cats were exercising, the role of limb afferent feedback on neural increases in ventilation could not be discounted. Thus, “fictive locomotion” was investigated in a subset of paralyzed cats whose end-tidal gases were maintained through the use of a ventilator. Stimulation of the subthalamic locomotor region under these conditions increased motor nerve activity (but not actual muscle contraction), while simultaneously increasing the magnitude and frequency of phrenic nerve activity. Entrainment during fictive locomotion has also been observed in curarized rabbits, birds, and lampreys [56, 67, 123], as well as following stimulation at ‘lower’ levels of the CNS (i.e., spinal cord). The results of these investigations demonstrate that automatic locomotion and proportional increases ventilation can be consistently invoked from stimulation of a single region of the CNS, even in the absence of peripheral feedback.
In addition to the central mechanisms, a variety of peripheral afferents may also contribute to the coupling of locomotion and respiration. Specifically, feedback from exercising limbs, chest wall, lung, and airway receptors, and central and/or peripheral chemoreceptors contribute to alterations in ventilation. In decerebrate geese, passive wing movements entrain breathing frequency to wing movements, even when neural feedback from the wings is blocked, and the only intact afferent feedback comes from the chest wall, lungs, and air sacs [67]. Morin and Viala [117] manipulated various parameters using an isolated brainstem–spinal cord preparation from a neonatal rat to further examine the neurogenic basis of LRC. Pharmacological activation of lumbar locomotor-generating networks led to an increase in ventilation; however, it did so only above a threshold locomotor frequency and did not result in any degree of phase coupling. On the other hand, when lumbar peripheral afferents were activated, there was a strict phase locking of LRC that occurred. The authors suggested that there is a direct functional connection between lumbar sensory inputs and higher respiratory centers. Overall, studies on the neural mechanisms of entrainment indicate an integrative response—whereby central feedforward signals from the brain and spinal cord are responsible for the parallel drive of locomotion and respiration, while peripheral afferent feedback serves to mediate or ‘fine-tune’ the response [11, 24, 137].
Implications of LRC
For such a variety of species, over a range of movements to exhibit LRC suggests that LRC may convey some advantages over dyssynchronous locomotion/respiration. In fact, Bramble and Carrier [28] suggested that locomotor–respiratory integration may well be a requirement for sustained aerobic activity among endothermic vertebrates. Attempts to determine what the aforementioned advantage is are not entirely conclusive, but there is evidence that an energetic and/or perceptual benefit to entrainment exists.
As mentioned previously, there is a strong phase locking between wing strokes and respiratory cycles in birds. Funk et al. [68] investigated whether or not this strict phase locking confers an energetic benefit by mechanically ventilating Canadian geese at a rate in-phase or out-of-phase the typical coordinated pattern seen during free flying (i.e., inspiration during upstroke, expiration during downstroke). At the 1:1 wingbeat to breath ratio, the energetic cost (as estimated via pressure–volume loops) of out-of-phase breathing was 26 % higher than in-phase synchronization. Similarly, at the less commonly employed 3:1 ratio, out-of-phase synchronization was still 9 % more costly than in-phase. This energetic saving from reducing mechanical interference between locomotion and ventilation is seen in exercising humans, as well.
Entrainment during cycling exercise, in particular, appears to consistently lower metabolic energy expenditure. While Yonge and Petersen found no difference in submaximal VO2 during entrained versus unentrained cycling [163], the majority of investigations have shown otherwise. Garlando et al. [69] had 30 healthy men and women cycle on an ergometer at 50 % of their maximal work capacity under spontaneous and acoustically triggered breathing conditions. Greater coupling was associated with significantly lower exercising VO2, though with considerable individual variation. Likewise, Villard et al. [155] found that as cycling exercise progressed, LRC became more stable (from a 3:1 to 2:1 ratio), in terms of dynamical systems theory. This increase in LRC stability was accompanied by a decrease in oxygen uptake. However, in a small study of eight healthy females, no decrease in total VO2 was observed with increased conscious entrainment during cycling. Interestingly, though when subjects were separated based upon the change in VO2RM, a significant correlation was observed between total VO2, and degree of entrainment was observed [147]. In other words, any decrease in total VO2 was largely determined by the decrease in VO2RM during entrainment trials. Using a metronome to stabilize LRC during cycling, Hoffman et al. [84] found significant reductions in energy expenditure with greater entrainment. Sixteen male athletes were instructed to synchronize either the breathing frequency or pedaling rate with the sound of a metronome. Under both experimental conditions, the stability of LRC increased and VO2 decreased (by ~4 %), with locomotor frequency tending to drive the respiratory rhythm more so than vice versa.
Examining the relationship between LRC and economy during other modes of exercise has been met with mixed results. Because of the increased vertical displacement (and subsequent movement of abdominal contents) during walking and running as opposed to cycling, as well as the higher incidence of LRC generally observed, a greater energetic benefit of entrainment in these modes could be hypothesized. Similarly, the near-obligatory coordination of locomotion and ventilation during rowing would suggest a certain energetic benefit to LRC. However, the few studies on the economical impact of coupling during walking, running, and rowing do not provide convincing evidence for this relationship. In a study of 16 untrained men, no differences were observed in VO2, while rowing using three distinct breathing patterns: spontaneous, inspire-drive, and inspire-recovery [104]. While walking, coordination between strides and breaths does not appear to impact the oxygen cost of locomotion [126, 150]. Specifically, as walking speed increased from 1 m s−1 (approximately 26 min mile−1) to 1.8 m s−1 (approx. 15 min mile−1), the coordination between locomotion and ventilation significantly increased; however, this increase in LRC was not associated with any change in VO2 [126]. Coordination while walking has also been shown to increase via an auditory cue; but, again, the change in LRC was not accompanied by a concomitant change in VO2 [150]. On the other hand, when Bernasconi et al. had subjects run in a variety of experimental conditions (e.g., speeds, inclines, physical manipulations, and sound cues), the best running economy was observed during the condition of the highest coordination [21]. Quantifying LRC during cycling (as opposed to walking/running) is more methodologically straightforward, and as such, much of the research on human LRC has used this mode of exercise, perhaps leading to more consistent findings. Additionally, the already-low energetic cost of walking and/or relatively slow running likely does not necessitate coupling; in other words, at higher workloads/speeds, energetic considerations are much more important for sustained performance compared to low-moderate intensities.
Alleviation of the respiratory discomfort associated with exercise could be an additional benefit of LRC. Dyspnea, a subjective sensation of uncomfortable and/or effortful breathing, is commonly cited as a factor limiting exercise [136]. A number of disorders can cause resting and exertional dyspnea, including airflow limitation, respiratory restriction, chest wall restriction, cardiac disease, anemia, obesity, psychogenetic disorders, and deconditioning, and with many of these conditions, breathing patterns can be disrupted [158]. The greater existence of dyspnea in lung disease patients who exhibit dyssynchronous breathing was a theoretical basis for entrainment studies examining measures of breathlessness. In the work by Takano et al., untrained cyclists who showed a decrease in VO2RM with entrainment had a corresponding reduced sense of dyspnea during the entrained condition [147]. When untrained individuals were instructed to row on an ergometer utilizing different breathing strategies, there was no effect on ratings of breathing discomfort [102]. However, the two “entrained” strategies in this study required subject to consciously adjust their breathing to different points in an unfamiliar task (i.e., rowing). The concentration required to maintain these unfamiliar coordination patterns likely led to a greater awareness of breathing, thus counterbalancing potential perceptual benefits of entrainment. In contrast, highly trained athletes are “associative” in terms of cognitive strategy during exercise, making them quite aware of changes in physiological measures like breathing [48, 116]. Additionally, because of their extensive training background, these athletes already tend to exhibit a great degree of entrainment. The combination of (a) tendency to display LRC, and (b) associative attentional strategies could make highly trained athletes particularly susceptible to increased dyspnea from locomotor–respiratory uncoupling.
Perturbations to LRC
To understand any concept more fully, it is useful to understand the counterpart to that concept. In the context of this review, understanding of the mechanisms underlying, robustness of, and implications regarding LRC in animals can be bolstered by examining disruptions to LRC. One possible perturbation to LRC is a stimulated increase in ventilation by altering inspired gases or dead space volume. Paterson et al. [121] examined entrainment in two Caucasian and four Nepalese men while running at natural altitudes ranging from 915 to 5030 m, as well as in seven subjects running in a sea-level laboratory breathing various oxygen–nitrogen mixtures. There was a significant inverse relationship between the level of hypoxia and degree of entrainment. The uncoupling appeared to be the result of a hypoxic-induced increase in ventilation/breathing frequency, as SF remained relatively constant throughout the trials. An uncoupling of LRC in hypoxia would indicate that in the hierarchy of inputs to respiratory drive, maintenance of blood gases takes precedence over entrainment of breathing and exercise rhythm. While Fabre et al. [61, 62] found a similar increase in VE and fB in rowers exercising in hypoxia compared to normoxia, the rowers also significantly increased their stroke rate under the hypoxic conditions. The concurrent increases in stroke and breathing frequencies resulted in no change in the SR/fB ratio, as well as consistency in the degree of entrainment. A similar response was seen in swimmers following induction of inspiratory muscle fatigue; both breathing frequency and stroke rate increased in parallel during subsequent swimming bouts [101]. The mechanics of rowing (as well as the synchronization requirements of swimming) are unique from running in that the respiratory musculature must both assist the propulsive force generation and be the effector of ventilation; thus, coordination of breathing and movement is essentially obligatory in order to favorably complete both tasks.
Seebauer et al. [134] also investigated entrainment in hypoxia, measuring coordination between breathing and cycling rhythms in 20 healthy women during moderate-to-vigorous exercise in normoxia and hypoxia. The single level of hypoxia utilized (14.5 % O2) corresponded closely to the intermediate level used in the previous study by [121]. The investigators found no difference in the degree of coordination, calculated as the percentage of breaths that began during the same phase of leg movement, between normoxic and hypoxic conditions. Additionally, there was no significant correlation between resting measures of hypoxic ventilatory responsiveness and LRC in normoxia, hypoxia, or the change from normoxia to hypoxia. At least three possibilities exist to at least partially explain the varying results of Paterson et al. [121] and Seebauer et al. [134]. First, the latter used cycling as the primary mode of exercise; however, LRC appears to be less prevalent during cycling as opposed to other modes of exercise, such as running. Second, the workloads used in the two studies were dissimilar: running at 40 % VO2max vs. cycling at 55, 75, and 95 % VO2max. As Seebauer et al. [134] propose, at lower workloads, both ventilatory and locomotor rhythms have greater degrees of freedom and may be more susceptible to other central and/or peripheral inputs. Certainly, evidence exists to support increasing levels of coordination with higher workloads, and thus the more vigorous workloads in work by Seebauer et al. [134] may have negated the impact of a hypoxic stimulus. Finally, differences exist in hormonal levels/activity between men and women, resulting in well-defined disparities in physiological measures. Another study by the same researchers [133] found evidence to suggest differences between the way in which men and women adjust LRC over a range of exercise intensities. Specifically, degree of entrainment with increasing cycling intensity increases in men, while remaining unchanged in women. Additionally, female limb and cardiac muscle are more resistant to fatigue than that of males; and recently, it has been shown that the same phenomenon is seen with regard to the diaphragm. It is possible that a difference in resistance to diaphragmatic (or other skeletal muscle) fatigue partially explains the difference in LRC patterns with increasing workload and/or hypoxia between men and women. On the other end of the perturbation spectrum, Villard et al. [155] manipulated oxygen transport capabilities of the cardiopulmonary system (using recombinant human erythropoietin) in order to determine if a greater independence between breathing and locomotion would manifest, but found no impact on LRC.
Using a novel task as opposed to whole-body exercise, Hodges et al. [83] challenged the coincident respiratory and postural functions of the diaphragm by observing invasive, diaphragmatic EMG activity while subjects made sustained, rapid arm movements under control and added dead space conditions. Power spectral densities of EMG and ventilation/arm movement frequencies were analyzed to assess the contribution of the diaphragm to ventilatory and postural control, respectively. Activity of the diaphragm associated with movement of the arm (postural control) was attenuated when ventilatory demand (via added dead space volume) was increased. The findings indicate that increased descending command from the pontomedullary respiratory center likely regulate the postural inputs to the phrenic motoneurons, compromising coordination between breathing and posture.
In a similar manner, cognitive tasks appear to mitigate limb–respiratory coordination. Bell and Duffin had subjects that solve a computer-based puzzle, while the respiratory response to passive lower limb movement (70 cycles per minute per leg) was measured [16]. In the control condition, where subjects simply relaxed in a tandem chair while wearing a blindfold and earmuffs, the initiation of passive leg movements resulted in a large, fast increase in breathing frequency, tidal volume, and ventilation. However, during the cognitive task trial, there was only an increase in breathing frequency (which was significantly smaller than that of the control condition) and no change in other ventilatory parameters. The reduced dependence of ventilatory frequency on limb movement frequency with elevated cognitive activity implies that there is competition among the inputs to the respiratory controller. As such, the ventilatory response to specific inputs (for example, afferent feedback) will depend on the state of other cortical inputs [11].
As mentioned previously, high levels of ventilation constitute a substantial amount of respiratory muscle work. Ultimately, increases in the work of breathing will lead to more rapid RMF. Fatigue of the diaphragm, the primary inspiratory muscle, can alter the respiratory muscle recruitment and breathing patterns, which could present an element of interference to LRC. Specifically, RMF tends to cause an overall increase in minute ventilation, achieved via a large increase in breathing frequency, and sometimes accompanied by a decrease in tidal volume. In instances of LRC, ventilation tends to “follow” locomotion more than vice versa. However, ventilation is also clearly the more vital process, and as such its maintenance (in terms of blood flow supply to the respiratory muscles) takes precedence over working limb muscles. Because RMF can occur during high-intensity, sustained exercise, attenuating this fatigue using respiratory muscle training (RMT) has been a subject of much research interest. Within this field of research, the mechanism underlying RMT has not been determined; however, particularly in sports where the respiratory muscles have dual purposes (e.g., rowing, swimming, etc.), maintenance of locomotor–respiratory coordination has been suggested as a potential benefit [110, 156].
There are various ways in which RMF may change the apparent relationship between locomotion and ventilation. An increase in the chemical drive to breathe, with no change in SF, would simply result in an uncoupling of or phase shift in the ratio between the two parameters. For example, if a subject ran at a stride rate of 70 strides min−1 while breathing 35 breaths min−1 (i.e., 2:1 frequency coupling), but increased breathing frequency to 40 breaths min−1 after RMF, he would likely no longer meet the criteria of entrainment. Alternatively, afferent feedback from the respiratory and locomotor muscles could impact both breathing and stride frequencies at the level of the brainstem or spinal CPGs. Finally, if the respiratory muscle metaboreflex exerts its proposed effects, then a cascade of events stemming from increased sympathetic efferent output (i.e., limb vasoconstriction, decreased oxygen transport to the limbs will decrease) could result in decreased central motor drive to the limbs and/or locomotor muscle fatigue. In this particular situation, rather than a change in ventilatory parameters disrupting LRC, an altered locomotor pattern could weaken said coordination. Ultimately, the impact of RMF on LRC is yet unknown, but could have implications for both highly trained endurance athletes and clinical populations in which LRC may be difficult to achieve—for example, conditions of obesity, pulmonary disease, or neuromuscular disorders.
Conclusion
It has been suggested that long-distance endurance was important for the evolution of the human species. Certainly, in other species, high-speed stamina is often necessary for survival. In order to accomplish such feats, it is possible that phenomena such as LRC were developed to minimize costs to the respiratory musculature and/or active skeletal muscle. Ventilation and locomotion are, individually, two quite complex processes; yet, remarkably, these two systems are temporally coordinated in species across the animal kingdom. The relative contributions of mechanical and neural underlying mechanisms to LRC are still being fully elucidated; however, it appears that bipedalism of the human gait attenuates mechanical constraints. Thus, in humans, an integrative neural response of central command and peripheral afferent feedback is primarily responsible for LRC. The control of this synchronization may be similar or related to that of locomotion and the cardiac cycle (cardio-locomotor coupling), respiratory and cardiac cycles (respiratory sinus arrhythmia), and/or other oscillating systems within the body.
Discrepancies in measurement techniques have likely clouded some potentially meaningful findings, but it is certainly conceivable that the widespread prevalence of LRC indicates that it is physiologically important. Potential benefits include improved gas exchange, minimized energetic costs at natural locomotor and/or respiratory frequencies, lowered respiratory muscle work, maximized mechanical assistance of locomotion to ventilation, and lowered perception of effort. If, indeed, LRC provides an energetic or perceptual benefit, then training strategies to increase the degree of, or prolong maintenance of, LRC could be profitable to both endurance athletes and patient populations alike. Continued research using consistent methodologies is needed to further elucidate the robustness and implications of LRC in a variety of study populations.
References
Aaron EA, Seow KC, Johnson BD, Dempsey JA (1992) Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol 72:1818–1825
Alexander R McNeill (1988) Elastic mechanisms in animal movement. Cambridge University Press, Cambridge England; New York
Amann M (2012) Pulmonary system limitations to endurance exercise performance in humans. Exp Physiol 97(3):311–318
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2010) Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109(4):966–976
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2011) Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol Lond 589(21):5299–5309
Anholm JD, Johnson RL, Ramanathan M (1987) Changes in cardiac output during sustained maximal ventilation in humans. J Appl Physiol 63(1):181–187
Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA (1995) Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol 78(5):1710–1719
Bai TR, Rabinovitch BJ, Pardy RL (1984) Near-maximal voluntary hyperpnea and ventilatory muscle function. J Appl Physiol 57(6):1742–1748
Bannister RG, Cunningham DJ, Douglas CG (1954) The carbon dioxide stimulus to breathing in severe exercise. J Physiol 125(1):90–117
Banzett RB, Mead J, Reid MB, Topulos GP (1992) Locomotion in men has no appreciable mechanical effect on breathing. J Appl Physiol 72(5):1922–1926
Bartlett D, Leiter JC (2012) Coordination of breathing with nonrespiratory activities. Compr Physiol 2(2):1387–1415
Baudinette RV, Gannon BJ, Runciman WB, Wells S, Love JB (1987) Do cardiorespiratory frequencies show entrainment with hopping in the Tammar Wallaby. J Exp Biol 129:251–263
Bear Mark F, Connors Barry W, Paradiso Michael A (2007) Neuroscience: exploring the brain, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Bechbache RR, Chow HH, Duffin J, Orsini EC (1979) The effects of hypercapnia, hypoxia, exercise and anxiety on the pattern of breathing in man. J Physiol 293:285–300
Bechbache RR, Duffin J (1977) The entrainment of breathing frequency by exercise rhythm. J Physiol 272(3):553–561
Bell HJ, Duffin J (2004) Respiratory response to passive limb movement is suppressed by a cognitive task. J Appl Physiol 97(6):2112–2120
Bellemare F, Grassino A (1982) Evaluation of human diaphragm fatigue. J Appl Physiol 53(5):1196–1206
Benditt JO (2006) The neuromuscular respiratory system: physiology, pathophysiology, and a respiratory care approach to patients. Respiratory Care 51(8):829–837
Berger M, Hart JS, Roy OZ (1970) Respiration, oxygen consumption and heart rate in some birds during rest and flight. Zeitschrift Fur Vergleichende Physiologie 66(2):201
Bernasconi P, Burki P, Buhrer A, Koller EA, Kohl J (1995) Running training and co-ordination between breathing and running rhythms during aerobic and anaerobic conditions in humans. Eur J Appl Physiol Occup Physiol 70(5):387–393
Bernasconi P, Kohl J (1993) Analysis of co-ordination between breathing and exercise rhythms in man. J Physiol 471:693–706
Berry MJ, Dunn CJ, Pittman CL, Kerr WC, Adair NE (1996) Increased ventilation in runners during running as compared to walking at similar metabolic rates. Eur J Appl Physiol 73(3–4):245–250
Bixler EO, Kales A, Vela-Bueno A, Jacoby JA, Scarone S, Soldatos CR (1982) Nocturnal myoclonus and nocturnal myoclonic activity in the normal population. Res Commun Chem Pathol Pharmacol 36(1):129–140
Boggs DF (2002) Interactions between locomotion and ventilation in tetrapods. Comp Biochem Physiol A 133(2):269–288
Bonsignore MR, Morici G, Abate P, Romano S, Bonsignore G (1998) Ventilation and entrainment of breathing during cycling and running in triathletes. Med Sci Sports Exerc 30(2):239–245
Brackenbury JH, Avery P (1980) Energy-consumption and ventilatory mechanisms in the exercising fowl. Comp Biochem Physiol A 66(3):439–445
Bramble DM (1989) Axial-appendicular dynamics and the integration of breathing and gait in mammals. Am Zool 29:171–186
Bramble DM, Carrier DR (1983) Running and breathing in mammals. Science 219(4582):251–256
Brocard F, Ryczko D, Fenelon K, Hatem R, Gonzales D, Auclair F, Dubuc R (2010) The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J Neurosci 30(2):523–533
Brooks George A (2000) Exercise physiology: human bioenergetics and its applications, 3rd edn. Mayfield Pub, Mountain View
Brown SP, Miller WC, Eason JM (2006) Exercise physiology: basis of human movement in health and disease. Lippincott Williams & Williams, New York
Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B84:308–319
Brown TG (1912) The factors in the rhythmic activity of the nervous system. Proc R Soc Lond B85:278–289
Bushnell T, Hunter I (2007) Differences in technique between sprinters and distance runners at equal and maximal speeds. Sports Biomech 6(3):261–268
Butler RJ, Crowell HP, Davis IM (2003) Lower extremity stiffness: implications for performance and injury. Clin Biomech, 18(6):511–517
Butler PJ, Woakes AJ (1980) Heart-rate, respiratory frequency and wing beat frequency of free flying barnacle geese branta-leucopsis. J Exp Biol 85(Apr):213–226
Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F (2006) Motor patterns in human walking and running. J Neurophysiol 95(6):3426–3437
Carrier DR (1987) Lung ventilation during walking and running in four species of lizards. Exp Biol 47(1):33–42
Carroll JL, Agarwal A (2010) Development of ventilatory control in infants. Paediatr Respir Rev 11(4):199–207
Cathcart EP (1925) The energy of expenditure. Sci Month 21(5):508–510
Cavagna GA, Saibene FP, Margaria R (1964) Mechanical work in running. J Appl Physiol 19:249–256
Cavanagh PR, Kram R (1985) The efficiency of human movement: a statement of the problem. Med Sci Sports Exerc 17(3):304–308
Cavanagh PR, Kram R (1989) Stride length in distance running: velocity, body dimensions, and added mass effects. Med Sci Sports Exerc 21(4):467–479
Cavanagh PR, Pollock ML, Landa J (1977) A biomechanical comparison of elite and good distance runners. Ann N Y Acad Sci 301:328–345
Cavanagh PR, Williams KR (1982) The effect of stride length variation on oxygen uptake during distance running. Med Sci Sports Exerc 14(1):30–35
Coast JR, Clifford PS, Henrich TW, Stray-Gundersen J, Johnson RL Jr (1990) Maximal inspiratory pressure following maximal exercise in trained and untrained subjects. Med Sci Sports Exerc 22(6):811–815
Coast JR, Haverkamp HC, Finkbone CM, Anderson KL, George SO, Herb RA (1999) Alterations in pulmonary function following exercise are not caused by the work of breathing alone. Int J Sports Med 20(7):470–475
Connolly CT, Janelle CM (2003) Attentional strategies in rowing: performance, perceived exertion, and gender considerations. J Appl Sport Psychol 15:195–212
Cooke DW, Thelen E (1987) Newborn stepping: a review of puzzling infant co-ordination. Dev Med Child Neurol 29(3):399–404
Cordain L, Rode EJ, Gotshall RW, Tucker A (1994) Residual lung volume and ventilatory muscle strength changes following maximal and submaximal exercise. Int J Sports Med 15(3):158–161
Dalleau G, Belli A, Bourdin M, Lacour JR (1998) The spring-mass model and the energy cost of treadmill running. Eur J Appl Physiol 77(3):257–263
De Troyer A, Brunko E, Leduc D, Jammes Y (1999) Reflex inhibition of canine inspiratory intercostals by diaphragmatic tension receptors. J Physiol 514(Pt 1):255–263
Dean JC, Kuo AD (2011) Energetic costs of producing muscle work and force in a cyclical human bouncing task. J Appl Physiol 110(4):873–880
Dempsey JA, Adams L, Ainsworth DM, Fregosi RF, Gallagher CG, Guz A, Johnson BD, Powers SK (1996) Airway, lung, and respiratory muscle function during exercise. Handbook of Physiology. Oxford University Press, New York, pp 448–514
Dickinson PS (1995) Interactions among neural networks for behavior. Curr Opin Neurobiol 5(6):792–798
Dickinson PS (2006) Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol 16(6):604–614
Duysens J, Van de Crommert HW (1998) Neural control of locomotion: the central pattern generator from cats to humans. Gait Posture 7(2):131–141
Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG (1985) Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59(3):313–337
Eldridge FL, Millhorn DE, Waldrop TG (1981) Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211(4484):844–846
Enoka RM, Duchateau J (2008) Muscle fatigue: what, why and how it influences muscle function. J Physiol 586(1):11–23
Fabre N, Perrey S, Arbez L, Rouillon JD (2007) Neuro-mechanical and chemical influences on locomotor respiratory coupling in humans. Respir Physiol Neurobiol 155(2):128–136
Fabre N, Perrey S, Passelergue P, Rouillon JD (2007) No influence of hypoxia on coordination between respiratory and locomotor rhythms during rowing at moderate intensity. J Sports Sci Med 6(4):526–531
Farmer CG, Carrier DR (2000) Pelvic aspiration in the American alligator (Alligator mississippiensis). J Exp Biol 203(Pt 11):1679–1687
Fink PW, Foo P, Jirsa VK, Kelso JAS (2000) Local and global stabilization of coordination by sensory information. Exp Brain Res 134(1):9–20
Forssberg H (1985) Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res 57(3):480–493
Frazier DT, Revelette WR (1991) Role of phrenic nerve afferents in the control of breathing. J Appl Physiol 70(2):491–496
Funk GD, Steeves JD, Milsom WK (1992) Coordination of wingbeat and respiration in birds. II. “Fictive” flight. J Appl Physiol 73(3):1025–1033
Funk G, Valenzuela II, Milsom W (1997) Energetic consequences of coordinating wingbeat and respiratory rhythms in birds. J Exp Biol 200(Pt 5):915–920
Garlando F, Kohl J, Koller EA, Pietsch P (1985) Effect of coupling the breathing and cycling rhythms on oxygen uptake during bicycle ergometry. Eur J Appl Physiol Occup Physiol 54(5):497–501
Gerasimenko YP, McKay WB, Pollo FE, Dimitrijevic MR (1996) Stepping movements in paraplegic patients induced by epidural spinal cord stimulation. Soc Neurosci Abstr 22:1372
Gleim GW, Stachenfeld NS, Nicholas JA (1990) The influence of flexibility on the economy of walking and jogging. J Orthop Res, 8(6): 814-823.
Griffin TM, Kram R, Wickler SJ, Hoyt DF (2004) Biomechanical and energetic determinants of the walk-trot transition in horses. J Exp Biol 207(Pt 24):4215–4223
Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B (2008) Neural bases of goal-directed locomotion in vertebrates: an overview. Brain Res Rev 57(1):2–12
Haas F, Distenfeld S, Axen K (1986) Effects of perceived musical rhythm on respiratory pattern. J Appl Physiol 61(3):1185–1191
Halsey LG, White CR (2012) Comparative energetics of mammalian locomotion: humans are not different. J Hum Evol 63(5):718–722
Hamnegard CH, Wragg S, Kyroussis D, Mills GH, Polkey MI, Moran J, Road J, Bake B, Green M, Moxham J (1996) Diaphragm fatigue following maximal ventilation in man. Eur Respir J 9(2):241–247
Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA (1997) Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82(5):1573–1583
Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA (1998) Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 85(2):609–618
Hay James G (1993) The biomechanics of sports techniques, 4th edn. Prentice-Hall, Englewood Cliffs
Heglund NC, Taylor CR, McMahon TA (1974) Scaling stride frequency and gait to animal size: mice to horses. Science, 186(4169):1112–1113
Heise GD, Martin PE (1998) “Leg spring” characteristics and the aerobic demand of running. Med Sci Sports Exerc 30(5):750–754
Hill JM (2000) Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856(1–2):240–244
Hodges PW, Heijnen I, Gandevia SC (2001) Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J Physiol 537(Pt 3):999–1008
Hoffmann CP, Torregrosa G, Bardy BG (2012) Sound stabilizes locomotor-respiratory coupling and reduces energy cost. PLoS ONE 7(9):e45206
Hoyt RW, Knapik JJ, Lanza JF, Jones BH, Staab JS (1994) Ambulatory foot contact monitor to estimate metabolic cost of human locomotion. J Appl Physiol, 76(4): 1818-1822.
Hoyt DF, Taylor CR (1981) Gait and the energetics of locomotion in horses. Nature 292(5820):239–240
Hultborn H, Petersen N, Brownstone P, Nielsen J (1993) Evidence of fictive spinal locomotion in the marmoset (Callithrix jacchus). Soc Neurosci Abstr 19:539
Hunter I, Smith GA (2007) Preferred and optimal stride frequency, stiffness and economy: changes with fatigue during a 1-h high-intensity run. Eur J Appl Physiol 100(6):653–661
Jasinskas CL, Wilson BA, Hoare J (1980) Entrainment of breathing rate to movement frequency during work at two intensities. Respir Physiol 42(3):199–209
Johnson BD, Babcock MA, Suman OE, Dempsey JA (1993) Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460:385–405
Johnson BD, Saupe KW, Dempsey JA (1992) Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73(3):874–886
Kaufman MP, Forster HV (1996) Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT (eds) Handbook of physiology section 12: exercise: regulation and integration of multiple systems. Oxford University Press, New York, pp 381–447
Kay JD, Petersen ES, Vejby-Christensen H (1975) Breathing in man during steady-state exercise on the bicycle at two pedalling frequencies, and during treadmill walking. J Physiol 251(3):645–656
Kelman GR, Watson AW (1973) Effect of added dead-space on pulmonary ventilation during sub-maximal, steady-state exercise. Q J Exp Physiol Cogn Med Sci 58(4):305–313
Kohl J, Koller EA, Jager M (1981) Relation between pedalling and breathing rhythm. Eur J Appl Physiol Occup Physiol 47(3):223–237
Kram R, Taylor CR (1990) Energetics of running: a new perspective. Nature, 346(6281): 265-267.
Krogh A, Lindhard J (1913) The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47(1–2):112–136
Lafortuna CL, Reinach E, Saibene F (1996) The effects of locomotor-respiratory coupling on the pattern of breathing in horses. J Physiol Lond 492(2):587–596
Le Ray D, Juvin L, Ryczko D, Dubuc R (2011) Chapter 4: supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res 188:51–70
Loke J, Mahler DA, Virgulto JA (1982) Respiratory muscle fatigue after marathon running. J Appl Physiol 52(4):821–824
Lomax M, Castle S (2011) Inspiratory muscle fatigue significantly affects breathing frequency, stroke rate, and stroke length during 200-m front-crawl swimming. J Strength Cond Res 25(10):2691–2695
Maclennan SE, Silvestri GA, Ward J, Mahler DA (1994) Does entrained breathing improve the economy of rowing? Med Sci Sports Exerc 26(5):610–614
Mador MJ, Acevedo FA (1991) Effect of respiratory muscle fatigue on breathing pattern during incremental exercise. Am Rev Respir Dis 143(3):462–468
Mador MJ, Acevedo FA (1991) Effect of respiratory muscle fatigue on subsequent exercise performance. J Appl Physiol 70(5):2059–2065
Mahler DA, Hunter B, Lentine T, Ward J (1991) Locomotor-respiratory coupling develops in novice female rowers with training. Med Sci Sports Exerc 23(12):1362–1366
Mahler DA, Shuhart CR, Brew E, Stukel TA (1991) Ventilatory responses and entrainment of breathing during rowing. Med Sci Sports Exerc 23(2):186–192
Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11(23):R986–R996
Martin BJ, Stager JM (1981) Ventilatory endurance in athletes and non-athletes. Med Sci Sports Exerc 13(1):21–26
McCloskey DI, Mitchell JH (1972) Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224(1):173–186
McConnell AK, Romer LM (2004) Respiratory muscle training in healthy humans: resolving the controversy. Int J Sports Med 25(4):284–293
McDermott WJ, Van Emmerik RE, Hamill J (2003) Running training and adaptive strategies of locomotor-respiratory coordination. Eur J Appl Physiol 89(5):435–444
McMahon TA, Valiant G, Frederick EC (1987) Groucho running. J Appl Physiol, 62(6): 2326-2337.
Millhorn DE, Eldridge FL, Waldrop TG, Kiley JP (1987) Diencephalic regulation of respiration and arterial pressure during actual and fictive locomotion in cat. Circ Res 61(4 Pt 2):I53–I59
Mitchell JH, Reardon WC, McCloskey DI (1977) Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol 233(3):H374–H378
Morgan DW, Martin PE (1986) Effects of stride length alteration on racewalking economy. Can J Appl Sport Sci 11(4):211–217
Morgan WP, Pollock ML (1977) Psychologic characterization of the elite distance runner. Ann N Y Acad Sci 301:382–403
Morin D, Viala D (2002) Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci 22(11):4756–4765
Nickerson BG, Keens TG (1982) Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. J Appl Physiol 52(3):768–772
O’Halloran J, Hamill J, McDermott WJ, Remelius JG, Van Emmerik REA (2012) Locomotor-respiratory coupling patterns and oxygen consumption during walking above and below preferred stride frequency. Eur J Appl Physiol 112:929–940
Paterson DJ, Wood GA, Morton AR, Henstridge JD (1986) The entrainment of ventilation frequency to exercise rhythm. Eur J Appl Physiol Occup Physiol 55(5):530–537
Paterson DJ, Wood GA, Marshall RN, Morton AR, Harrison AB (1987) Entrainment of respiratory frequency to exercise rhythm during hypoxia. J Appl Physiol 62:1767–1771
Persegol L, Jordan M, Viala D (1991) Evidence for the entrainment of breathing by locomotor pattern in human. J Physiol (Paris) 85(1):38–43
Persegol L, Jordan M, Viala D, Fernandez C (1988) Evidence for central entrainment of the medullary respiratory pattern by the locomotor pattern in the rabbit. Exp Brain Res 71(1):153–162
Philippson M (1905) L’autonomie et la centralisation dans le systeme nerveux des animaux. Trav Lab Physiol Inst Solvay 7:1–208
Polimanti O (1912) Uber den Beginn der Atmung bei den Embryonen von Scyllium. Z Biol 57:237–251
Rabler B, Kohl J (1996) Analysis of coordination between breathing and walking rhythms in humans. Respir Physiol 106(3):317–327
Remmers JE (1970) Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol 10(3):358–383
Remmers JE, Marttila I (1975) Action of intercostal muscle afferents on the respiratory rhythm of anesthetized cats. Respir Physiol 24(1):31–41
Roberts TJ, Azizi E (2011) Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. J Exp Biol 214(Pt 3):353–361. doi:10.1242/jeb.038588
Rossignol S (1996) Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT (eds) Handbook of physiology, vol 12. American Physiological Society, Oxford, pp 173–216
Satchell GH (1968) A neurological basis for co-ordination of swimming with respiration in fish. Comp Biochem Physiol 27(3):835
Saunders PU, Pyne DB, Telford RD, Hawley JA (2004) Factors affecting running economy in trained distance runners. Sports Med 34(7):465–485
Seebauer M, Sidler MA, Kohl J (2003) Gender differences in workload effect on coordination between breathing and cycling. Med Sci Sports Exerc 35(3):495–499
Seebauer M, Siller T, Kohl J (2003) Influence of hypoxia on coordination between breathing and cycling rhythms in women. Eur J Appl Physiol 89(1):90–94
Selionov VA, Kazennikov OV, Levik YS, Gurfinkel VS (1997) Experimental investigation of locomotor-like movements elicited by vibration in human. In: Paper presented at the International Symposium on Brain and Movement, St. Petersburg, Moscow
Sheel AW (2002) Respiratory muscle training in healthy individuals: physiological rationale and implications for exercise performance. Sports Med 32(9):567–581
Sheel AW, Romer LM (2012) Ventilation and respiratory mechanics. Compr Physiol 2:1093–1142
Sherman MF, Lam T, Sheel AW (2009) Locomotor-respiratory synchronization after body weight supported treadmill training in incomplete tetraplegia: a case report. Spinal Cord 47(12):896–898
Shik ML, Severin FV, Orlovskii GN (1966) Control of walking and running by means of electric stimulation of the midbrain. Biofizika 11(4):659–666
Siegmund GP, Edwards MR, Moore KS, Tiessen DA, Sanderson DJ, McKenzie DC (1999) Ventilation and locomotion coupling in varsity male rowers. J Appl Physiol 87(1):233–242
Simons RS (1999) Running, breathing and visceral motion in the domestic rabbit (Oryctolagus cuniculus): testing visceral displacement hypotheses. J Exp Biol 202(Pt 5):563–577
Sliwinski P, Yan S, Gauthier AP, Macklem PT (1996) Influence of global inspiratory muscle fatigue on breathing during exercise. J Appl Physiol 80(4):1270–1278
Spengler CM, Knopfli-Lenzin C, Birchler K, Trapletti A, Boutellier U (2000) Breathing pattern and exercise endurance time after exhausting cycling or breathing. Eur J Appl Physiol 81(5):368–374
St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA (2000) Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529(Pt 2):493–504
Steinacker JM, Both M, Whipp BJ (1993) Pulmonary mechanics and entrainment of respiration and stroke rate during rowing. Int J Sports Med 14:S15–S19
Takano N (1995) Phase relation and breathing pattern during locomotor/respiratory coupling in uphill and downhill running. Jpn J Physiol 45(1):47–58
Takano N, Deguchi H (1997) Sensation of breathlessness and respiratory oxygen cost during cycle exercise with and without conscious entrainment of the breathing rhythm. Eur J Appl Physiol Occup Physiol 76(3):209–213
Taylor CR (1978) Why change gaits: recruitment of muscles and muscle-fibers as a function of speed and gait. Am Zool 18(1):153–161
Thelen E, Cooke DW (1987) Relationship between newborn stepping and later walking: a new interpretation. Dev Med Child Neurol 29(3):380–393
van Alphen J, Duffin J (1994) Entrained breathing and oxygen consumption during treadmill walking. Can J Appl Physiol 19(4):432–440
Van de Crommert HWAA, Mulder T, Duysens J (1998) Neural control of locomotion: sensory control of the central pattern generator and its relation to treadmill training. Gait Posture 7(3):251–263
Vaughan CL (1984) Biomechanics of running gait. Crit Rev Biomed Eng 12(1):1–48
Verges S, Notter D, Spengler CM (2006) Influence of diaphragm and rib cage muscle fatigue on breathing during endurance exercise. Respir Physiol Neurobiol 154(3):431–442
Vilensky JA, Moore AM, Eidelberg E, Walden JG (1992) Recovery of locomotion in monkeys with spinal-cord lesions. J Mot Behav 24(3):288–296
Villard S, Casties JF, Mottet D (2005) Dynamic stability of locomotor respiratory coupling during cycling in humans. Neurosci Lett 383(3):333–338
Volianitis S, McConnell AK, Koutedakis Y, McNaughton L, Backx K, Jones DA (2001) Inspiratory muscle training improves rowing performance. Med Sci Sports Exerc 33(5):803–809
Von Holst E (1937) Vom Wesen der Ordnung im Zentralnervensystem. Naturwissenschaften 40:641–647
Wasserman K (1982) Dyspnea on exertion. Is it the heart or the lungs? JAMA 248(16):2039–2043
West John B (2005) Respiratory physiology: the essentials, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Weyand PG, Sternlight DB, Bellizzi MJ, Wright S (2000) Faster top running speeds are achieved with greater ground forces not more rapid leg movements. J Appl Physiol 89(5):1991–1999
Williams KR, Cavanagh PR (1987) Relationship between distance running mechanics, running economy, and performance. J Appl Physiol 63(3):1236–1245
Yokota T, Hirose K, Tanabe H, Tsukagoshi H (1991) Sleep-related periodic leg movements (nocturnal myoclonus) due to spinal cord lesion. J Neurol Sci 104(1):13–18
Yonge RP, Petersen ES (1983) Entrainment of breathing in rhythmic exercise. In: Whipp BJ, Wiberg DM (eds) Modelling and control of breathing. Elsevier Science, New York, pp 197–203
Young IS, Alexander RM, Woakes AJ, Butler PJ, Anderson L (1992) The synchronization of ventilation and locomotion in horses (Equus caballus). J Exp Biol 166:19–31
Author information
Authors and Affiliations
Corresponding author
Additional information
Endorsed by Robert F. Chapman, PhD.
Rights and permissions
About this article
Cite this article
Stickford, A.S.L., Stickford, J.L. Ventilation and Locomotion in Humans: Mechanisms, Implications, and Perturbations to the Coupling of These Two Rhythms. Springer Science Reviews 2, 95–118 (2014). https://doi.org/10.1007/s40362-014-0020-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40362-014-0020-4