Abstract
Purpose
The present review aims to discuss the role of the brain 18F-FDG–PET and 18F-FDG–PET/CT (FDG–PET) in diagnosis and follow-up of the autoimmune encephalitis (AE) patients, highlighting the main findings and the new perspectives on use of these methods in the study of the disease.
Methods
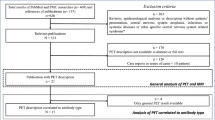
The literature search was performed in the following databases: PubMed/MEDLINE, Scopus, Web of Science, Embase, and Google Scholar, according to the PRISMA statement. The main terms of search were: “autoimmune encephalitis” AND “18F-FDG–PET OR 18F-FDG–PET/CT”, or the combination between the term “18F-FDG–PET” OR “18F-FDG–PET/CT” AND the antibodies receptors abbreviations (e.g., “NMDA”, “VGKC”, etc.). The methodological quality of the publications was assessed according to the QUADAS-2 criteria.
Results
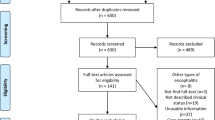
The search of the articles found 56 main articles. These articles encompassed 1,462 patients with AE positive antibodies, from which 808 had brain FDG–PET images with 714 (88.67%) showing alterations. Furthermore, some AE antibodies have specific metabolic signatures, detected in the images, which are discussed in the text. Moreover, patients at different stages of the disease may present different brain metabolic patterns. The areas of more common hypermetabolism were basal ganglia, hippocampus, amygdala, and cerebellum. The areas of more common hypometabolism were the visual cortex and a diffuse cortical metabolism.
Conclusions
This extensive literature review shows the high sensitivity of FDG–PET and FDG–PET/CT in patients with AE. FDG–PET detects findings of hyper and hypometabolism which are suggestive of AE. Besides, AE caused by the different antibodies may present specific alterations which may be suggestive of each one. However, more prospective studies are necessary for these images become a standard diagnostic method of AE.







Similar content being viewed by others
Data availability
Not applicable.
Change history
28 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40336-023-00592-2
References
Oppenheim H (1888) Über Hirnsymptome bei Carcinomatose ohne nachweisbare Veränderungen im Gehirn. Charité-Annalen (Berlin) 13:335–344
Brierley JB, Corsellis JAN, Hierons R, Nevin S (1960) subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 83(3):357–368
Corsellis JAN, Goldberg GJ, Norton AR (1968) “Limbic encephalitis” and its association with carcinoma. Brain 91(3):481–496
Machado S, Pinto A, Irani S (2012) What should you know about limbic encephalitis? Arq Neuropsiquiatr 70(10):817–822
Russel D (1961) Encephalomyelitis and carcinomatous neuropathy. In: van Bogaert LRJ, Hozay J, Lowenthal, (eds) The encephalitidies. Elsevier, Amsterdam
Wilkinson P (1964) Serological findings in carcinomatous neuromyophathy. Lancet London 1:7346
Trotter J, Hendin B, Osterland C (1976) Cerebellar degeneration with hodgkin disease an immunological study. Archives Neurol. 33(9):660
Graus F, Cordon-Cardo C, Posner J (1985) Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology 35(4):22
Patel A, Meng Y, Najjar A, Lado F, Najjar S (2022) Autoimmune encephalitis: a Physician’s guide to the clinical spectrum diagnosis and management. Brain Sci 12(9):1130
Graus F, Titulaer M, Balu R, Benseler S, Bien C, Cellucci T et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi J, Voloschin A et al (2007) Paraneoplastic anti-N-Methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Anna Neurol. 61(1):25
Hughes E, Peng X, Gleichman A, Lai M, Zhou L, Tsou R et al (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci off J Soci Neurosci. 30(17):5866
Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J et al (2010) Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 9(1):67
Irani S, Michell A, Lang B, Pettingill P, Waters P, Johnson M et al (2011) Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 69(5):892–900
Lai M, Huijbers M, Lancaster E, Graus F, Bataller L, Balice-Gordon R et al (2010) Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 9(8):776
Vincent A, Buckley C, Schott J, Baker I, Dewar B, Detert N et al (2004) Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. J Neurol 127(3):33
Irani S, Pettingill P, Kleopa K, Schiza N, Waters P, Mazia C et al (2012) Morvan syndrome: clinical and serological observations in 29 Cases. Anna Neurol. 72(2):214
Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P et al (2001) Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain J Neurol. 124(12):2417
Lai M, Hughes E, Peng X, Zhou L, Gleichman A, Shu H et al (2009) AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Annals Neurol. 65(4):424
Dale R, Merheb V, Pillai S, Wang D, Cantrill L, Murphy T et al (2012) Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain J Neurol. 135(11):3453
Boronat A, Gelfand J, Gresa-Arribas N, Jeong H, Walsh M, Roberts K et al (2013) Encephalitis and antibodies to dipeptidyl-Peptidase-Like protein-6, a Subunit of Kv42 potassium channels. Annals Neurol. 73(1):120–128
Tobin W, Lennon V, Komorowski L, Probst C, Clardy S, Aksamit A et al (2014) DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 83(20):1797–1803
Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blöcker I et al (2014) progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 82(17):1521–1528
Lancaster E, Dalmau J (2012) Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 8(7):380–390
Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R et al (2008) Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain Journal Neurol 131(Pt 10):2553–2663
Hutchinson M, Waters P, McHugh J, Gorman G, O’Riordan S, Connolly S et al (2008) Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 71(16):1291–1302
McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto J, Harvey R, McEvoy K et al (2013) Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol 70(1):44–50
Carvajal-González A, Leite M, Waters P, Woodhall M, Coutinho E, Balint B et al (2014) Glycine receptor antibodies in PERM and Related syndromes: characteristics, clinical features and outcomes. Brain J Neurol 137(Pt 8):2178–2192
Wuerfel E, Bien C, Vincent A, Woodhall M, Brockmann K (2014) Glycine receptor antibodies in a boy with focal epilepsy and episodic behavioral disorder. J Neurol Sci 343(1–2):180–182
Borges-Rosa J, Oliveira-Santos M, Silva R, Gomes A, de Almeida J, Costa G et al (2022) [18 F]FDG-PET in cardiac sarcoidosis: a single-centre study in a southern European population. Int J cardiol 347:22
Rodríguez-Alfonso B, Ruiz Solís S, Silva-Hernández L, Pintos Pascual I, Aguado Ibáñez S, Salas AC (2021) 18 F-FDG-PET/CT in SARS-CoV-2 Infection and its Sequelae. Revista Espanola de Med Nucl Imagen Mol. 40(5):299
Rosen R, Fayad L, Wahl R (2006) Increased 18F-FDG Uptake in degenerative disease of the spine: characterization with 18F-FDG PET/CT. J Nucl Med offi Publ Soc Nucl Med. 47(8):3
Tang Y, Liow JS, Zhang Z, Li J, Long T, Li Y et al (2018) the evaluation of dynamic FDG-PET for detecting epileptic foci and analyzing reduced glucose phosphorylation in refractory epilepsy. Front Neurosci 12:993
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E et al (2017) Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 4:352
Zupanc M, Handler E, Levine R, Jahn T, ZuRhein G, Rozental J et al (1990) Rasmussen encephalitis: epilepsia partialis continua secondary to chronic encephalitis. Pediat neurol. 6(6):397
Bernsen R, Jong B (1997) Limbic encephalitis, specifically depicted by PET - Bernsen 1997 European Journal of Neurology Wiley Online Library. Eur J Neurol 4:507–511
Provenzale J, Barboriak D, Coleman R (1998) Limbic encephalitis: comparison of FDG PET and MR imaging findings. AJR Amer J Roentgenol. 170(6):1659
Fakhoury T, Abou-Khalil B, Kesller RM (1999) Limbic encephalitis and hyperactive foci on PET scan. Seizure 8:427–430
Fiorella D, Provenzale J, Coleman R, Crain B, Al-Sugair A (2001) (18)F-fluorodeoxyglucose positron emission tomography and MR imaging findings in Rasmussen encephalitis. Amer J Neuroradiol. 22(7):2
McInnes M, Moher D, Thombs B, McGrath T, Bossuyt P, Clifford T et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 319(4):388–396
Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma J et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Lagarde S, Lepine A, Caietta E, Pelletier F, Boucraut J, Chabrol B et al (2016) Cerebral (18)FluoroDeoxy-Glucose positron emission tomography in paediatric anti n-methyl-d-aspartate receptor encephalitis: a case series. Brain develop 38(5):461
Wegner F, Wilke F, Raab P, Tayeb SB, Boeck A-L, Haense C et al (2014) Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol. https://doi.org/10.1186/1471-2377-14-136
Park S, Choi H, Cheon G, Wook Kang K, Lee D (2015) 18F-FDG PET/CT in anti-LGI1 encephalitis: initial and follow-up findings. Clin Nucl Med 40(2):156
Celicanin M, Blaabjerg M, Maersk-Moller C, Beniczky S, Marner L, Thomsen C et al (2017) Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies - a national cohort study. Eur J Neurol 24(8):999–1005
Zhao X (2019) The different metabolic patterns of brain 18F-FDG PET in anti-NMDA, anti-LGi-1 and anti-GABAb encephalitis. J Nucl Med 60(1):1475
Probasco J, Solnes L, Nalluri A, Cohen J, Jones K, Zan E et al (2017) Decreased occipital lobe metabolism by FDG-PET/CT: An anti-NMDA receptor encephalitis biomarker. Neurol Neuroimmunol Neuroinflam. 5(1):413
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T et al (2012) Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol, Neurosurgand psychiat. 83(7):681
Fisher R, Patel N, Lai E, Schulz P (2012) Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clinical Nucl Med. 37(9):213
Baumgartner A, Rauer S, Mader I, Meyer P (2013) Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 260(11):2744
Sarkis R, Nehme R, Chemali Z (2014) Neuropsychiatric and seizure outcomes in nonparaneoplastic autoimmune limbic encephalitis. Epilepsy Behav 39:21–25
Turpin S, Martineau P, Levasseur M-A, Meijer I, Décarie J-C, Barsalou J et al (2019) 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imag 46(6):1309–1324
Yuan J, Guan H, Zhou X, Niu N, Li F, Cui L et al (2016) Changing Brain Metabolism Patterns in Patients With ANMDARE: Serial 18F-FDG PET/CT Findings. Clin Nucl Med 41(5):366–370
Qian C (2017) 18F FDG-PET features in anti-NMDA receptor encephalitis. J Nucl Med 58(1):223
Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A et al (2017) Diagnostic Value of 18F-FDG PET/CT Versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med 58:1307
Tripathi M, Tripathi M, Roy S, Parida G, Ihtisham K, Dash D et al (2018) Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology 60(2):1307
Strohm T, Steriade C, Wu G, Hantus S, Rae-Grant A, Larvie M (2019) FDG-PET and MRI in the evolution of new-onset refractory status epilepticus. Am J Neuroradiol 40(2):238–244
Ge J, Deng B, Guan Y, Bao W, Wu P, Chen X et al (2021) Distinct cerebral 18 F-FDG PET metabolic patterns in anti-N-methyl-D-aspartate receptor encephalitis patients with different trigger factors. Therap Adv Neurol Dis. 14:1756
Nissen M, Ørvik M, Nilsson A, Ryding M, Lydolph M, Blaabjerg M (2021) NMDA-receptor encephalitis in Denmark from 2009 to 2019: a national cohort study. J Neurol 269:1618
Moreno-Estébanez A, Durán SB, Bilbao MM, Díaz-Cuervo I, Agirre-Beitia G, Martínez LC et al (2021) Autoimmune encephalitis and related disorders: a retrospective study of 43 cases in a tertiary hospital. Neurol Perspect 1(4):197–205
Solimena M, Folli F, Denis-Donini S, Comi G, Pozza G, De Camilli P et al (1988) Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. New Engl J Med. 318(16):1012
Solimena M, Folli F (1988) Stiff-man syndrome and type I diabetes mellitus: a common autoimmune pathogenesis? Annali dellIstituto superiore di sanita. 24(4):583
Kim T, Lee S, Shin J, Moon J, Lim J, Byun J et al (2014) Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol 270(1–2):45–50
Zhu F, Shan W, Lv R, Li Z, Wang Q (2020) Clinical characteristics of Anti-GABA-B receptor encephalitis. Frontiers Neurol. https://doi.org/10.3389/fneur.2020.00403
Shen K, Xu Y, Guan H, Zhong W, Chen M, Zhao J et al (2018) Paraneoplastic limbic encephalitis associated with lung cancer. Sci Rep 8(1):2
Steriade C, Moosa A, Hantus S, Prayson R, Alexopoulos A, Rae-Grant A (2018) Electroclinical features of seizures associated with autoimmune encephalitis. Seizure. 60:22
Wen X, Wang B, Wang C, Han C, Guo S (2021) A retrospective study of patients with GABA B R encephalitis: therapy, disease activity and prognostic factors. Neuropsychiatr Dis Treat 17:99–110
van Coevorden-Hameete MH, de Bruijn MA, de Graaff E, Bastiaansen DA, Schreurs MW, Demmers JA et al (2019) The expanded clinical spectrum of anti-GABABR encephalitis and added value of KCTD16 autoantibodies. Brain 142(6):1631–1643
Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B et al (2004) Clinical analysis of anti-Ma2-associated encephalitis. Brain 127(8):1831–1844
Linke R, Schroeder M, Helmberger T, Voltz R (2004) Antibody-positive paraneoplastic neurologic syndromes: value of CT and PET for tumor diagnosis. Neurology 63(2):282–286
De Leiris N, Ruel B, Vervandier J, Boucraut J, Grimaldi S, Horowitz T et al (2021) Decrease in the cortex/striatum metabolic ratio on [18 F]-FDG PET: a biomarker of autoimmune encephalitis. Europ J Nucl Med Mol Imag. 221:223
Wang Y, Sadaghiani M, Tian F, Fitzgerald K, Solnes L, Newsome S (2021) Brain and muscle metabolic changes by FDG-PET in stiff person syndrome spectrum disorders. Front Neurol. https://doi.org/10.3389/fneur.2021.692240
Darnell R, Posner J (2006) Paraneoplastic syndromes affecting the nervous system. Semi Oncol. 33(3):270
Crimì F, Camporese G, Lacognata C, Fanelli G, Cecchin D, Zoccarato M (2018) Ovarian Teratoma or Uterine Malformation? PET/MRI as a Novel Useful Tool in NMDAR Encephalitis. In vivo (Athens, Greece) 32(5):1231–1233
Zaborowski M, Spaczynski M, Nowak-Markwitz E, Michalak S (2015) Paraneoplastic neurological syndromes associated with ovarian tumors. J Cancer Res Clin Oncol 141(1):99
Aydos U, Arhan E, Akdemir Ü, Akbaş Y, Aydin K, Atay L et al (2020) Utility of brain fluorodeoxyglucose PET in children with possible autoimmune encephalitis. Nucl Med Commun 41(8):800
Kerik-Rotenberg N, Diaz-Meneses I, Hernandez-Ramirez R, Muñoz-Casillas R, Reynoso-Mejia C, Flores-Rivera J et al (2020) A metabolic brain pattern associated with Anti-N-Methyl-D-aspartate receptor encephalitis. Psychosomatics 61(1):39
Moreno-Ajona D, Prieto E, Grisanti F, Esparragosa I, Orduz LS, Pérez-Larraya JG et al (2020) 18F-FDG-PET imaging patterns in autoimmune encephalitis: impact of image analysis on the results. Diagnostics 10(6):356
Chen C, Wang X, Zhang C, Cui T, Shi W, Guan H et al (2017) Seizure semiology in leucine-rich glioma-inactivated protein 1 antibody-associated limbic encephalitis. Epilepsy Behav 77:90
Dodich A, Cerami C, Iannaccone S, Marcone A, Alongi P, Crespi C et al (2016) Neuropsychological and FDG-PET profiles in VGKC autoimmune limbic encephalitis. Brain Cogn 108:81
Lv R, Pan J, Zhou G, Wang Q, Shao X, Zhao X et al (2019) Semi-quantitative FDG-PET analysis increases the sensitivity compared with visual analysis in the diagnosis of autoimmune encephalitis. Front Neurol. https://doi.org/10.3389/fneur.2019.00576
Liu X, Shan W, Zhao X, Ren J, Ren G, Chen C et al (2020) The clinical value of 18 F-FDG-PET in autoimmune encephalitis associated with LGI1 antibody. Front Neurol. https://doi.org/10.3389/fneur.2020.00418
Deuschl C, Rüber T, Ernst L, Fendler W, Kirchner J, Mönninghoff C et al (2020) 18F-FDG-PET/MRI in the diagnostic work-up of limbic encephalitis. PLoS ONE 15(1):2279
Malter M, Helmstaedter C, Urbach H, Vincent A, Bien C (2010) Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Anna Neurol. 67(4):470
Newey C, Sarwal A, Hantus S (2016) [(18)F]-Fluoro-Deoxy-glucose positron emission tomography scan should be obtained early in cases of autoimmune encephalitis. Auto Dis. 2016:1
Ances B, Vitaliani R, Taylor R, Liebeskind D, Voloschin A, Houghton D et al (2005) Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain J Neurol. 128(8):1764
Flanagan EP, Kotsenas AL, Britton W et al (2015) Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol-Neuroimmunol Neuroinflam. 2:6
Yin Y, Wu J, Wu S, Chen S, Cheng W, Li L, et al. 2021 Usefulness of brain FDG PET/CT imaging in pediatric patients with suspected autoimmune encephalitis from a prospective study. European Journal of nuclear medicine and molecular imaging.
Jang Y, Lee S, Bae J, Kim T, Jun J, Moon J et al (2018) LGI1 expression and human brain asymmetry: insights from patients with LGI1-antibody encephalitis. J Neuroinfl. https://doi.org/10.1186/s12974-018-1314-2
Day GS, Gordon BA, Jackson K, Christensen JJ, Rosana Ponisio M, Su Y et al (2017) Tau-PET binding distinguishes patients with early-stage posterior cortical atrophy from amnestic alzheimer disease dementia. Alzheimer Dis Assoc Disord 31(2):87–93
Day G, Gordon B, McCullough A, Bucelli R, Perrin R, Bezinger T et al (2021) Flortaucipir (tau) PET in LGI1 antibody encephalitis-Day-2021-annals of clinical and translational neurology - wiley online library. Anna Clin Translat Neurol 8:491–497
McGinnity C, Koepp M, Hammers A, Riaño Barros D, Pressler R, Luthra S et al (2015) NMDA receptor binding in focal epilepsies. J Neurol Neurosurg Psychiatry 86:1150–1157
Harada R, Hayakawa Y, Ezura M, Lerdsirisuk P, Du Y, Ishikawa Y et al (2021) 18F-SMBT-1: A selective and reversible PET tracer for monoamine oxidase-B imaging. J Nucl Med 62:253
Neelamegam R, Kumar D (2021) Automated radiosynthesis and in vivo PET evaluation of VEGFR2 ligand [11C]BTFP. J Nucl Med 62:1205
Acknowledgements
The authors would like to thank the whole team of the Nuclear Medicine Division of the Clinical Hospital—UNICAMP, the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), and the Department of Neurology—Campinas Medical School – UNICAMP.
Funding
None.
Author information
Authors and Affiliations
Contributions
Literature search and review, manuscript writing, meta-analysis and content planning, manuscript writing and editing (journal suggestion): BS, AE. Study conception and design: BS, AW. Acquisition of data: BSW. Analysis and interpretation of data: BS. Drafting of manuscript: BS, AE. Critical revision: EAC. Approval of the final version: BS, AWE, Cendes.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the affiliation 6 for Mauricio Baldissin was missing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baldissin, M.M., de Souza, E.M., Watanabe, N. et al. FDG–PET in patients with autoimmune encephalitis: a review of findings and new perspectives. Clin Transl Imaging 12, 15–30 (2024). https://doi.org/10.1007/s40336-023-00581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-023-00581-5