Abstract
Radical prostatectomy (RP) with or without pelvic lymph node dissection (PLND) is the most frequent approach to treat men affected by prostate cancer (PCa), together with primary radiotherapy. Generally, patients performed computed tomography (CT) and bone scintigraphy to stage the disease prior to surgery. However, specific inhibitors directed to the prostate-specific membrane antigen (PSMA) have been recently proposed as radiopharmaceutical for positron emission tomography (PET) imaging. PSMA-PET proved higher diagnostic accuracy to stage high-risk PCa compared to conventional imaging, even if its impact on overall survival is yet to be confirmed. One of the main limitations for PSMA-PET in staging PCa is represented by the low sensitivity in identifying metastatic lymph node, namely in case of nodes smaller than 4–5 mm. Radioguided surgery (RGS) is based on the intraoperative detection of radiation emitted by the specific radiopharmaceutical. Recently, the possibility of performing RGS using cancer-specific radiotracer with high diagnostic accuracy (e.g. PSMA inhibitors) gained attention. In this review, we aimed to explore the value of PSMA-RGS in PCa, aimed at improving the surgery accuracy to remove nodal metastasis. Furthermore, we evaluated different radiation detectors (gamma rays probes vs. beta positron probes) and the diagnostic accuracy of these probes compared to PSMA-PET. A comprehensive literature review was performed in December 2022 with a non-systematic approach. After the first literature screening, a total of 16 studies have been selected and a comprehensive qualitative narrative synthesis of the articles has been performed. First studies showed promising results for PSMA-RGS, and prospective trials demonstrated good concordance of in vivo PSMA-positive detected nodes with histopathology analysis of the specimens. High sensitivity and specificity of the RGS approach were found. Whilst gamma probes have been more broadly explored, the clinical use of beta probes has been tested in feasibility studies only. Finally, Cerenkov luminescence imaging, micro-image guidance and augmented reality/virtual reality approaches in surgery are currently rising attention and are generating future perspectives for PSMA-guided surgery in PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background: in vivo PSMA-PET imaging in prostate cancer
PCa is the most frequent malignancy in men, and it is still considered one of the three “big killers” together with lung cancer and breast cancer. The main curative treatment for PCa is represented by radical prostatectomy (RP) with or without pelvic lymphadenectomy (PLND) and primary radiotherapy. Nevertheless, 30–40% of patients might experience disease recurrence after radical treatment. Recently, the prostate-specific membrane antigen (PSMA) was proposed as molecular target to specifically identify PCa cells, and small peptides inhibitors have been developed as radiopharmaceuticals for positron emission tomography (PET) imaging. PSMA-PET proved higher diagnostic accuracy to stage high-risk PCa compared to conventional imaging, namely, to correctly identify metastatic lymph nodes [1]. Basically, PSMA-PET identifies more lesions and, subsequently, gives a more accurate evaluation of disease burden. However, the impact of PSMA-PET on overall survival is yet to be evaluated. Thus, at present, it is not well established if performing PSMA-PET prior to radical treatment will have a positive impact on cancer-specific mortality [1,2,3,4]. Nevertheless, the impact of PSMA-PET on clinical decision-making process is not negligible and PSMA-PET is currently considered a game changer in men affected by PCa. Finally, PSMA can be used also as theranostics agent, as PSMA-based radio-ligand therapy already proved its efficacy in reducing the risk of death and the radiological disease progression in metastatic castration-resistant PCa (Table 1).
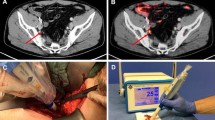
One of the main limitations for PSMA-PET in staging PCa is represented by the low sensitivity in identifying metastatic lymph nodes. In studies using histopathology as standard-of-truth, the sensitivity ranged from 40 to 60% [2, 5], and the presence of metastatic lymph nodes smaller than 4–5 mm represents the main drawback as PET scanner cannot discriminate PSMA-positive nodes from the background. The availability of a technique able to correctly identify metastatic lymph nodes might have the potential to improve surgery performance and clinical outcomes. However, the intraoperative identification and removal of metastatic lymph nodes is really challenging, because these lesions might be atypically located, or too small to be removed, or even morphologically undetectable (Fig. 1).
A patient with high-risk PCa (Gleason score 4 + 5, initial PSA = 8 ng/ml), enrolled in a phase II trial currently ongoing at our institution, underwent a 68 Ga-PSMA-11 whole-body PET/CT scan for the initial staging of the disease prior to RALP. PSMA-PET showed pathological and heterogeneous uptake in the whole prostate, with suspicion of extra-capsular extension, two PSMA-positive left obturator lymph nodes (A, red square) and one PSMA-positive extra-pelvic lymph node. The patient was referred to radical prostatectomy with extended PLND with a PSMA-RGS approach. After the intravenous injection of 90 MBq of 68 Ga-PSMA-11 administered in the surgery theatre, a DROP-IN beta probe was used during surgery (B). A TBR cut-off algorithm was implemented to establish a positivity criterion for the detection of PSMA-positive lymph nodes. Ex vivo measurements were made to cross-validate in vivo findings (C). Histopathological analysis and PSMA staining confirmed the presence of prostate cancer cells in all surgical specimens (D)
Radio guided surgery (RGS) is a surgery procedure mostly based on the intraoperative injection of specific radiopharmaceuticals and the detection of the emitted radiation by dedicated intraoperative probes. Recently, the possibility of performing RGS using cancer-specific radiotracer with high diagnostic accuracy (e.g. PSMA inhibitors) gained attention. However, RGS is still size dependent and the reduced amount of radiopharmaceutical uptake in micro-metastases leads to an insufficient signal-to-background ratio to be detected by specific probes. PSMA-RGS can be performed with gamma-ray probes, detecting single photon emitting isotopes (e.g. Technetium-99 m or Indium-111 labelled with PSMA inhibitors) or with beta-ray probes, detecting the radiation emitted by routinely used PET radiopharmaceuticals (e.g. Gallium-68 or Fluorine-18 labelled with PSMA inhibitors).
In this narrative review, we aimed to explore the value of PSMA-RGS in PCa and to assess if this novel approach might improve surgery accuracy in removing metastatic lymph nodes. A comprehensive critical literature review was performed, focussing the attention also to analyse different detectors and future technological perspectives.
Background: technical aspects and development of gamma and beta probes for radioguided surgery
RGS is based on intraoperative detection of radiation emitted by the radiopharmaceutical, detected by a probe made of scintillating materials. When a photon crosses such materials, it interacts with its components, releasing energy to electrons. This energy is then converted into scintillating light, detected by dedicated instruments like photo multipliers tubes (PMT) or silicon photo multipliers (SiPM). The amount of scintillating light is proportional to the amount of energy deposited in the detector by the original particle and can be used to estimate the flux of particles that impinges on it. However, probes perform differently depending on the injected radiopharmaceutical, and its proper emission (gamma vs. beta emitters) [6]. At present, the majority of RGS applications are based on the intraoperative detection of gamma particles (photons), as those emitted by the commonly used Technetium-99 m (99mTc), emitting gamma rays at 140 keV. To detect this kind of particles, inorganic scintillating materials are typically used. In fact, being electrically neutral, photons tend to interact weakly with matter, and high-density materials are, thus, needed to maximise this interaction. Similarly, also gamma probes for RGS are typically composed by an inorganic scintillating crystal. The active area is covered by lateral shielding that ensures directionality in the probe sensitive area. Consequently, gamma probes typically result to be heavy instruments, whose lateral dimension cannot be too reduced, in order not to jeopardise their efficiency. These probes can be used also with annihilation photons when performing RGS with PET isotopes. However, the higher energy of these beta photons (511 keV) requires a different, more extensive collimation of the probe, making the detector even more cumbersome, and limit its application in minimally invasive surgery.
Recently, the possibility of performing RGS with beta-emitting radioisotopes gained attention, namely considering the high diagnostic accuracy of PSMA inhibitors used for PET imaging. The detection of beta radiation presents some peculiarities, due to the different interaction of this kind of particles with matter [7]. Beta particles tend to interact continuously with matter, experiencing abundant scattering, and thus resulting in a tortuous path. As a result, small active volumes are typically sufficient to absorb, and thus detect these particles. The use of inorganic scintillators is not optimal due to their high density that limits the detection of beta and gamma particles and the clinical application of these probes for RGS in clinical practise. Therefore, organic scintillating materials have been developed [8], having low density (and low detection of photons) and very high sensitivity for beta radiation. Moreover, considering the short penetration range of beta radiation, minimal lateral shielding is needed, resulting in small dimensions devices.
Evidence acquisition
This critical review aimed to explore the value of PSMA-RGS in PCa and if this novel approach might improve the surgery accuracy in removing nodal metastasis. Furthermore, we evaluated different radiation detectors (gamma-ray probes vs. beta positron probes) and the diagnostic accuracy of these probes compared to PSMA-PET.
A comprehensive literature review was performed in December 2022 with a non-systematic approach. The search was performed using the Ovid platform and a comparison of the Embase and Medline databases, using the following string: (“prostate specific membrane antigen” OR “PSMA”) AND (“Radio-guided surgery” OR “radio guided surgery” or RGS or “live surgery”) AND (“prostate cancer” OR PCa). FM, LM, GZ performed the literature research. Disagreements have been resolved by consensus. All the original articles published in English over the last 10 years were considered. Retrospective and prospective series, as well as randomised and non-randomised clinical trials have been considered. Abstract, narrative review, case reports or case series, editorials and letter to editors have been excluded. For clinical studies, all PSMA radiopharmaceuticals were considered; the most frequent PET tracer used was 68 Ga-PSMA-11, followed by 18F-DCFPyL and 18F-PSMA-1007. The literature search was updated until December 2022. After the first literature screening, a total of 16 studies have been selected. Authors tabulated and organised relevant studies and performed a comprehensive qualitative narrative synthesis of both tabulated studies and non-tabulated articles.
Evidence summary
RGS with single photon emitter PSMA radiopharmaceuticals
The first experiences with PSMA-RGS were made with gamma-emitting radionuclides such as Indium-111 (111In) (E γ = 171.3, 245.4 keV). However, the sub-optimal nuclear properties of 111In, the high cost of the isotopes and limited availability of 111InCl3, the relatively poor SPECT quality, and the high burden of radiation, sensibly limited the clinical application of this surgery approach. Consequently, PSMA-RGS quickly moved towards PSMA inhibitors labelled with Technetium-99 m (99mTc) (E γ = 140.5 keV) [9].
The 99mTc-PSMA-RGS procedure involves several steps. Radiotracer is injected intravenously the day before the surgery. Subsequently, SPECT/CT imaging is performed to cross-validate PSMA-PET findings. Then RP and PLND were performed with the intraoperative use of a gamma probe. According to Maurer et al. [10] 99mTc-labelling with PSMA inhibitors (e.g. PSMA I&S or MIP-1404) represents a readily available and cost-effective method to obtain gamma emission detectable by surgical probes. Intraoperatively, gamma probes facilitate in vivo guidance and enable ex vivo measurements to confirm successful resection of metastatic lesions, with a specificity of more than 95% for 99mTc-PSMA-I&S radiotracer. Positive identification was defined as measurements exceedingly at least twice the background level of the patient’s non-cancerous fatty tissue.
In a cohort of 31 patients with biochemical relapse after primary RP [11], a sensitivity of 83.6% and a specificity of 100% in the correct identification and removal of metastatic lymph nodes were demonstrated. Even if all lesions identified on preoperative 68 Ga-PSMA-11 PET were also detected by positive gamma probe measurements and all positive tissue specimens on gamma probe measurements contained metastatic PCa tissue, low-volume small-sized PC lesions might be detected neither by preoperative 68 Ga-PSMA-11 PET nor by intraoperative gamma probe measurements, indicating that the detection rate in both approaches is clearly size dependent. Therefore, the main advantage of 99mTc-PSMA-RGS was the possibility of immediate confirmation of successful removal of metastatic lesions, rather than the possibility of removing a higher number of lesions than those detected by PSMA-PET. Similar results were obtained in a larger cohort (n = 121) retrospective analysis by T. Horn et al. [12], where PSMA-RGS (either performed with 11In-PSMA I&T or 99mTc-PSMA I&S) did not clearly outperform PSMA-PET detection of metastatic nodes in a salvage lymphadenectomy setting, identifying just 18.8% of the lesions that were positive on histopathological analysis and negative on PSMA-PET. However, in two-third of patients a complete biochemical response was reached. At 1-year follow-up, 64% of patients remained without any prostate-cancer-directed treatment. Concerning preoperative parameters, low PSA values and single localization of recurrence in PSMA-PET were associated with longer biochemical recurrence-free survival rates. On the contrary, Gleason score at the time of primary radical prostatectomy, prior radiation treatment, time from RP and localisation of recurrence had no significant impact on this end point.
Regarding short-term outcomes of PSMA-RGS in salvage lymphadenectomy, a match-comparison analysis by Knipper et al. suggested that the short-term biochemical-free survival rate was significantly more favourable in the RGS approach in their cohort of 13 patients, compared to the control cohort composed by 29 patients who underwent a standard procedure [13]. Long-term outcomes are still unclear and further prospective studies are needed to confirm these preliminary results [14].
Recently, further studies were performed in primary staging setting to evaluate the feasibility of PSMA-RGS in laparoscopic robot-assisted RP (RALP). In a recent analysis by Gandaglia et al. [15], authors enrolled intermediate- and high-risk patients referred to RP and PLND in a primary setting to undergo PSMA-RGS in phase 2 prospective clinical trial (n = 18). The interim analysis of this study (n = 12) showed a high specificity of the RGS approach with 99mTc-PSMA I&S on a per-region and per-patient basis (99% and 100%, respectively). However, a lack of sensitivity regarding the detection of small lymph nodes has been observed. 99mTc-PSMA-RGS diagnostic accuracy did not clearly overcome PSMA-PET in the correct identification of PCa nodal metastasis, not identifying any additional suspicious area in the pelvic nodal region. Therefore, the risk of underestimation associated with the use of PSMA-PET before surgery in patients with a high nodal burden might also apply to PSMA-RGS. A slightly higher sensitivity but a lower specificity on a per-region basis was found by Gondoputro et al. [16] in a small (n = 12) cohort of high-risk patients that underwent PSMA-RGS in a primary setting with 99mTc-PSMA I&S. Both the population’s characteristics and the threshold considered for PSMA-RGS positivity were the potential reasons of these discrepancies.
The additional value of PSMA-RGS is mainly evident in atypically located lesions that are not easily accessible using the standard lymphadenectomy template. Therefore, probes handling is one of the most critical issues in RGS. DROP-IN probes, inserted directly in the surgery trocar, facilitate the implementation of RGS in the RALP. DROP-IN probes allow the surgeon to independently control the device using the robot console and thus integrating the RGS approach with minimally invasive surgery. A recent prospective study evaluated whether the DROP-IN gamma probe facilitates minimally invasive, robot-assisted, 99mTc-PSMA radioguided surgery in a cohort of 20 patients with recurrent PCa, referred to salvage lymphadenectomy. Even if a learning curve was required for optimal execution of PSMA-RGS, the preliminary results were promising. In the 90% of cases (19/21), the lesions identified as suspected for recurrent PCa in PSMA-PET were successfully removed. The sensitivity and specificity of PSMA-RGS were 86% and 100%, respectively, and all false-negative nodes were smaller than 3 mm, which corresponds to the detection limit of the gamma probe [17].
The proper patient selection was considered crucial in the largest series published in recurrent PCa patients, as influence PSMA-RGS approach and the related oncologic outcomes [18]. PSMA-RGS is developing as a logical extension to the theranostic approach that is based on the statement “we treat what we see”. Therefore, PSMA-RGS can transfer information derived by PSMA-PET images (both qualitative as site and number of lesions or quantitative as the PSMA expression) to the surgical theatre. In addition, other RGS techniques might work in parallel. Sentinel lymph node biopsy can be proposed as additional tool for selecting patients who might benefit from whole-pelvis radiotherapy after primary surgical treatment [19].
However, PSMA-RGS is not exempt from false-positive findings. PSMA is not a cancer-specific radiopharmaceuticals and non-oncological processes over-expressing PSMA (e.g. inflammation) might lead to an incorrect intraoperative identification of a metastatic lesions. Nevertheless, the specificity and the positive predictive value are optimal for PSMA imaging and, accordingly, the impact of false-positive nodes in PSMA-RGS should be minimal.
Future perspectives
A high-energy γ-emitter has a higher tissue penetration, improving the accuracy of preoperative imaging, but also results in a higher background signal measured during RGS. Therefore, to overcome limitations derived from gamma rays’ wavelength, positron emitter detectors (beta probes) are currently being introduced in clinical practise. The higher tumour to background ratio (shorter penetration depth of beta rays compared to gamma rays) will theoretically increase the precision of intraoperatively PSMA-positive lesions detection. Moreover, beta probes allow the use of already clinically approved radiopharmaceuticals, routinely used in daily PET imaging practise, (e.g. 68 Ga-PSMA-11). In an ongoing phase II clinical trial (NCT05596851) designed to evaluate the feasibility and the diagnostic performance of β + emission PSMA-RGS with DROP-IN probes in primary lymph node dissection, preliminary results proved to be promising. The DROP-IN approach has been implemented in robotic surgery without side effects, and without significantly affecting the overall length of the surgery procedure. Finally, to maximise the advantage of having a low emitting background, an adaptive algorithm has been developed to identify intraoperatively a specific cut-off able to distinguish malignant lesions from the background measured in surrounding tissues [20]. Therefore, the use of a DROP-IN beta probe in combination with 68 Ga-PSMA-11 showed some potential advantages. First, the possibility to use a widely available PET tracer, second the possibility to perform an accurate scanning of superficial lesion due to the limited tissue penetration of beta particles. This could allow the assessment of surgical margins’ invasion. On the other hand, an intrinsic limitation of this technique is the beta radiation attenuation when > 1.5 mm of healthy tissue is located above the lesion that is intended to detect. This could interfere with the performance of the PSMA-RGS. Another potential limitation is the radiation dose for the surgical staff, that should be standardised and evaluated more extensively in further studies [21]. The DROP-IN beta probe can be used also in other oncological disease. Recently, the use of this innovative device has been proposed also in patients affected by neuroendocrine tumours of the ileum, and a feasibility phase II trial is currently ongoing (NCT05448157).
In addition to beta (Emax = 1899 keV) and gamma (Emax = 511 keV) emission tracing, 68 Ga-PSMA-11 can potentially be traced via Cerenkov light (> 250 nm) [9]. Therefore, luminescence imaging RGS is currently being evaluated in clinical practise. Cerenkov luminescence imaging (CLI) with 68 Ga-PSMA-11 showed promising results in a first-in-men feasibility study in ten patients, about the detection of positive prostate’s surgical margins, providing a good correlation to histopathologic examination. The main drawback might be related to a high signal level without histopathologic evidence of PCa tissue at the resection margin. This is critical for two reasons: first, CLI intensity can emerge from PSMA expression and tracer uptake in non-cancerous tissues. Second, Cerenkov luminescence can be produced from a few millimetres underneath the tissue’s surface [22]. In this perspective, the use of an optical by-pass filter or the implementation of CLI with 18F-PSMA compounds might further improve the detection rate of positive surgical margins, increasing the signal detectability of more superficial lesions. Fluorine-18 has, indeed, a significantly shorter positron range in tissues than 68 Ga (0.54 vs. 2.83 mm) [23].
Alongside the development of probes for PSMA-RGS, small new PET scanners have been recently developed. PET/CT specimen imagers have been developed for a molecular-based evaluation of surgical margins and first live experiences were promising. The first analysis of this novel imaging approach in patients undergoing RALP with extended lymphadenectomy in PCa proved that the extremely high resolution of these images could offer a deeper understanding of the biodistribution of PSMA in prostate cancer lesions and might improve surgeon’s confidence in ensuring complete removal of the primary tumour when prostate’s margin involvement is assessed [24]. According to preliminary data [24], PET/CT specimen imagers hold high sensitivity, and cancer lesions smaller than 1 mm might be detected.
The high demand of molecular imaging solutions in minimally invasive surgical strategies is also linked to the development of new image-guided surgical approaches based on augmented reality and artificial intelligence that are gaining popularity alongside radioguided approaches based on in vivo detection of radioactivity. The main advantage of the implementation of PET data in augmented reality robotic surgery approaches lies in its software-based nature, avoiding the need of preoperative injection of radiotracers, adjunctive imaging and intraoperative use of adjunctive medical devices. Segmented PET data are then over-imposed upon the in vivo anatomy and visualised on the screen of the surgical robot, allowing PSMA-based guidance [25].
Conclusion
The clinical implementation of precision PSMA-RGS in PCa deserves to be further explored, as holds the potential to improve oncological outcomes by improving the surgery accuracy in removing metastatic lymph nodes. However, there is still a lack of data derived from clinical trial powered for efficacy and many challenges are still to be solved. The first studies about PSMA-RGS with gamma probes showed promising results regarding the in vivo identification of metastatic lymph nodes, even if the sensitivity is still sub-optimal. In this perspective, PSMA-RGS does not seem to overcome PSMA-PET limitations regarding the detection of micro-metastasis. Therefore, the main advantage lies in the correct and immediate identification of lesions during surgery. Some limitations related to the use of gamma probes might be solved by the introduction of beta probes, even if data are too preliminary and limited to feasibility studies only and reliable conclusion cannot be drawn at present. CLI, PET/CT specimen imager and AR/VR approaches in surgery are currently arising opening new perspectives in image-guided surgery.
Data availability
All data supporting the findings of this study are available within the paper in the section “References”.
References
Hofman MS, Lawrentschuk N, Francis RJ et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395(10231):1208–1216. https://doi.org/10.1016/S0140-6736(20)30314-7
Hope TA, Eiber M, Armstrong WR et al (2021) Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol 7(11):1635–1642. https://doi.org/10.1001/jamaoncol.2021.3771
Emmett L, Buteau J, Papa N et al (2021) The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol 80(6):682–689. https://doi.org/10.1016/j.eururo.2021.08.002
Fendler WP, Calais J, Eiber M et al (2019) Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 5(6):856–863. https://doi.org/10.1001/jamaoncol.2019.0096
Pienta KJ, Gorin MA, Rowe SP et al (2021) A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J Urol 206(1):52–61. https://doi.org/10.1097/JU.0000000000001698
Collamati F, van Oosterom MN, Hadaschik BA, Fragoso Costa P, Darr C (2021) Beta radioguided surgery: towards routine implementation? Q J Nucl Med Mol Imaging 65(3):229–243. https://doi.org/10.23736/S1824-4785.21.03358-6
Camillocci ES, Baroni G, Bellini F et al (2015) A novel radioguided surgery technique exploiting β− decays. Sci Rep 4(1):4401. https://doi.org/10.1038/srep04401
Angelone M, Battistone G, Bellini F et al (2014) Properties of para-Terphenyl as a detector for α, β and γ radiation. IEEE Trans Nuclear Sci 61(3):1483–1487. https://doi.org/10.1109/TNS.2014.2322106
Hensbergen AW, van Willigen DM, van Beurden F et al (2020) Image-guided surgery: are we getting the most out of small-molecule prostate-specific-membrane-antigen-targeted tracers? Bioconjug Chem 31(2):375–395. https://doi.org/10.1021/acs.bioconjchem.9b00758
Maurer T, Graefen M, van der Poel H et al (2020) Prostate-specific membrane antigen-guided surgery. J Nucl Med 61(1):6–12. https://doi.org/10.2967/jnumed.119.232330
Maurer T, Robu S, Schottelius M et al (2019) 99mTechnetium-based prostate-specific membrane antigen-radioguided surgery in recurrent prostate cancer. Eur Urol 75(4):659–666. https://doi.org/10.1016/j.eururo.2018.03.013
Horn T, Krönke M, Rauscher I et al (2019) Single lesion on prostate-specific membrane antigen-ligand positron emission tomography and low prostate-specific antigen are prognostic factors for a favorable biochemical response to prostate-specific membrane antigen-targeted radioguided surgery in recurrent prostate cancer. Eur Urol 76(4):517–523. https://doi.org/10.1016/j.eururo.2019.03.045. (Epub 2019 Apr 12 PMID: 30987843)
Knipper S, Tilki D, Mansholt J et al (2019) Metastases-yield and prostate-specific antigen kinetics following salvage lymph node dissection for prostate cancer: a comparison between conventional surgical approach and prostate-specific membrane antigen-radioguided surgery. Eur Urol Focus 5(1):50–53. https://doi.org/10.1016/j.euf.2018.09.014
Knipper S, Budäus L, Graefen M, Maurer T (2021) Prostate-specific membrane antigen radioguidance for salvage lymph node dissection in recurrent prostate cancer. Eur Urol Focus 7(2):294–296. https://doi.org/10.1016/j.euf.2021.01.015
Gandaglia G, Mazzone E, Stabile A et al (2022) Prostate-specific membrane antigen radioguided surgery to detect nodal metastases in primary prostate cancer patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection: results of a planned interim analysis of a prospective phase 2 study. Eur Urol 82(4):411–418. https://doi.org/10.1016/j.eururo.2022.06.002
Gondoputro W, Scheltema MJ, Blazevski A et al (2022) Robot-assisted prostate-specific membrane antigen-radioguided surgery in primary diagnosed prostate cancer. J Nucl Med 63(11):1659–1664. https://doi.org/10.2967/jnumed.121.263743
de Barros HA, van Oosterom MN, Donswijk ML et al (2022) Robot-assisted prostate-specific membrane antigen-radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol 82(1):97–105. https://doi.org/10.1016/j.eururo.2022.03.002
Knipper S, Mehdi Irai M, Simon R et al (2023) Cohort study of oligorecurrent prostate cancer patients: oncological outcomes of patients treated with salvage lymph node dissection via prostate-specific membrane antigen-radioguided surgery. Eur Urol 83(1):62–69. https://doi.org/10.1016/j.eururo.2022.05.031
De Barros HA, Duin JJ, Mulder D et al (2023) Sentinel node procedure to select clinically localized prostate cancer patients with occult nodal metastases for whole pelvis radiotherapy. Eur Urol Open Sci 49:80–89. https://doi.org/10.1016/j.euros.2022.12.011
Radio-guided Surgery With DROP-IN Beta Probe for 68Ga-PSMA, in High-risk Prostate Cancer Patients Eligible for Robotic-assisted Radical Prostatectomy, National Clinical Trial Identifier: NCT05596851.
Collamati F, van Oosterom MN, De Simoni M et al (2020) A DROP-IN beta probe for robot-assisted 68Ga-PSMA radioguided surgery: first ex vivo technology evaluation using prostate cancer specimens. EJNMMI Res 10(1):92. https://doi.org/10.1186/s13550-020-00682-6
Darr C, Harke NN, Radtke JP et al (2020) Intraoperative 68Ga-PSMA cerenkov luminescence imaging for surgical margins in radical prostatectomy: a feasibility study. J Nucl Med 61(10):1500–1506. https://doi.org/10.2967/jnumed.119.240424. (Epub 2020 Feb 14)
Fragoso Costa P, Püllen L, Kesch C et al (2022) F18-PSMA Cerenkov luminescence and flexible autoradiography Imaging in a prostate cancer mouse model and first results of a radical prostatectomy feasibility study in men. J Nucl Med. https://doi.org/10.2967/jnumed.122.264670
Muraglia L, Mattana F, Travaini LL et al (2023) First live-experience session with PET/CT specimen imager: a pilot analysis in prostate cancer and neuroendocrine tumor. Biomedicines 11(2):645. https://doi.org/10.3390/biomedicines11020645
Wendler T, van Leeuwen FWB, Navab N, van Oosterom MN (2021) How molecular imaging will enable robotic precision surgery: The role of artificial intelligence, augmented reality, and navigation. Eur J Nucl Med Mol Imaging 48(13):4201–4224. https://doi.org/10.1007/s00259-021-05445-6
Acknowledgements
Lorenzo Muraglia is the recipient of a grant supported by the European Institute of Oncology Foundation (FIEO).
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muraglia, L., Mattana, F., Zuccotti, G. et al. Prostate-specific membrane antigen (PSMA) radioguided surgery in prostate cancer: An overview of current application and future perspectives. Clin Transl Imaging 11, 255–261 (2023). https://doi.org/10.1007/s40336-023-00558-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-023-00558-4