Abstract
Arterial hypertension represents an important risk factor for the development of cardiac, vascular and renal events, predisposing to heart failure, acute coronary syndromes, peripheral artery disease, stroke, and chronic renal disease. Arterial hypertension leads to the development of subclinical hypertension mediated organ damage (HMOD) which has prognostic relevance and may influence the choice of treatment options. Alterations of cardiac structure and function represent the more widely assessed form of HMOD. This manuscript will focus on the diagnostic opportunities, prognostic significance and treatment of diastolic dysfunction alterations
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diastolic dysfunction is defined as an alteration of left ventricular diastolic filling, due to a decrease of myocardial relaxation and to an increase in LV stiffness. It is highly prevalent in the aging population and in patients with hypertension, diabetes and coronary artery disease. Multiple mechanisms may influence the development of myocardial relaxation and fibrosis, including structural and functional changes of coronary microcirculation, even in the absence of LVH or systolic dysfunction. Despite the increasing availability and accuracy of imaging techniques, the diagnosis of diastolic dysfunction remains challenging. In the future multimodality imaging, possibly in combination with biomarkers, will better detect and define the presence of diastolic dysfunction. LV diastolic dysfunction (LVDD) is recognized as a predictor of cardiovascular (CV) events, and in particular of heart failure (HF). The improvement of LVDD has been observed during antihypertensive treatment, although the favourable effect of blood pressure (BP) control and of specific classes of antihypertensive drugs treatment on LVDD improvement should be better addressed in future studies. In addition it remains to be defined whether improvement in diastolic dysfunction “per se” may carry a better CV outcome in hypertensive patients.
2 Diagnosis
Echo-Doppler echocardiography is the most simple and at the same time accurate technique for the investigation of cardiac and vascular remodeling and diastolic dysfunction [1, 2].
The recent guidelines of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) [3] include echocardiography among the recommended methods to be considered in hypertensive patients.
Echocardiography is a relatively easy method, is repeatable, is specific and more sensitive measure of left ventricular hypertrophy (LVH) than electrocardiography although more expensive than electrocardiography. Echocardiography provides additional information on cardiac function including systolic, diastolic functions and cardiac mechanics. In addition, it can be performed before starting antihypertensive therapy, but also during treatment [4].
Other advanced imaging methods, including speckle tracking, three-dimensional (3D) echocardiography, cardiac magnetic resonance imaging (CMR), computed tomography (CT) and PET-computed tomography (PET-CT), can further improve the early detection of changes in the myocardium and in the coronary circulation induced by pressure overload [5, 6].
In hypertensive patients, diastolic dysfunction is characterized by the impairment of left ventricular (LV) relaxation and filling and by the increase in LV stiffness [7].
LVDD may precede abnormalities of systolic function [8] and develops several years before the onset of symptomatic HF [9].
The assessment of echocardiographic Doppler transmitral flow velocities was initially proposed for the evaluation of LV diastolic function in asymptomatic hypertensive patients [10]. The influence of several factors such as age, gender, heart rate and BP values on transmitral flow velocities have been extensively evaluated, in order to assess the technical variability of Doppler flow velocities changes.
Transmitral flow velocities, evaluated by Doppler echocardiography, reflect LV early filling (E wave velocity) as well as atrial contraction and emptying (A wave velocity). Three patterns of transmitral flow velocities can be recognized, representing progressive worsening of diastolic LV filling: (a) “slowed relaxation”, with a reversed E/A ratio, slowed deceleration time and increased isovolumic relaxation time; (b) “pseudonormalization”, with a preserved ratio E/A, but a shortened deceleration time due to abnormalities of both relaxation and compliance (analysis of the pulmonary venous filling patterns may be indicated to this regard); (c) ”restrictive pattern” with an increase of E/A ratio (> 1.5–2) associated to a very abrupt deceleration time, suggestive of an elevated atrial pressure.
A systematic analysis performed by Aurigemma et al [11] indicated that even when the acquisition and interpretation of ultrasound images is performed by skilled and expert specialists, the assignment of LV filling patterns is not possible in up to one-third of patients. An improvement in the study of LV diastolic function has been provided by the simultaneous assessment of Doppler trans-mitral flow velocities and by pulsed Doppler tissue imaging (DTI).
The analysis of myocardial velocities at the mitral annulus may reveal an increase in LV filling pressure; in respect to Doppler trans-mitral flow velocities, DTI velocities show no ‘‘pseudonormalization’’ pattern [12, 13]. The average value of DTI velocities at the septal and lateral sides of the mitral annulus should be used for the assessment of global LV diastolic function. The E/e′ ratio represents a reliable estimate of LV filling pressures, and different cut-off values have been proposed for the definition of normal or progressively higher LV filling pressure. E/e′ ratio > 14 indicates a severe increase in LV filling pressure [1, 2]. E/e′ ratio may be measured in patients in sinus rhythm, but also in the presence of atrial fibrillation [1].
The tricuspid valve (TV) regurgitation peak velocity (assessed from multiple windows in order to measure the highest velocity) is used to calculate pulmonary artery systolic pressure (PAPs) [1, 2].
Left atrial (LA) size is a further parameter to be assessed in the evaluation of diastolic function [14]. An accurate measurement of LA size should be an integral part of the standard echocardiogram in hypertensive patients. LA enlargement may reflect the increase in LV filling pressure; in patients with preserved systolic function, it may be a marker of diastolic dysfunction and is predictive of an increased risk of atrial fibrillation, stroke, HF and mortality. This has been shown by the measurement of LA anteroposterior linear dimension by M-mode from the parasternal long axis view and by the more accurate evaluation of LA volume from 2D images [1, 2, 6]. The measurement of LA volume is recommended both in clinical practice and in research studies and most guidelines recommend the biplane area-length method. The EAE/ASE guidelines recommend a cut off value of > 34 ml/m2 for LA enlargement [1, 2]. The 2018 ESH/ESC Guidelines for the management of arterial hypertension [3] recommend the indexation of LA volume by the height and indicate different cut off values in men (> 18.5 mL/m2) and women (> 16.5 mL/m2), according to Kuznetsova et al [15].
Compared to the conventional 2-dimensional approach, 3D echocardiography appears superior in the assessment of LA volume; at present, however, this application is limited to research studies. Current software packages used to quantify LV size and function may provide volume-time curves and show the dynamic LV volume change throughout the cardiac cycle; by the examination of the diastolic part of these curves, several indices of LV filling may be measured to differentiate patients with normal LV diastolic function from patients with different degrees of diastolic dysfunction. The same consideration applies to several other parameters of LA function, based on 2D- or 3D-measures, conventional and tissue Doppler or strain rate imaging [16, 17].
LA reservoir and conduit function are assessed by volumetric and strain analysis and have been reported to be significantly impaired in hypertensive patients as compared to normotensive controls.
Some authors have found that all LA functions are reduced in hypertensive patients, whereas other investigators reported decreased conduit and enhanced LA reservoir and pump function [17]. High values of LA pump strain identified normal LV filling pressure with good accuracy in patients with normal systolic function.
According to Sitges et al. [18] LV global longitudinal strain and LA strain have been more frequently selected among the proposed additional parameters to assess LV systolic and diastolic function and their use is considered particularly in the “indeterminate diastolic function” group patients or in patients with other clinical diseases such as atrial fibrillation and mitral regurgitation.
LA reservoir strain has been included in echocardiographic estimation of LV filling pressure in the latest guidelines for multimodality evaluation of HF with preserved ejection fraction (HFpEF) [19].
In conclusion, a comprehensive echocardiographic evaluation of LV filling uses measurements of Doppler mitral inflow (and pulmonary venous flow), of tissue Doppler early diastolic mitral annular velocities, of peak velocity of tricuspid valve regurgitation, and of LA size (Table 1) [20].
3 Prevalence and Determinants
The reported prevalence of LVDD varies from 12 to 84%. This wide range of prevalence is due to the use of different recommendations [11] and methodological approaches to grade LV diastolic function using echocardiographic indexes in patients or cohorts’ individuals with different clinical characteristics [21].
In consideration of the influence that several factors, such as gender, body mass index, heart rate and BP, and in particular age, may exert on Doppler flow velocities, Klein et al assessed normal values for Doppler parameters according to age groups in a relatively small sample of 117 subjects [22].
In addition, the use of different standardized approaches may identify a different number of patients with normal, impaired or indeterminate diastolic function, as reported by Almeida J et al [23]. These authors compared the recommendations of the American Society of Echocardiography (ASE) and European Association of Echocardiography (EAE) in 2009 and the second set of recommendations of the ASE and EACVI published in 2016. In this analysis, the use of the ASE/EACVI 2016 algorithm was associated with an increase in prevalence of patients with unclassifiable diastolic function from 5 to 15%.
Diastolic dysfunction is typically observed in patients with hypertension, mainly in the presence of LV hypertrophy (LVH) [24] or concentric geometry [25] and in patients with coronary artery disease.
In fact, in patients with LV hypertrophy or remodelling, LV relaxation is usually slowed, with a decrease in early diastolic filling; in the presence of normal LA pressure, a greater proportion of LV filling is shifted from early to late diastole after atrial contraction. LVDD can also occur in other various different clinical disorders, with a particularly high prevalence in the elderly [25, 26].
More interestingly, the prevalence of abnormal relaxation (and of low systolic myocardial function) is greater in patients with inappropriate LV mass, suggesting that this condition may represent an accelerated phase of transition from compensatory LVH towards HF [27, 28].
According to ASE/EACVI 2016 guidelines, the indicators of myocardial disease embody the presence of abnormal LV structure and/or systolic function (including more subtle parameters of systolic function such as global longitudinal strain), the presence of LA enlargement, the presence of coronary artery disease and/or of multiple wall motion abnormalities. The identification of a myocardial disease translates in a higher incidence of diastolic dysfunction, which in all these circumstances may be diagnosed only based on mitral inflow and mitral annulus diastolic velocities as shown recently by Sorrentino et al [29].
Few studies have observed that, in the presence of diastolic dysfunction, systolic function, although normal at rest, is proportionally impaired during stress [30, 31].
A gender-related difference in normal cardiac adaptation to physical activity has been observed, suggesting that impaired diastolic relaxation should have a larger impact on exercise capacity in hypertensive female with LVH than in men [32]. This is in accordance with recent reports that identify elderly women as those most candidate for HFpEF [33].
The role of renin angiotensin system on diastolic function has been extensively evaluated in patients with secondary hypertension (primary aldosteronism and renovascular hypertension) and in essential hypertensive patients [34]. Aldosterone exerts pro-fibrotic effects directly stimulating cardiac myocytes and fibroblast proliferation, promoting the expression pro-fibrotic molecules such as transforming growth factor-β1 (TDGF- β1) and endothelin-1 (ET-1) [35].
Aldosterone mediated cardiac organ damage includes impaired LV relaxation, evaluated by either tissue Doppler imaging or speckle-tracking echocardiography. Impaired LV relaxation results in diastolic dysfunction, reflected by a lower e′ and a higher E/e′ ratio in patients with PA compared with those affected by essential hypertension [36, 37].
In a recent study, renin-independent aldosterone production was associated with alterations of cardiac structure and diastolic function, as assessed among the participants to the ARIC (the Atherosclerosis Risk in Communities Study) cohort. Renin suppression was associated with greater LV mass, LV volumes, LA volume index, and a lower E/A ratio (adjusted P < 0.001 for all), while higher aldosterone was associated with greater LV mass, lower global longitudinal strain and lateral E' [38].
The role of sympathetic nervous system activation on diastolic function impairment has been highlighted by the evaluation of patients with pheochromocytoma/paragangliomas (PPGLs). Impaired baseline values in several parameters of diastolic function (e.g., E/A ratio, e′ velocity, and E/e′ ratio) and improvements after curative resection of PPGLs have been observed in some but not in all studies. In a recent meta-analysis of echocardiographic studies in patients with different types of secondary hypertension, LV diastolic function was not impaired [39]. Controversial data are confirmed when cardiac magnetic resonance was performed in patients with PPGLs [39].
In addition, obesity, diabetes, dietary intake, inducing myocardial and epicardial steatosis and alterations in myocardial fatty acid metabolism, can affect diastolic function, with some sex-related physiological differences [40,41,42,43].
4 Prognostic Significance
The presence of LVDD is a potential precursor to CV events, particularly in hypertensive patients.
A significant increase in cardiac mortality and in cardiovascular morbidity and mortality, was associated with changes of the E/A ratio in 2 large cohort of hypertensive patients independently of LV mass and of ambulatory BP [44, 45].
Data from the Olmsted county epidemiological study indicate that the grading suggested by the EAE/ASE recommendations is an important predictor of all-cause mortality [46].
In hypertensive patients at high CV risk participating into the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) echocardiographic sub-study, E/e′ ratio was the strongest predictor of first cardiac events, independent of LVM and geometry [47].
The prognostic significance of progressive impairment of LV relaxation and filling, defined including the measurements of TDI parameters [48], was confirmed in the prediction of HF [49] and of all-cause mortality [50].
In a cohort of Chinese hypertensive patients, assessed for a mean follow-up of 5.4 years, LVDD was a stronger predictor of MACE (HR: 2.5; 95% CI: 1.20 to 5.25; c- statistics 0.805) than E/e′ ratio (HR: 1.13; 95% CI: 1.04 to 1.22) [51] in multivariable Cox regression analyses.
Other studies, taking into account the presence of LVH and/or concentric remodeling, have shown that the prognostic significance of LVDD, as defined by the E/A ratio [52] or by the ASE/EACVI 2016 recommendations [53] is lost, when other confounders, and in particular LV mass, are considered.
More over the role of diastolic heart dysfunction as a novel risk factor for chronic kidney disease (CKD) was suggested by the results of the KNOW-CKD (Korean Cohort Study for Outcome in Patients with Chronic Kidney Disease). In patients with non-dialysis renal failure, the increment of early diastolic mitral inflow velocity/early diastolic mitral annulus velocity ratio (E/e′ ratio) was associated to the risk of CKD progression [54].
Very recently, Inciardi et al have assessed LA structure (LA maximal and minimal volume indexed by body surface area) and function (LA emptying fraction, LA reservoir, conduit, and contraction strain) in almost 5000 participants to the ARIC (Atherosclerosis Risk In Communities) study (mean age 75 ± 5 years, 81% hypertensive) without prevalent HF. It was shown that LA measures were associated with NT-proBNP values and were prognostic for both incident HFpEF or death and incident HF with reduced ejection fraction or death [55].
5 Effect of Treatment
Guidelines indicate that the choice of antihypertensive therapy should be based on patients’ target organ damage and/or comorbidities such as coronary artery disease or diabetes. The effect of antihypertensive treatment on diastolic function has been evaluated in several studies, although the clinical significance remains to be established [56]. In addition, in most cases, a combination strategy for antihypertensive therapy may be necessary to target differing mechanisms of diastolic dysfunction [3].
Diastolic dysfunction and LA dilatation exhibit a relationship with BP and the decrease of BP values should improve “per se” diastolic function. Accordingly, the intensity of antihypertensive therapy and the achievement of BP control to a lower threshold could impact cardiac structure and function. Solomon et al showed that a more pronounced reduction of BP values by treatment with an ACE-inhibitor/calcium channel blocker combination was associated with a parallel improvement in E transmitral flow velocity [57].
Chen et al compared hypertensive patients with target BP between 110 and 130 mmHg (intensive group) and with BP threshold between 130 and 150 mmHg (standard group). At 1 year, no significant difference in conventional echocardiographic parameters of LV systolic function or LV structure was observed but an improved E/e′ ratio and global longitudinal strain in the intensive group was found [58]. On the opposite, the results of the SPRINT-HEART study suggest that antihypertensive treatment has a clear beneficial effect on LV structural and functional abnormalities, and the improvement in global longitudinal strain was mainly related to the reduction in LV mass index rather than to the achieved level of SBP [59].
Several studies have reported an improvement of diastolic function parameters (and of midwall FS) in response to antihypertensive therapy [60, 61]. Among all these studies two have shown no favorable changes in diastolic filling or E/e′ ratio, despite adequate BP control; in these studies, however, a limited, despite statistically significant, decrease of LVM and no change in RWT were noted [62, 63].
Some investigations using speckle tracking echocardiography reported significant improvement of parameters of LV diastolic function, as well as of subclinical systolic dysfunction (measured by the LV global longitudinal strain) in hypertensive patients followed 6 and 12 months, respectively, after introduction of antihypertensive therapy. Therefore a mild deterioration of LV function seems to be reversible with appropriate and timely prescribed therapy [64, 65]. All studies that used speckle-tracking echocardiography have been recently reviewed by Tadic et al in a meta-analysis [39]. The average E/e′ ratio was significantly reduced by treatment from 8.6 + 0.4 at baseline to 8.1 + 0.4 at the end of follow-up period (P < 0.0001), although some differences were observed among the 7 examined studies. In more detail, one study evaluating the effect of two different calcium-channel blockers (azelnidipine and amlodipine) demonstrated no significant improvement in LV structure and diastolic function (LV mass index and E/e′) after treatment [66], while an improvement in LV mass index and global longitudinal strain, but not E/e′ was observed when treatment with two different combinations of an ACE inhibitor and a diuretic (trandolapril/ hydrochlorothiazide or trandolapril/indapamide) were compared in hypertensive and diabetic patients [67].
It is possible that the partial dissociation between structural and functional changes during antihypertensive treatment reflect, at least in part, the effect of treatment on several factors influencing diastolic function, including heart rate, humoral changes and extracellular matrix composition.
Myocardial interstitial fibrosis may contribute to LVDD and HF with preserved LV ejection fraction by directly increasing LV stiffness and impairing LV filling during early diastole. The deposition of perivascular and interstitial fibrosis has been evaluated by some non-invasive ultrasound methods, showing that drugs interfering with the renin-angiotensin-aldosterone system may be particularly effective in inducing changes that might reflect a decrease of myocardial tissue collagen content [68]. More recently, accurate and non-invasive assessment of regional myocardial fibrosis may be obtained by cardiac magnetic resonance using late Gadolinium enhancement, and diffuse interstitial myocardial fibrosis may be assessed with post-contrast T1 mapping [69]. However, the studies using CMR have included patients with hypertensive heart disease. Biochemical markers of collagen synthesis and degradation have been proposed for the evaluation of myocardial fibrosis as reviewed [70]. The main limitation to the use these circulating biomarkers on a wider clinical ground is that they may reflect fibrosis in other organs or tissues of the body, lacking of specificity for the myocardium.
In addition to pharmacological treatment, it has been demonstrated that renal denervation may be associated to reversal of diastolic dysfunction, as assessed by echocardiography [71] or CMR [72, 73], with favorable changes in extracellular collagen volume in only one study [72].
More recent investigations have focused on systolic and diastolic function changes during chronic treatment with glucagon-like peptide-1 receptor agonists (GLP-1Ra) and sodium glucose transporter 2 inhibitors (SGLT-2i) in subjects with diabetes with and without HF. Chronic treatment with liraglutide in T2D subjects without established CV disease improved diastolic function by reducing filling pressures by 20% (reduced E-wave and E/e′ ratio), possibly related to the decrease in body mass index (BMI) but not of BP [74, 75].
Diastolic function improvement during treatment with SGLT-2i was assessed in patients with diabetes mellitus type 2 and HF and/or coronary artery disease. An improvement around 10–15% was shown, similar among all studies, although more pronounced in patients with lower ejection fraction [76].
In conclusion during effective antihypertensive treatment modifications of LV and atrial structure and function may be observed, and the independent role of BP control, of LV mass and geometry and LA dimensions on changes of different diastolic dysfunction parameters remains difficult to establish.
6 Conclusions
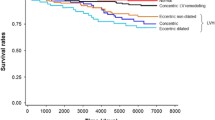
LV diastolic dysfunction is highly prevalent in the aging population and hypertensive patients. Multiple mechanisms may have a role in influencing the development of myocardial dysfunction (relaxation) and fibrosis, including structural and functional changes of coronary microcirculation, even in the absence of LVH or systolic dysfunction. Multimodality imaging, possibly in combination with biomarkers, will better detect and define the presence of diastolic dysfunction. The favourable effect of BP control and antihypertensive drugs treatment on LVDD improvement should be addressed in future studies, possibly defining whether improvements in diastolic dysfunction may carry a better cardiovascular outcome in hypertensive patients (see Fig. 1).
References
Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE)dagger. Eur Heart J Cardiovasc Imaging. 2015;16:577–605.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Nagueh SF. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. 2020;13:228–44.
Schumann CL, Jaeger NR, Kramer CM. Recent advances in imaging of hypertensive heart disease. Curr Hypertens Rep. 2019;21:3.
Perrone-Filardi P, Coca A, Galderisi M, Paolillo S, Alpendurada F, et al. Non-invasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients: A consensus paper from the European Association of Cardiovascular Imaging (EACVI), the European Society of Cardiology Council on Hypertension, and the European Society of Hypertension (ESH). Eur Heart J Cardiovasc Imaging. 2017;18:945–60.
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50.
Perlini S, Chung ES, Aurigemma GP, Meyer TE. Alterations in early filling dynamics predict the progression of compensated pressure overload hypertrophy to heart failure better than abnormalities in midwall systolic shortening. Clin Exp Hypertens. 2013;35:401–11.
Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, et al. Hypertension and heart failure with preserved ejection fraction: position paper by the European Society of Hypertension. J Hypertens. 2021;39:1522–45.
Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
Narayanan A, Aurigemma GP, Hill JC, McNamee A, Tighe DA. High prevalence of ‘Unclassifiable’ diastolic dysfunction using current criteria. Circulation. 2008;118:787.
Nagueh SF. Echocardiographic assessment of left ventricular relaxation and cardiac filling pressures. Curr Heart Fail Rep. 2009;6:154–9.
Wang J, Nagueh SF. Echocardiographic assessment of left ventricular filling pressures. Heart Fail Clin. 2008;4:57–70.
Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5.
Kuznetsova T, Haddad F, Tikhonoff V, Kloch-Badelek M, Ryabikov A, et al. Impact and pitfalls of scaling of left ventricular and atrial structure in population-based studies. J Hypertens. 2016;34:1186–94.
Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1961–77.
Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, et al. Association of left atrial reservoir function with left atrial structural remodeling related to left ventricular dysfunction in asymptomatic patients with hypertension: evaluation by two-dimensional speckle-tracking echocardiography. Clin Exp Hypertens. 2015;37:155–65.
Sitges M, Ajmone Marsan N, Cameli M, D’Andrea A, Carvalho RF, et al. EACVI survey on the evaluation of left ventricular diastolic function. Eur Heart J Cardiovasc Imaging. 2021;22:1098–105.
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23:e34–61.
Nagueh SF. Diastology: 2020-A practical guide. Echocardiography. 2020;37:1919–25.
Selmeryd J, Henriksen E, Leppert J, Hedberg P. Interstudy heterogeneity of definitions of diastolic dysfunction severely affects reported prevalence. Eur Heart J Cardiovasc Imaging. 2016;17:892–9.
Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, et al. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994;69:212–24.
Almeida JG, Fontes-Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. 2018;19:380–6.
Wachtell K, Smith G, Gerdts E, Dahlof B, Nieminen MS, et al. Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Losartan Intervention For Endpoint. Am J Cardiol. 2000;85:466–72.
Zanchetti A, Cuspidi C, Comarella L, Rosei EA, Ambrosioni E, et al. Left ventricular diastolic dysfunction in elderly hypertensives: results of the APROS-diadys study. J Hypertens. 2007;25:2158–67.
Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–52.
Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, et al. Inappropriate left ventricular mass changes during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2007;49:1077–83.
Palmieri V, Wachtell K, Bella JN, Gerdts E, Papademetriou V, et al. Usefulness of the assessment of the appropriateness of left ventricular mass to detect left ventricular systolic and diastolic abnormalities in absence of echocardiographic left ventricular hypertrophy: the LIFE study. J Hum Hypertens. 2004;18:423–30.
Sorrentino R, Esposito R, Santoro C, Vaccaro A, Cocozza S, et al. Practical impact of new diastolic recommendations on noninvasive estimation of left ventricular diastolic function and filling pressures. J Am Soc Echocardiogr. 2020;33:171–81.
Cuocolo A, Sax FL, Brush JE, Maron BJ, Bacharach SL, et al. Left ventricular hypertrophy and impaired diastolic filling in essential hypertension. Diastolic mechanisms for systolic dysfunction during exercise. Circulation. 1990;81:978–86.
Gerdts E, Bjornstad H, Toft S, Devereux RB, Omvik P. Impact of diastolic Doppler indices on exercise capacity in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy). J Hypertens. 2002;20:1223–9.
Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–74.
Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–7.
Catena C, Verheyen N, Pilz S, Kraigher-Krainer E, Tomaschitz A, et al. Plasma aldosterone and left ventricular diastolic function in treatment-naive patients with hypertension: tissue-Doppler imaging study. Hypertension. 2015;65:1231–7.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–69.
Chang YY, Liao CW, Tsai CH, Chen CW, Pan CT, et al. Left ventricular dysfunction in patients with primary aldosteronism: a propensity score-matching follow-up study with tissue Doppler imaging. J Am Heart Assoc. 2019;8: e013263.
Yang Y, Zhu LM, Xu JZ, Tang XF, Gao PJ. Comparison of left ventricular structure and function in primary aldosteronism and essential hypertension by echocardiography. Hypertens Res. 2017;40:243–50.
Brown JM, Wijkman MO, Claggett BL, Shah AM, Ballantyne CM, et al. Cardiac structure and function across the spectrum of aldosteronism: the atherosclerosis risk in communities study. Hypertension. 2022;79:1984–93.
Tadic M, Sala C, Carugo S, Mancia G, Grassi G, et al. Left ventricular global longitudinal strain in secondary hypertension: a meta-analysis of echocardiographic studies. Eur J Intern Med. 2022;96:81–9.
Daneii P, Neshat S, Mirnasiry MS, Moghimi Z, Dehghan Niri F, et al. Lipids and diastolic dysfunction: recent evidence and findings. Nutr Metab Cardiovasc Dis. 2022;32:1343–52.
Catena C, Colussi G, Verheyen ND, Novello M, Fagotto V, et al. Moderate alcohol consumption is associated with left ventricular diastolic dysfunction in nonalcoholic hypertensive patients. Hypertension. 2016;68:1208–16.
Russo C, Jin Z, Homma S, Rundek T, Elkind MS, et al. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur J Heart Fail. 2010;12:454–61.
Frohlich ED, Varagic J. Sodium directly impairs target organ function in hypertension. Curr Opin Cardiol. 2005;20:424–9.
Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, et al. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol. 2002;39:2005–11.
Wachtell K, Palmieri V, Gerdts E, Bella JN, Aurigemma GP, et al. Prognostic significance of left ventricular diastolic dysfunction in patients with left ventricular hypertrophy and systemic hypertension (the LIFE Study). Am J Cardiol. 2010;106:999–1005.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202.
Sharp AS, Tapp RJ, Thom SA, Francis DP, Hughes AD, et al. Tissue Doppler E/E’ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31:747–52.
Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, et al. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;3: e000789.
Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63.
Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–8.
Zhou D, Yan M, Cheng Q, Feng X, Tang S, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in community hypertension patients. BMC Cardiovasc Disord. 2022;22:265.
de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–7.
Kuznetsova T, Cauwenberghs N, Sabovcik F, Kobayashi Y, Haddad F. Evaluation of diastole by echocardiography for detecting early cardiac dysfunction: an outcome study. ESC Heart Fail. 2022;9:1775–83.
Kang E, Lee SW, Ryu H, Kang M, Kim S, et al. Left ventricular diastolic dysfunction and progression of chronic kidney disease: analysis of KNOW-CKD data. J Am Heart Assoc. 2022;11: e025554.
Inciardi RM, Claggett B, Minamisawa M, Shin SH, Selvaraj S, et al. Association of left atrial structure and function with heart failure in older adults. J Am Coll Cardiol. 2022;79:1549–61.
Nadruz W, Shah AM, Solomon SD. Diastolic dysfunction and hypertension. Med Clin North Am. 2017;101:7–17.
Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55:241–8.
Chen X, Yang Q, Fang J, Guo H. Effects of different systolic blood pressure targets on myocardial function: a one-year follow-up in geriatric hypertension. Int J Gen Med. 2021;14:3775–85.
Upadhya B, Rocco MV, Pajewski NM, Morgan T, Blackshear J, et al. Effect of intensive blood pressure reduction on left ventricular mass, structure, function, and fibrosis in the SPRINT-HEART. Hypertension. 2019. https://doi.org/10.1161/HYPERTENSIONAHA.119.13073.
Ginelli P, Bella JN. Treatment of diastolic dysfunction in hypertension. Nutr Metab Cardiovasc Dis. 2012;22:613–8.
Perlini S, Muiesan ML, Cuspidi C, Sampieri L, Trimarco B, et al. Midwall mechanics are improved after regression of hypertensive left ventricular hypertrophy and normalization of chamber geometry. Circulation. 2001;103:678–83.
Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–87.
Barron AJ, Hughes AD, Sharp A, Baksi AJ, Surendran P, et al. Long-term antihypertensive treatment fails to improve E/e’ despite regression of left ventricular mass: an Anglo-Scandinavian cardiac outcomes trial substudy. Hypertension. 2014;63:252–8.
Cheng S, Shah AM, Albisu JP, Desai AS, Hilkert RJ, et al. Reversibility of left ventricular mechanical dysfunction in patients with hypertensive heart disease. J Hypertens. 2014;32:2479–86 (discussion 86-7).
Uzieblo-Zyczkowska B, Krzesinski P, Gielerak G, Skrobowski A. Speckle tracking echocardiography and tissue Doppler imaging reveal beneficial effect of pharmacotherapy in hypertensives with asymptomatic left ventricular dysfunction. J Am Soc Hypertens. 2017;11:334–42.
Motoki H, Koyama J, Izawa A, Tomita T, Miyashita Y, et al. Impact of azelnidipine and amlodipine on left ventricular mass and longitudinal function in hypertensive patients with left ventricular hypertrophy. Echocardiography. 2014;31:1230–8.
Vinereanu D, Dulgheru R, Magda S, Dragoi Galrinho R, Florescu M, et al. The effect of indapamide versus hydrochlorothiazide on ventricular and arterial function in patients with hypertension and diabetes: results of a randomized trial. Am Heart J. 2014;168:446–56.
Brilla CG. Renin-angiotensin system mediated mechanisms: cardioreparation and cardioprotection. Heart. 2000;84(Suppl 1):i18–9 (discussion i50).
Maceira AM, Mohiaddin RH. Cardiovascular magnetic resonance in systemic hypertension. J Cardiovasc Magn Reson. 2012;14:28.
Diez J, Butler J. Growing heart failure burden of hypertensive heart disease: a call to action. Hypertension. 2022. https://doi.org/10.1161/HYPERTENSIONAHA.122.19373.
Schirmer SH, Sayed MM, Reil JC, Ukena C, Linz D, et al. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol. 2014;63:1916–23.
Delacroix S, Chokka RG, Nelson AJ, Wong DT, Pederson S, et al. Effects of renal sympathetic denervation on myocardial structure, function and perfusion: a serial CMR study. Atherosclerosis. 2018;272:207–15.
Doltra A, Messroghli D, Stawowy P, Hassel JH, Gebker R, et al. Potential reduction of interstitial myocardial fibrosis with renal denervation. J Am Heart Assoc. 2014;3: e001353.
Yagi K, Imamura T, Tada H, Chujo D, Liu J, et al. Diastolic Cardiac function improvement by liraglutide is mainly body weight reduction dependent but independently contributes to B-type natriuretic peptide reduction in patients with type 2 diabetes with preserved ejection fraction. J Diabetes Res. 2021;2021:8838026.
Bizino MB, Jazet IM, Westenberg JJM, van Eyk HJ, Paiman EHM, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol. 2019;18:55.
Natali A, Nesti L, Trico D, Ferrannini E. Effects of GLP-1 receptor agonists and SGLT-2 inhibitors on cardiac structure and function: a narrative review of clinical evidence. Cardiovasc Diabetol. 2021;20:196.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
All authors disclose that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Author Contributions
All authors contributed to the manuscript. MLM had the idea for the article, SC, DS, CA performed the literature search and data analysis, and CAR, FB and GB drafted the manuscript and MLM, MS, AP, CDC critically revised the work. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bertacchini, F., Agabiti Rosei, C., Buso, G. et al. Subclinical HMOD in Hypertension: Left Ventricular Diastolic Dysfunction. High Blood Press Cardiovasc Prev 29, 585–593 (2022). https://doi.org/10.1007/s40292-022-00548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-022-00548-z