Abstract
Introduction
The accurate detection of genetic variants such as single substitutions (IDH1/2, TERT), chromosomal abnormalities (CDKN2A, 1p/19q deletions, and EGFR amplifications), or promoter methylations (MGMT) is critical for glioma patient management, as emphasized in the World Health Organization's (WHO’s) most recent classification in 2021 (WHO CNS5). The purpose of this study was to evaluate novel innovative methods for determining IDH1/2 status in the context of WHO CNS5.
Methods
Multiple biomarkers were simultaneously screened using next-generation sequencing (NGS) on 34 glioma samples. In cases where the IDH1/2 status determined by immunohistochemistry (IHC) or multiplex ligation-dependent probe amplification (MLPA) was inconsistent with the NGS results, quantitative polymerase chain reaction (qPCR) and Sanger sequencing were performed to resolve the adjudicated discrepancy.
Results
IDH1/2 NGS results differ from IHC (7/13 samples) as well as MLPA reports (1/4 samples). All NGS findings were confirmed by qPCR and Sanger sequencing. WHO CNS5 requires assessment of multiple mutations for glioma classification.
Conclusions
We demonstrated that qPCR or NGS performed in reference genetic laboratories, rather than IHC, is the most reliable method for IDH1/2 analysis. Clinicians should be aware of discrepancies in MLPA or IHC results and seek reconsultation in facilities with extensive access to advanced molecular technologies. Moreover, we proposed a new algorithm for the molecular diagnostic procedures in glioma patients based on the WHO CNS5.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Next-generation sequencing (NGS) and quantitative polymerase chain reaction (qPCR) are the most reliable methods for IDH1/2 detection. |
We propose a novel algorithm for the molecular diagnosis of astrocytomas. |
Final glioma diagnosis should be made in reference centers with access to advanced molecular technologies. |
1 Introduction
Gliomas are the most common primary central nervous system (CNS) tumors, with an incidence of 1.9–9.6 per 100,000, depending on age, sex, ethnicity, and geographic location [1, 2]. The 2021 World Health Organization Classification of Tumors of the Central Nervous System (WHO CNS5) has fundamentally changed the classification of gliomas and incorporated many molecular biomarkers [3]. In this classification system, the primary genetic markers for gliomas are IDH1/2 mutation status, 1p/19q co-deletion, H3F3A alterations, ATRX gene mutations, MGMT promoter methylation status, loss of CDKN2A, and EGFR amplification, a combined gain of chromosome 7 and loss of chromosome 10, and TERT promoter pathogenic variants. In the new classification, there are three genetic parameters, TERT promoter mutation, EGFR gene amplification, or combined gain of entire chromosome 7 and loss of entire chromosome 10 [+7/−10]), that, in the case of IDH-wildtype diffuse astrocytic tumor in adults, change the diagnosis to glioblastoma (see Fig. 1) [3, 4].
Astrocytoma grading based on genetic alterations according to WHO CNS5 2021. Cyclin-dependent kinase inhibitor 2A (CDKN2A) homozygous deletion, in IDH-mutant astrocytomas, is a marker of the highest malignancy grade. In IDH-wildtype diffuse astrocytic gliomas, the presence of one or more of three genetic parameters (EGFR gene amplification, TERT promoter pathogenic variants, or a combination of chromosome 7 gain and 10 loss [+ 7/− 10]) obligate to assign the highest CNS WHO grade and classify them as glioblastoma. Glioblastoma, IDH-mutant, does not exist in the current classification. WHO CNS5 2021 the fifth edition of the WHO Classification of Tumors of the Central Nervous System, TERT human telomerase reverse transcriptase, WHO World Health Organization
Depending on the genetic aberration, numerous molecular methods can be used, such as fluorescence in situ hybridization (FISH), quantitative polymerase chain reaction (qPCR) including methylation-specific polymerase chain reaction (MS-PCR), and next-generation sequencing (NGS) extended with copy number variation (CNV) analysis. The resolution, sensitivity, and specificity of molecular techniques vary. The limit of detection (LOD) for NGS and qPCR depends on the workflow and type of targeted genomic change. Usually, the detection limit (LOD) varies between 1% and 5% for qPCR and 5% and 10% for NGS; however, the LOD for qPCR- and NGS-based analyses is not consistently defined or determined and usually varies between 1% and 10% and 2% and 15%, respectively [5]. The LOD for NGS and qPCR depends on the workflow and type of targeted genomic change. Sanger sequencing methodology allows the detection of variants at a frequency of 15–20% and above [6]. It is critical to choose an appropriate approach for detecting genetic changes and to conduct molecular analysis in an oncological reference center. The use of monoclonal antibodies specific to the mutant protein enabled immunohistochemistry (IHC) to be incorporated in standard IDH1/2 point mutation screening protocols [7]. Even though the cost of analyzing a single sample is significantly higher for NGS than for other methods, it is NGS that enables comprehensive analysis, and thus methods such as FISH, qPCR, Sanger sequencing, or mutant-specific IHC will be replaced in the near future in favor of NGS extended for CNV [8,9,10].
Taking into account the latest diagnostic recommendations and the growing number and significance of genetic markers in clinical management, the purpose of this study was to consider NGS as the major method that allows for the analysis of many genetic biomarkers in glioma in one simultaneous step. In addition, we compare NGS and IHC methods for determining IDH1/2 status in the context of WHO CNS5.
2 Materials and Methods
2.1 Patient Selection
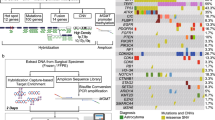
A retrospective analysis of the medical records of 86 patients diagnosed with gliomas referred to the Oncology Center in Bydgoszcz between January 2017 and June 2021 was conducted (Table S1, see the electronic supplementary material). Histopathological evaluation and IHC were performed in non-reference, non-oncology centers with an anatomical pathology unit, however, without access to advanced technologies such as NGS and without significant exposures to genetic analysis of glioma. Whereas, data such as age, gender, prior radiotherapy and chemotherapy, as well as the results of genetic tests come from the oncology reference center. NGS was performed on 34 patients’ samples. The inclusion criteria for NGS examination were as follows: patients diagnosed with glioma; biological archive material (the same used for histopathological evaluation) that was of adequate quality for analysis. Exclusion criteria were as follows: a lack of biological material or double stranded DNA (dsDNA) not of sufficient quality and quantity for NGS analysis. From 34 gliomas that were tested using NGS, 17 had previous IDH1/2 status assessed in non-oncological centers using IHC or multiplex ligation-dependent probe amplification (MLPA) (Fig. 2).
The research evaluated surgical materials diagnosed in 12 different facilities with varying levels of IHC and genetic testing availability. The Ethics Committee of the Nicolaus Copernicus University, Collegium Medicum in Bydgoszcz, Poland, approved the study protocol (KB 265/2012).
2.2 DNA Isolation
The percentage of tumor cells in material qualified by a pathologist ranged from 5 to 95%. DNA was isolated from brain cancer tissue fixed in formalin and embedded in paraffin (FFPE) using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. The initial concentration and quality of DNA were measured using NanoDrop1000 (Thermo Scientific, Waltham, MA, USA).
2.3 DNA Quality Assessment
The quality and quantity of DNA were evaluated using real-time polymerase chain reaction (PCR) with Fragmentation Quantification Assay (FQA) CE-IVD (EntroGen) or/and by fluorometric methods using Quantus (Promega) with QuantiFluor® dsDNA System (Promega). The FQA assay uses three amplicon sizes (37, 150, 301 bp) of a reference gene and five standards to calculate the absolute copy number of amplifiable DNA and determine sample integrity through the fragmentation ratio (F-ratio). The two F-ratios, 150 bp/37 bp and 301 bp/37 bp, are indicative of sample quality and help predict the optimal amount of input sample for downstream assays. Archive FFPE material was found to be significantly degraded, but the lack of other biological material forced us to use DNA with an F-ratio of < 0.5, below the manufacturer’s recommendations.
2.4 Library Preparation and NGS
Libraries for NGS were prepared according to the manufacturer's instructions using a 16-gene panel, the EntroGen NGS Targeted Hotspot Panel (CE-IVD). It includes hotspot exons for genes: BRAF, EGFR, ERBB2, HRAS, C-KIT, IDH1, IDH2, JAK2, KRAS, MET, NRAS, PDGFRA, PIK3CA, RET, the promoter region of TERT, and the entire exome of TP53. The libraries were quantified using the QuantiFluor dsDNA System (Promega) on Quantus (Promega), denatured, and diluted to 5 pM together with the control library PhiX. Sequencing was performed on a MiniSeq platform (Illumina) using the MiniSeq Mid Output Kit, 2 × 150 cycles, to obtain reads for both strands. Sequencing results were analyzed using EntroGen Variant Reporter v1.3 and Illumina Variant Studio 3.0 [11]. Single nucleotide variant (SNV) pathogenic alterations detected by NGS are listed in the supplementary data: Table S2 (see the electronic supplementary material).
2.5 NGS IDH1/2 Validation by Two Independent Methods, qPCR and Sanger Sequencing
In all cases where the IDH1/2 status was determined by IHC or MLPA, qPCR and Sanger sequencing were performed to resolve adjudicated discrepancies between different methods. IDH1/2 genotyping was validated using allele-specific qPCR (IDH-RT38, EntroGen) and Sanger resequencing. For qPCR analysis, the IDH1/2 Mutation Analysis Kit (EntroGen) was used, which is a screening test for the detection of the most prevalent mutations in codons 100 and 132 of the IDH1 gene and in codons 140 and 172 of the IDH2 gene. Sanger sequencing was performed using AmpliTaq Gold, Fast PCR Master Mix (ThermoScientific, USA), ExoSAP-ITTM (ThermoScientific, USA), BigDye™ Terminator v3.1 Cycle Sequencing Kit (ThermoScientific, USA), BigDye XTerminator™ Purification Kit (ThermoScientific, USA), and sequencing primers (forward: 5′ TCA CCA AAT GGC ACC ATA CGA 3′; reverse: 5′ TGT GTT GAG ATG GAC GCC TAT TTG TA 3′), receiving a 353-bp amplicon size sequenced on SeqStudio Genetic Analyzer (ThermoScientific, USA). Verified and validated pathogenic variants were used for subsequent WHO CNS5 classification.
2.6 Assessment of CDKN2A Homozygous Deletion
The biallelic deletion of the CDKN2A gene was performed using the Vysis Paraffin Pretreatment Reagent Kit (Abbott Molecular) according to the manufacturer’s protocol described before [12]. In hybridization, applied Vysis CDKN2A/CEP 9 FISH Probe Kit (Abbott Molecular). Assessment of copy number variants to evaluate the presence or absence of CDKN2A homozygous deletion through NGS was performed on Nextseq550 using SOLIDaccuTest CE-IVD (NGeneBio Co) according to the manufacturer’s protocol.
3 Results
3.1 Frequency of Pathogenic and Likely Pathogenic Variants
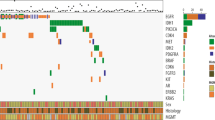
We performed NGS analysis using DNA isolated from 34 glioma patients. The TERT promoter pathogenic variants were the most prevalent (found in 23/34, 67.6% of patients), with 19 out of 23 patients having NC_000005.9:g.1295228G>A (commonly called C228T) and four out of 23 having NC_000005.9:g.1295250G>A (C250T). C250T and C228T variants were mutually exclusive (Table S2, see the electronic supplementary material). The highest rate of TERT promoter pathogenic variants was observed in oligodendroglioma (80%, 4/5 tested patients), followed by glioblastoma (72%, 13/18 patients). The IDH1/2 pathogenic variants were the second most prevalent in our sample (present in 13/34, 38.2% of patients), followed by the TP53 pathogenic variants (11/34, 32.3% of patients) and PIK3CA pathogenic variants (3/34, 8.8% of patients) (see Fig. 3). Figure 4 shows the coexistence of pathogenic variants. We can note that the pathogenic variants in EGFR did not occur in any case with the pathogenic variants in IDH1/2. In addition, pathogenic variants in IDH1/2 often coexisted with pathogenic variants in TP53. Examples of the most common pathogenic variants detected by NGS can be found in Fig. 5.
Examples of the most common pathogenic variants detected by NGS. The black arrows show the position of the detected variant. Abnormalities were assessed using TIER 2017 Variant Classification and AMCG 2015. A TERT promoter upstream gene variant NC_000005.9: g.1295228G>A TIER I; pathogenic. B TERT promoter upstream gene variant NC_000005.9:g.1295250G>A TIER I; pathogenic. C IDH1 R132H missense variant NM_005896.2:c.395G>A (p.Arg132His) TIER I. D IDH1 R132S missense variant NM_005896.2:c.394C>A (p.Arg132Ser) TIER II; Likely pathogenic. E PIK3CA missense variant NM_006218.2:c.1633G>A (p.Glu545Lys) TIER I; pathogenic. F BRAF missense variant NM_004333.4:c.1799T>A (p.Val600Glu) TIER I; pathogenic. NGS next-generation sequencing
3.2 Comparison of IHC or MLPA Results with NGS, qPCR, and Sanger Sequencing for IDH1/2 Status Assessment
To determine the optimal method for assessing IDH1/2 status, NGS findings acquired at the reference F. Łukaszczyk Oncology Center were compared to IHC or MLPA reports obtained at 12 different hospitals in Poland, none of which were oncology reference centers. The status of IDH1/2 was previously assessed using IHC in 13 cases and MLPA in four. The same FFPE material used for IHC or MLPA testing at other hospitals was used for NGS analysis (EntroGen NGS Targeted Hotspot Panel, CE-IVD) at the oncology reference center.
According to IHC reports from a non-oncological center, four samples were classified as IDH-wildtype, while NGS results revealed pathogenic variants. Moreover, three samples were classified as IDH-mutant, but no pathogenic variants were detected during the NGS examination (Table 1). Additionally, the reports did not specify the kind of antibody that was used in the IHC tests, nor whether they were CE-IVD antibodies. On the other hand, in the MLPA reports, there was one out of four findings, which was not consistent with the NGS. In this case, the IDH-wildtype result was obtained using MPLA, whereas NGS revealed the presence of the pathogenic variant not tested by MLPA.
Due to the fact that IDH1/2 NGS results differ from IHC (7/13 samples) as well as MLPA reports (1/4 samples), NGS results were validated using two independent methods: qPCR and Sanger sequencing. Firstly, the IDH1/2 Mutation Detection Kit (Entrogen CE-IVD), which allows the detection of eight IDH1 and nine IDH2 variants, was used. Secondly, the Sanger sequencing was conducted using the same set of primers [7], but a newer SeqStudio Genetic Analyzer. A direct comparison of NGS, qPCR, and Sanger sequencing with 5, 1, and 20% LOD, respectively, revealed 100% concordance, 100% specificity, and 100% sensitivity for the 34 (qPCR) and 31 (Sanger sequencing) samples analyzed in the oncology reference center by laboratory medical genetics specialists. However, we were unable to confirm the status of IDH1/2 with Sanger sequencing of three samples due to a lack of amplicons, as DNA was significantly degraded and quality was not sufficient for Sanger sequencing. However, the same three samples passed internal controls for qPCR and those qPCR final results were consistent with NGS analysis.
Moreover, we compared four available MLPA reports to our NGS, qPCR, and Sanger sequencing results. Due to the limited scope of the tested mutations by MLPA (R132C, R132H, R172K, and R172M), one of four was a false-negative result, as the R132L variant was not included in the commercial test to be detected by MLPA.
The number of IDH1/2 variants identified by the MLPA detection kit is limited, resulting in a concordance with NGS of 75%, a sensitivity of 66.6%, and a specificity of 100%. However, those results are not representative, because of the limited number of MLPA samples (only four cases; Table 2).
Unfortunately and surprisingly, for IHC reports, we observed a concordance with NGS of only 38.5%, with a sensitivity as low as 33.3% and a specificity as low as 42.9%. Due to the limited study sample and the fact that the reports came from five different facilities, estimated sensitivity and specificity values do not adequately reflect the IHC method's reliability (Fig. 6).
Examples of IHC and MLPA reports from different non-oncological reference centers versus NGS, qPCR, and Sanger sequencing results from the oncology reference center. Two false positives were detected by MLPA and IHC, and one false negative by IHC. Pathogenic variants detected by NGS were additionally validated by two independent methods: Sanger sequencing and qPCR. The black arrows indicate mutations detected using next-generation sequencing (NGS Targeted Hotspot Panel, Entrogen), Sanger sequencing (Applied Biosystems SeqStudio Genetic Analyzer), and qPCR (IDH1/2 Mutation Detection Kit, Entrogen), with a limit of detection of 5, 20, and 1%, respectively. Two red curves represent (1) positive control and (2) amplicon with mutation detected (arrow). The patient no. 13 – MLPA report indicates IDH-wildtype, while NGS, qPCR, and Sanger sequencing confirmed mutation in the IDH1 gene. IHC immunohistochemistry, MLPA multiplex ligation-dependent probe amplification, NGS next-generation sequencing, qPCR quantitative polymerase chain reaction
3.3 Reevaluation of Diagnosis Based on WHO CNS5
Taking into account the growing significance of genetic markers and WHO CNS5 diagnostic recommendations, NGS and FISH, if needed, were conducted to reevaluate the diagnosis.
TERT promoter pathogenic variants were identified in all (3/3) patients with WHO grade 3 IDH-wildtype glioma using NGS. The new WHO CNS5 classification recommends classifying these patients as grade 4 glioma rather than grade 3 (see Table 3). CDKN2A analysis was performed on samples derived from patients diagnosed with astrocytoma grade 2 (A2) or A3 who had IDH1/2 pathogenic variants.
Due to the fact that neither of the two tested samples indicated a homozygous deletion, no adjustment in classification was necessary in the analyzed group of glioma patients (Fig. 7). Changes from WHO grade 2 to grade 4 are crucial in terms of treatment and follow-up strategies. However, we were not able to provide an example of IDH-wildtype and TERT promoter mutated grade 2 glioma in our group.
Examples of FISH and NGS CNV for homozygous deletion of CDKN2A gene in glioma. A Representative FISH photo of a single glioma cell with CDKN2A homozygous deletion. A′ CDKN2A, normal fluorescent signals in glioma cell. B CNV analysis for CDKN2A. Lack of homozygous deletion. CNV copy number variation, FISH fluorescence in situ hybridization, NGS next-generation sequencing
4 Discussion
4.1 The Superiority of the NGS Method over the IHC or MLPA
IDH1/2 status can be assessed in multiple ways. Ten years ago, our group examined the prognostic value of IDH1/2 using various techniques, including IHC and molecular genetic testing (PCR, Sanger) [7]. Over the years, the majority of laboratories have implemented IHC, pyrosequencing, qPCR, or MLPA as routine glioma diagnosis procedures. In this study, we used NGS for detecting all known and novel variations and compared it to technologies that rely on IHC, Taqman-specific probes (qPCR), hybridization (MLPA), and Sanger sequencing. While evaluating final reports from non-reference non-oncological centers where IDH status was obtained (IHC or MLPA), we identified clinically substantial discordance that had an impact on WHO classification and treatment. Following the NGS analysis, we observed differences in the IDH1/2 status in seven out of 13 and one out of four of the patients determined by IHC and MLPA, respectively. To better understand how this discrepancy may affect clinical decisions, qPCR and Sanger sequencing were performed, and all NGS-based diagnoses were confirmed.
It is vital to realize that non-canonical IDH mutations occur at an 80% incidence in infratentorial astrocytomas, compared to 10% in supratentorial astrocytomas [13]. As a result, procedures such as IHC, MLPA, or qPCR may be employed as screening tests, with negative results requiring re-evaluation by NGS. In fact, in our study, there was a patient who had a non-canonical mutation; therefore, no mutation was found by IHC.
We found that the IDH1/2 status of seven out of 13 IHC results obtained in separate non-reference non-oncological centers was incorrectly classified. While the stereotactic biopsy is frequently used to diagnose brain tumors, only a tiny amount of material is collected. This could interfere with the IHC assessment, leading to false-negative results [14]. Moreover, preanalytical standardization of human tissues (both surgery or stereotactic biopsy), as well as histopathological staining and IHC processes, is extremely difficult to implement in hospitals [15]. Therefore, artifacts in IHC findings and challenges in tissue fixation and embedding decrease the sensitivity of the IHC test and make it more difficult to interpret the data unequivocally [16]. In contrast, during NGS, Sanger sequencing, and qPCR, DNA is amplified, enabling the detection of alterations in small tumor specimens. It could explain the inconsistent results in three cases where IHC identified them as IDH-wildtype. However, NGS and qPCR detected mutations in the IDH1/2 genes.
Taking into account the current 2021 WHO classification, which requires the analysis of multiple genetic alterations, clinical practice should be based on high-throughput technologies such as NGS in glioma management instead of time-consuming cascade diagnostics. Moreover, it is possible that the total cost of different routinely used techniques such as IHC, Sanger sequencing, PCR, or FISH (to evaluate a broad range of somatic IDH1/2, TERT, EGFR, CDKN2A, and 1p19q variants) will be more costly than single NGS analysis. On the other hand, implementing NGS in some clinical molecular diagnostic laboratories may be challenging and is unlikely to be accessible for smaller pathology laboratories in general [17]. Therefore, we recommend that a complete glioma diagnosis should be performed using NGS in molecular genetics laboratories located in reference oncological centers and supervised by experienced specialists in laboratory medical genetics. Collaboration between experts in neurosurgery, medical genetics, and pathomorphology is essential in the context of the WHO 2021 classification. Focusing on the strengths and minimizing the disadvantages is the best strategy for any individual specialist.
4.2 Integrated Interdisciplinary Diagnostics Algorithm in Adult Astrocytoma Patients
Advanced molecular techniques have developed significantly over the years. Therefore, molecular parameters have been added for more precise glioma grading and prognosis estimation. In 2016, the WHO CNS classification for the first time used molecular markers to classify gliomas, and in 2021, placed even more emphasis on them [3, 18]. Due to the fact that WHO CNS5 was published relatively recently, there is no standard for the molecular diagnosis of astrocytomas in the literature. In response to this need, we propose the following NGS evidence-based algorithm (Fig. 8).
Algorithm of integrated interdisciplinary diagnostics in astrocytoma patients. CDKN2A cyclin-dependent kinase inhibitor 2A homozygous deletion, CNV copy number variation, EGFR-amp epidermal growth factor receptor amplification, FISH fluorescence in situ hybridization, HE hematoxylin and eosin staining, IDH isocitrate dehydrogenase, IHC immunohistochemistry, MGMT O6-methylguanine-DNA methyltransferase, MS-PCR methylation-specific PCR, NGS next-generation sequencing, PCR polymerase chain reaction, STB stereotactic biopsy, TERT telomerase reverse transcriptase promoter pathogenic variants, mt mutant, wt wildtype, + 7/− 10 combination of chromosome 7 gain and 10 loss. *Not elsewhere classified (NEC); NEC diagnosis is facilitated by the use of layered integrated reports; integrated diagnosis combined tissue-based histological and molecular diagnosis
The first step in diagnosing a brain tumor is to obtain reliable tissue material during surgery. When microsurgical excision is not feasible, stereotactic biopsy should be performed due to its safeness, effectiveness, and high concordance with open surgical specimens in acquiring diagnostic material. In the era of the WHO CNS5 2021 classification, collecting representative samples for histological and molecular investigations is essential [19, 20]. Next, the IDH gene status assessment seems to be a crucial step in the management of a patient with astrocytoma. Based on the IDH1/2 genetic analysis and histopathological findings, the direction of further molecular diagnostics should be set. The diagnostics of patients with astrocytoma should begin with a histopathological grade assessment, especially to differentiate grade 4 gliomas (in the presence of necrosis and microvascular proliferation) from grade 2 or 3. In the case of grade 2 or 3 astrocytoma, we propose performing NGS as the first method of choice. Analysis should include as broad a range of glioma biomarkers as possible. The NGS panel must include IDH1/2, and TERT and additional analyses of PIK3CA and TP53 are welcome but not obligatory. We also propose the evaluation of CNV in CDKN2A, EGFR, and +7/−10 chromosomes by NGS analysis. In cases where NGS is chosen without a CNV panel, genetic evaluation of IDH-mutant astrocytoma must be followed by FISH to determine the presence of CDKN2A homozygous deletion or EGFR amplification.
On the other hand, in IDH-wildtype astrocytoma, further management depends on the presence of TERT promoter pathogenic variants and EGFR amplification. Therefore, simultaneous NGS analysis of IDH1/2 and TERT promoter together with EGFR amplification allows quick, proper WHO CNS5 2021 classification. If TERT or EGFR alteration was found in previously classified IDH-wildtype astrocytoma, then glioblastoma IDH-wildtype should be diagnosed. In contrast, the absence of TERT promoter pathogenic variants and EGFR amplification obliges analysis of the presence of + 7/− 10 chromosomal abnormalities, which can be tested during the first NGS analysis with CNV or separately by FISH. While the existence of the combination of aberrations allows for the diagnosis of IDH-wildtype glioblastoma, the absence of + 7/− 10 obliges the diagnosis of IDH-wildtype astrocytoma. In the case of grade 4 astrocytoma, it is sufficient to determine only the presence of an IDH gene mutation. If the mutation is identified, WHO CNS5 recommends classifying these tumors as IDH-mutant grade 4 astrocytoma. However, in the absence of the mutation, it is required to diagnose IDH-wildtype glioblastoma.
Last but not least, additional prognostic and predictive biomarkers, such as the assessment of MGMT methylation can be performed, especially in glioblastoma IDH-wildtype and A4 IDH-mutant. Finally, by integrating histopathological findings and molecular testing, a reliable and unequivocal diagnosis that enables targeted treatment can be made.
In non-reference hospitals, where NGS is not available, we suggest modifying the algorithm. Then, we recommend that the first-choice test should be a qPCR analysis of the IDH gene status. Subsequently, in the case of IDH-wildtype, qPCR would be used to test TERT and EGFR status. Considering patients with histological diagnosis of grade 4 astrocytoma, when the availability of advanced molecular techniques is limited, assessing the existence of the IDH1/2 mutations using qPCR would be sufficient to establish a precise diagnosis.
Although in grade 4 glioma patients WHO CNS5 only recommends evaluating the IDH gene, which could be done with qPCR, we suggest performing NGS using a panel that consists of the most important and the most frequently mutated genes in astrocytomas (such as IDH1/2, TERT, EGFR, TP53, and PIK3CA). This enables the diagnosis to be made not only based on histological findings (necrosis and vascular invasion), but also on molecular changes (the presence of genetic alterations that are proven to be related to shorter survival and are included in the WHO CNS5). As a result, assessing many genes using NGS enables a more precise prognosis.
According to the European Association of Neuro-Oncology (EANO) guidelines published before WHO CNS5, molecular analysis of IDH needs to be undertaken if the IHC result was IDH-wildtype [21]. It is critical to remember that these IHC guidelines were established prior to the introduction of the entire list of novel genetic biomarkers in WHO CNS5. As a result, these recommendations should be modified to incorporate NGS, which enables not only extensive analysis but also single-phase usage of the material. Therefore, in the case of an increasing number of biomarkers that need to be examined for the proper classification of gliomas, a comprehensive analysis should be conducted upfront, especially with a limited amount of biological material.
In conclusion, we suggest that NGS should be primarily used, firstly to assess IDH status, as our observations indicate that this method provides more reliable results [21], and secondly to assess a possibly broad range of biomarkers. It is expected that CDKN2A homozygous deletion, the combination of + 7/− 10, or EGFR amplification usually evaluated by FISH, will be replaced by single-step NGS CNV in the near future.
4.3 Limitations
Our study has several limitations. We did not have information about the methods of IDH1/2 determination in every patient, as the material was evaluated in 12 different centers. Since the type of IHC antibody was not specified in the histopathology data, we are unable to determine which specific antibody was responsible for the false-positive and false-negative results. Moreover, we performed NGS only on 34 glioma samples because only those materials met the quality criteria. The diagnostic algorithm is based on experience working with a small 16-gene panel (EntroGen NGS Targeted Hotspot CE-IVD) and a broader CNV-targeted (SOLIDaccuTest CE-IVD) NGS 84 gene panel for adult glioma; we did not focus on FGFR, the NTRK gene family, NF1, or H3 H3F3A, which are also a necessary focus, particularly for pediatric glioma classification.
5 Conclusions
Integration of histopathological findings and genetic aberrations provides an accurate and unequivocal diagnosis that enables targeted treatments. We evaluated how the use of NGS could be considered as a novel method to improve the classification and molecular characterization of glioma samples, particularly astrocytomas. Indeed, the WHO CNS5 guidelines express and confirm the significance of an integrated histogenetic classification of malignant gliomas, which should combine histological tumor subtyping and grading with various genetic biomarkers, such as gene mutations and CNVs. Additionally, we developed a new algorithm for molecular diagnosis of glioma patients using NGS based on the WHO CNS5.
References
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science”. Review n.d.:18.
Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15:405–17. https://doi.org/10.1038/s41582-019-0220-2.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncol. 2021. https://doi.org/10.1093/neuonc/noab106.
Wen PY, Packer RJ. The 2021 WHO Classification of Tumors of the Central Nervous System: clinical implications. Neuro-Oncol. 2021;23:1215–7. https://doi.org/10.1093/neuonc/noab120.
Singh RR. Next-generation sequencing in high-sensitive detection of mutations in tumors: challenges, advances, and applications. J Mol Diagn. 2020;22:994–1007. https://doi.org/10.1016/j.jmoldx.2020.04.213.
Tsiatis AC, Norris-Kirby A, Rich RG, Hafez MJ, Gocke CD, Eshleman JR, et al. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. J Mol Diagn. 2010;12:425–32. https://doi.org/10.2353/jmoldx.2010.090188.
Lewandowska MA, Furtak J, Szylberg T, Roszkowski K, Windorbska W, Rytlewska J, et al. An analysis of the prognostic value of IDH1 (isocitrate dehydrogenase 1) mutation in polish glioma patients. Mol Diagn Ther. 2014;18:45–53. https://doi.org/10.1007/s40291-013-0050-7.
Mardis ER. A decade’s perspective on DNA sequencing technology. Nature. 2011;470:198–203. https://doi.org/10.1038/nature09796.
D’Haene N, Meléndez B, Blanchard O, De Nève N, Lebrun L, Van Campenhout C, et al. Design and validation of a gene-targeted, next-generation sequencing panel for routine diagnosis in gliomas. Cancers. 2019;11:773. https://doi.org/10.3390/cancers11060773.
Priesterbach-Ackley LP, Wesseling P, Snijders TJ, de Vos FYFL, de Leng WWJ. Molecular tools for the pathologic diagnosis of central nervous system tumors. Neuro-Oncol Pract. 2019;6:4–16. https://doi.org/10.1093/nop/npy041.
Szczerba E, Kamińska K, Mierzwa T, Misiek M, Kowalewski J, Lewandowska MA. BRCA1/2 mutation detection in the tumor tissue from selected polish patients with breast cancer using next generation sequencing. Genes. 2021;12:519. https://doi.org/10.3390/genes12040519.
Chrzanowska NM, Kowalewski J, Lewandowska MA. Use of fluorescence in situ hybridization (FISH) in diagnosis and tailored therapies in solid tumors. Molecules. 2020;25:1864. https://doi.org/10.3390/molecules25081864.
Banan R, Stichel D, Bleck A, Hong B, Lehmann U, Suwala A, et al. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol (Berl). 2020;140:569–81. https://doi.org/10.1007/s00401-020-02194-y.
Gelpi E, Birner P, Haberler C, Koperek O, Budka H, Hainfellner JA. Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol (Berl). 2004;108:24–30. https://doi.org/10.1007/s00401-004-0856-9.
Dunstan RW, Wharton KA, Quigley C, Lowe A. The use of immunohistochemistry for biomarker assessment—can it compete with other technologies? Toxicol Pathol. 2011;39:988–1002. https://doi.org/10.1177/0192623311419163.
Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–64. https://doi.org/10.1200/JCO.2000.18.21.3651.
Jabbar KJ, Luthra R, Patel KP, Singh RR, Goswami R, Aldape KD, et al. Comparison of next-generation sequencing mutation profiling with BRAF and IDH1 mutation-specific immunohistochemistry. Am J Surg Pathol. 2015;39:454–61. https://doi.org/10.1097/PAS.0000000000000325.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl). 2016;131:803–20. https://doi.org/10.1007/s00401-016-1545-1.
Furtak J, Śledzińska P, Bebyn MG, Szylberg T, Krajewski S, Birski M, et al. Infratentorial stereotactic biopsy of brainstem and cerebellar lesions. Brain Sci. 2021;11:1432. https://doi.org/10.3390/brainsci11111432.
Furtak J, Mielczarek M, Szylberg M, Harat M. Biomarker concordance between molecular stereotactic biopsy and open surgical specimens in gliomas. Neurol Neurochir Pol. 2019;53:435–41. https://doi.org/10.5603/PJNNS.a2019.0056.
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–86. https://doi.org/10.1038/s41571-020-00447-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Conceptualization: PŚ, MAL, and MH; methodology: PŚ and ES; software: PŚ and MAL; formal analysis: PŚ and MAL; investigation: ES, NO, KK, and PŚ; resources: ES and MAL; data curation: PŚ and MAL; writing—original draft preparation: PŚ; writing—review and editing: PŚ and MAL; visualization: PŚ and MB; supervision: MAL; funding acquisition: JK, MAL, and JF; conceptualization related only to the clinical context of TERT mutation: MH; CDKN2A FISH analysis: NO; CDKN2A NGS analysis: KK. All authors read the final version and agreed to the published version of the manuscript.
Funding
This research was supported by funds for statutory research from the Ludwik Rydygier Collegium Medicum Nicolaus Copernicus University (UMK CM 2018 WL103).
Ethics and publication participation & consent
The study was conducted in accordance with the Declaration of Helsinki, and the Ethics Committee of the Nicolaus Copernicus University, Collegium Medicum in Bydgoszcz, Poland approved the study protocol (KB 265/2012).
Informed consent statement
Informed consent was obtained from all participants involved in the study.
Data availability statement
All data relevant to this study are reported within the manuscript.
Code availability
Not applicable
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Śledzińska, P., Bebyn, M., Szczerba, E. et al. Glioma 2021 WHO Classification: The Superiority of NGS Over IHC in Routine Diagnostics. Mol Diagn Ther 26, 699–713 (2022). https://doi.org/10.1007/s40291-022-00612-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00612-3