Abstract
Background
Sarcoidosis features non-necrotizing granulomas consisting mainly of activated CD4-lymphocytes. T-cell activation is regulated by immune checkpoint (IC) molecules. The present study aimed to compare IC expression on CD4, CD8 and NK cells from peripheral, alveolar and lung‐draining lymph node (LLN) samples of sarcoidosis patients.
Methods
Flow-cytometry analysis was performed to detect IC molecules and a regression decision tree model was constructed to investigate potential binary classifiers for sarcoidosis diagnosis as well as for the IC distribution.
Results
Fourteen patients (7 females) were consecutively recruited in the study; all enrolled patients showed hilo-mediastinal lymph node enlargement and lung parenchyma involvement with chest X-rays and high resolution computed tomography. CD4+PD1+ and CD8+PD1+ were higher in bronchoalveolar lavage (BAL) than in LLN (p = 0.0159 and p = 0.0439, respectively). CD4+ T-cell immunoglobulin and ITIM domain (TIGIT)+ were higher in BAL than in peripheral blood mononuclear cells (PBMCs) (p = 0.0239), while CD8+TIGIT+ were higher in PBMC than in BAL (p = 0.0386). CD56+TIGIT+ were higher in LLN than in PBMC (p = 0.0126). The decision-tree model showed the best clustering cells of PBMC, BAL and LLN: CD56, CD4/CD8 and CD4+TIGIT+ cells. Considering patients and controls, the best subset was CD4+CTLA-4+.
Conclusion
High expression of PD1 and TIGIT on T cells in BAL, as well as CTLA-4 and TIGIT on T cells in LLN, suggest that inhibition of these molecules could be a therapeutic strategy for avoiding the development of chronic inflammation and tissue damage in sarcoidosis patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immune checkpoint (IC) molecules are key regulators of T cells. |
Sarcoidosis consists mainly of activated CD4-lymphocytes. |
Knowledge about the distribution of IC expressed on T cells at the peripheral, alveolar and lymph node level could be translated in a therapeutic strategy for avoiding the development of chronic inflammation and tissue damage in sarcoidosis patients. |
1 Introduction
Sarcoidosis is a systemic granulomatous disorder of unknown origin that can develop in any organ, but usually affects the lungs [1, 33, 34]. It features non-necrotizing granulomas consisting mainly of activated CD4+ T helper lymphocytes, which accumulate in affected tissue. Pulmonary sarcoidosis is therefore associated with an elevated CD4+/CD8+ ratio in bronchoalveolar lavage (BAL) fluid. This ratio is useful for diagnosis. Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) is currently the preferred initial method for evaluating intrathoracic lymphadenopathy in cases of suspected sarcoidosis. Previous studies reported that the elevated CD4/CD8 T-cell ratio in BAL could not be demonstrated in lung‐draining lymph node (LLN) samples obtained by EBUS‐TBNA [2]. T-cell activation is regulated by co-stimulatory factors, such as CD28, and co-inhibitory factors, such as immune checkpoint (IC) molecules. Suppression of T cells occurs during binding of the T-cell receptor to antigen/major histocompatibility complex with the involvement of IC molecules [3].
Autoimmunity was recently postulated as a possible cause of sarcoidosis [4]. Co-inhibitory receptors play a central role in regulating autoimmune disease and have been linked genetically to such diseases [5, 6]. IC molecules have also come to the forefront in cancer [7] and chronic viral infections [8], where these receptors are highly expressed and are being targeted clinically to improve T-cell responses. Kotetsu and colleagues speculated that T-cell BAL expressing programmed cell death 1 (PD-1) and T-cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) are involved in the pathogenesis of sarcoidosis and are associated with computed tomography (CT) evidence of spontaneous improvement [9]. IC molecules are also overexpressed on natural killer (NK) cells, inducing cell exhaustion. NK and NKT cells are gradually becoming focal points in research efforts to unravel the pathways influencing granuloma resolution and persistence [10,11,12,13,14]. To our knowledge, a detailed comparison of the expression of IC molecules on T cells in different anatomical compartments is still lacking. Here we focus on the following proteins: PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) as negative regulators of activated T cells, and the T cell immunoglobulin and ITIM domain (TIGIT) as the next generation of IC molecules which also suppressively regulate immune responses of T cells through their unique signals. Taken together, these findings underline the importance of performing comparative studies in different anatomical compartments side by side in the same patient in order to better elucidate the immune responses associated with the development and progression of sarcoidosis.
The aim of the present study was to analyse and compare expression of IC molecules on CD4, CD8 and NK cells from peripheral, alveolar and LLN samples of sarcoidosis patients. We also compared their expression in peripheral samples from sarcoidosis patients and a group of healthy volunteers.
2 Materials and Methods
2.1 Study Design, Patient Characteristics and Controls
Patients who were subjected to contemporary BAL, LLN and peripheral blood collection and newly diagnosed with sarcoidosis at Siena University Hospital and Perugia Hospital between November 2020 and November 2021 were eligible for enrolment in this study. All patients were referred for bronchoscopy. They were diagnosed with sarcoidosis according to WASOG (World Association of Sarcoidosis and other Granulomatous Disorders) guidelines based on clinical signs, chest radiography findings, and non‐caseating granulomas in lymph nodes and/or endobronchial biopsy specimens. Patients with calcific lymph nodes as well as those patients with simultaneous peripheral blood and bronchoscopy were excluded. At the time of bronchoscopy, all patients still had enlarged lymph nodes, as assessed by computer tomography prior to bronchoscopy, to conduct EBUS‐TBNA. Informed consent was obtained prior to bronchoscopy. Five healthy volunteers (median age [interquartile range, IQR] 52 [39–55] years, 2 males) were also enrolled. They had no history of concomitant pathologies and were not on any medication. BAL and EBUS-TBNA were not performed on healthy volunteers for ethical reasons. The study was approved by the regional ethical review board of Siena, Italy (C.E.A.V.S.E. Markerlung 17431) and complied with the declaration of Helsinki.
2.2 Bronchoscopy
Venous blood (32 mL) was drawn from each patient into anticoagulant EDTA tubes (BD Vacutainer®, BD Biosciences, CA, USA) before they were treated with fentanyl 100 μg and midazolam (3–5 mg) IV 15–30 minutes prior to bronchoscopy. Lidocaine was administered to the larynx and bronchi for topical anaesthesia. A Pentax bronchoscope EB15-J10 (Pentax Medical Company, PENTAX Europe GmbH, Hamburg Germany) was inserted through the mouth to avoid blood contamination. BAL with 3 × 50-mL saline solution was instilled in the middle or lingual lobe. EBUS‐TBNA using linear EBUS EB-1970UK (Pentax Medical Company, PENTAX Europe GmbH, Hamburg Germany) was then performed to sample mediastinal and hilar LLNs. LLN aspirate was obtained using Echotip Ultra (ECHO-HD-22-EBUS, Cook Medical, Ireland). A single-pass sample (5 mL) was obtained for laboratory analysis.
2.3 Single Cell Preparations From Blood, Bronchoalveolar Lavage Fluid and Lymph Nodes
Peripheral blood mononuclear cells (PBMCs) were isolated from blood collected in EDTA tubes according to the manufacturer's protocol. BAL samples were kept on ice, filtered through a 100-μm nylon filter (Syntab) and centrifuged at 400×g for 15 min. LLN aspirates were filtered through a 40 μm nylon cell strainer and centrifuged at 300×g for 10 min. Lysis of red blood was performed with 1 × BD FACS lysing solution (BD Biosciences, CA, USA) for 5 min; the cells were then centrifuged at 300×g for 5 min. Cells were counted manually and Trypan Blue was used to assess viability as previously reported [15]. All samples reported at least 95% of cell viability.
2.4 Flow Cytometry
Multicolour flow cytometric analysis was performed using the following fluorochrome-labelled monoclonal anti-human antibodies against surface molecules: TIGIT-FITC (MBSA43, Invitrogen, Thermo Fisher Scientific, US), PD1-PE (PD1.3.1.3, Miltenyi Biotec, Germany), CTLA4-PeCy7 (14D3, Invitrogen, Thermo Fisher Scientific, US), CD3-APC (UCHT1, Biolegend, US), CD4-APC-Cy7 (RPA-T4, Biolegend, US), CD8-BV421 (SK1, Biolegend, US) and CD56-PerCPCy5.5 (5.1H11, Biolegend, US). Cells were stained for 15 mins at 4 °C, measured with a Facs CantoII (BD Biosciences, CA, USA) flow cytometer and analysed with Kaluza Analysis 2.1 software (Beckman and Coulter Life Sciences). The gating strategy is reported in Figure s1 (see electronic supplementary material [ESM]).
2.5 Cytometric Bead Array Analysis
Interleukin (IL)-10, tumour necrosis factor-α (TNFα) and interferon (IFN)-γ were analysed in blood from controls and sarcoidosis patients and in BAL from patients drawn at the same time as that for cell analysis, as previously reported [15, 16]. Analysis of samples was performed according to the manufacturer’s instructions [17] using a Facs CantoII flow cytometer and LEGENDPlex™ V8.0 software (Biolegend). Before analysis, the cytometer was calibrated using set-up beads according to the manufacturer’s protocol. An example of a dot-plot (cytogram) is shown in Fig. s2 (see ESM). LEGENDPlex™ V8.0 software was used to calculate mean fluorescence intensity (MFI) for each population of molecules that bound a given cytokine. Concentrations were expressed in pg/mL and were entered in the database.
2.6 Statistical Analysis
All data is reported as median and interquartile range (IQR) or mean and standard error of the mean (M ± SEM). Significant differences between BAL, LLN and PBMC samples from controls and patients were determined by Student’s t-test or the Mann-Whitney U test. Correlations were determined by Spearman correlation coefficient. Probability values < 0.05 were considered significant. Statistical analysis was performed by GraphPad Prism 9.3 and XLSTAT 2021 software. Unsupervised principal component analysis (PCA) was employed to reduce the dimensionality of data hyperspace. The data matrix was constructed with Microsoft Excel. The cell subsets of BAL, LLN and PBMC from patients and controls were used to build a decision-tree model to determine the best clustering variables according to the Gini criterion.
3 Results
3.1 Study Population
We enrolled 41 patients with suspected sarcoidosis at the first visit. We consecutively and prospectively analysed 14 patients between the ages of 50 and 60 years (50% female) from whom we collected, at the same time, PBMC, LLN and BAL. Demographic, immunological and clinical data is reported in Table 1. Seven patients were females and there was a prevalence of never smokers (57%). All patients were in radiological Scadding stage II. None were on treatment at the time of bronchoscopy and sampling. Lymphocytosis (median [IQR]: 20 [9–38]) and high CD4/CD8 ratio (median [IQR]: 4.79 [2.36–6.75]) were recorded in BAL samples from all patients.
3.2 PD1, TIGIT and CTLA-4 Expression on T and NK Cells and Cytokine Concentrations in Sarcoidosis Patients
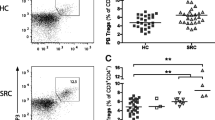
Higher CD4 cell percentages were observed in LLN than in PBMC samples (p = 0.0150) (Fig. 1). Higher CD4+PD1+ expression was observed in BAL than in LLN samples (p = 0.0159). TIGIT was expressed on a higher fraction of CD4+ cells in BAL than in LLN samples (p = 0.0010) (Fig. 1). CD4+TIGIT+ cells were therefore more abundant in BAL than in PBMC samples (p = 0.0239) (Fig. 1a). CTLA-4 expression on CD4-positive cells was higher in LLN samples than in BAL samples (p = 0.0296) (Fig. 1).
PD-1, CTLA-4 and TIGIT distribution on CD4, CD8 and NK cells from BAL, LLN and PBMC samples of sarcoidosis patients. All p-values are reported in the text. BAL bronchoalveolar lavage, CTLA-4 cytotoxic T-lymphocyte antigen 4, LLN lung‐draining lymph node, NK natural killer, PBMC peripheral blood mononuclear cells, PD-1 programmed cell death 1, SP sarcoidosis patients, TIGIT T-cell immunoglobulin and ITIM domain
CD8+ cell percentages were lower in LLN than in PBMC and BAL samples (p = 0.0469 and p = 0.0304, respectively) (Fig. 1). PD1 expression was higher on CD8+ cells from BAL samples than from LLN and PBMC samples (p = 0.0439 and p = 0.0386, respectively) (Fig. 1). Higher CD8+CTLA-4+ cell percentages were observed in LLN samples than in BAL samples (p = 0.0163) (Fig. 1). TIGIT was expressed on a higher fraction of CD8+ cells in PBMC than in BAL samples (p = 0.0312) (Fig. 1). However, TIGIT was not expressed on CD8-positive cells of LLN samples.
NK-CD56+ cell percentages were higher in PBMC than in LLN and BAL samples (p < 0.0001 and p = 0.0006, respectively) (Fig. 1). PD1 was not expressed on CD56-positive cells in LLN and BAL samples, while a small fraction of CD56+PD1+ cells was detected in PBMC samples. There was a higher fraction of CD56+TIGIT+ cells in LLN samples than in PBMC samples (p = 0.0126) (Fig. 1).
TNF-α and IFN-γ concentrations were higher in BAL than in serum samples (p = 0.0104 and p = 0.045, respectively).
3.3 Immune Checkpoint Expression and Cytokine Concentrations in PBMC From Sarcoidosis Patients and Controls
CD4+ cell percentages were lower in sarcoidosis patients than in controls (p = 0.0008), while CD4+CLTA-4+ and CD4+TIGIT+ cell percentages were higher in patients than controls (p = 0.0008 and p = 0.0020, respectively) (Fig. 2).
PD-1, CTLA-4 and TIGIT distribution on CD4, CD8 and NK cells from PBMC samples of sarcoidosis patients and healthy controls. All p-values are reported in the text. CTLA-4 cytotoxic T-lymphocyte antigen 4, HC healthy controls, NK natural killer, PBMC peripheral blood mononuclear cells, PD-1 programmed cell death 1, SP sarcoidosis patients, TIGIT T-cell immunoglobulin and ITIM domain
CD8+ and CD8+CTLA-4+ cell percentages were higher in sarcoidosis patients than controls (p = 0.0008 and p = 0.0364, respectively) (Fig. 2), while TIGIT and PD1 were not expressed on CD8-positive cells in PBMC samples from controls.
CD56-positive and CD56+TIGIT+ cell percentages were higher in sarcoidosis patients than controls (p = 0.0020 and p = 0.0152, respectively) (Fig. 2), while PD1 was not expressed on CD56-positive cells from PBMC samples.
Serum concentrations of IL-10, TNF-α and IFN-γ were higher in sarcoidosis patients than controls (p < 0.0001).
3.4 Correlation Analysis
Figure s3a (see ESM) shows the correlation matrix of IC expression on CD4, CD8 and NK cells from LLN, BAL and PBMC samples of sarcoidosis patients. In LLN samples, CD4+CTLA-4+ cell percentages were inversely correlated with CD56+TIGIT+ (r = − 1.000, p = 0.001) and CD56+CTLA-4+ cell percentages were inversely correlated with CD4+PD1+ and CD8+PD1+ cell percentages (r = − 1.000, p = 0.016 and r = − 1.000, p = 0.003, respectively). A direct correlation was observed between CD4+PD1+ and CD8+PD1+ cell percentages (r = 1.000, p = 0.013).
In BAL samples, CD56+CTLA-4+ cell percentages were inversely correlated with CD56+TIGIT+ cell percentages (r = − 0.999, p = 0.023). CD8+ cell percentages were inversely correlated with alveolar concentrations of IL-10 (r = − 0.822, p = 0.019), and CD56+ cell percentages were inversely correlated with alveolar concentrations of TNF-α (r = − 0.685, p = 0.042), while BAL-CD56+ cell percentages were directly correlated with alveolar concentrations of IL-10 (r = 0.773, p = 0.024).
In PBMC samples, CD8+CTLA-4+ cells were inversely correlated with CD8+PD1+ cell percentages (r = − 0.998, p = 0.036). CD8+CTLA-4+ and CD56+CTLA-4+ cell percentages were directly correlated with serum concentrations of IFN-γ (r = 0.757, p = 0.015 and r = 0.660, p = 0.037, respectively).
Figure s3b (see ESM) shows the correlation matrix of IC expression on CD4, CD8 and NK cells from PBMC samples of controls and sarcoidosis patients. A direct correlation was observed in the control group between CD56+TIGIT+ and CD8+CTLA-4+ cell percentages (r = 1.000, p < 0.0001). PBMC-CD56+ percentages from the control group were inversely correlated with serum concentrations of IFN-γ (r = − 0.975, p = 0.033), while a direct correlation was observed between CD8+ cells and serum concentrations of IFN-γ (r = 0.975, p = 0.033).
3.5 Multivariate Analysis
Analysis of variance of cell populations in PBMC from sarcoidosis patients showed a homogeneous distribution with low variance (red boxes) (Fig. s4, see ESM), while LLN, BAL and PBMC samples of controls showed high interindividual variance (green boxes) for many of the cell populations analysed (Fig. s4).
PCA plots were performed to distinguish the three groups (LLN, BAL and PBMC). The analysis showed separation of the three groups on the basis of cell phenotype (Fig. 3). The first and second components explained 41.99% of the total variance.
PCA analysis was also performed to distinguish controls and sarcoidosis patients on the basis of IC expression on peripheral CD4, CD8 and NK cells (Fig. 4). The first and second components explained 49.76% of the total variance.
The cell subsets of PBMC, BAL and LLN were used to build a decision-tree model (with cross-validation by confusion matrix) to determine the best clustering variables (Fig. 5). The model showed an improvement of 3.115 using a CD56 cut-off value of 6.71% for PBMC followed by 2.033 improvement using a CD4/CD8 cut-off of 5.77 and 1.5 improvement using CD4+TIGIT+ cut-off of 5.47%.
In order to determine the best clustering variables to distinguish sarcoidosis patients and the healthy control group, a decision-tree model (with cross-validation by confusion matrix) was used. The model obtained using PBMC cell subsets (Fig. 6) showed an improvement of 7.059 using a CD4+CTLA4+ cut-off value of 5.98% to identify sarcoidosis patients.
4 Discussion
This is the first time that expression of PD1, CTLA-4 and TIGIT on CD4, CD8 and NK cells in three different anatomical compartments of sarcoidosis patients have been analysed and compared, and that expression of IC molecules on T and NK cells in PBMC from patients and controls has been compared.
Elevated CD4/CD8 cell ratio in BAL is a criterion for the diagnosis of sarcoidosis, but has not been observed in LLN samples obtained by EBUS‐TBNA [2]. Although our study confirmed this in our sarcoidosis population, we observed higher CD4-positive and lower CD8-positive cell percentages in LLN samples than in PBMC and BAL samples of these patients, while NK cell percentages were higher in PBMC than in BAL and LLN samples from the same patients. Accordingly, assessing a decision-tree model, our study showed a CD4/CD8 ratio cut-off value of 5.77 followed by a NK cut-off value of 0.36% to identify BAL samples. The exaggerated effector T-cell responses are associated with deficiencies in regulatory T-cell and invariant natural killer T-cell numbers and function in sarcoidosis patients [18, 19].
Reciprocal interactions between NK cells and T lymphocytes further contribute to the regulation of innate and adaptive immune response [11, 20, 21]. However, the balance of activating and inhibitory signals determines whether they are tolerated or activated against their target cells.
PD-1 downregulates immune responses to prevent immune-mediated tissue damage and contributes to immune privilege of certain tissues and prevention of autoimmunity [22]. It has been reported that expression of PD-1 on CD4+ T cells in BAL is higher in patients with sarcoidosis than in healthy controls [23]. Our study confirmed these findings, showing higher PD1 expression on CD4-positive and CD8-positive cells in BAL samples from sarcoidosis patients.
Since PD-1 can down-regulate T-cell responses, higher expression of PD-1 on CD4+ and CD8+ T cells may down-regulate Th-1 cytokines (such as TNF-α and IFN-γ) and decrease mitotic activity so as to induce apoptosis, resulting in resolution of the granuloma [24]. Both cytokines were higher in our BAL samples than in serum, and higher peripheral concentrations were recorded in patients than in controls. Although these findings suggest that the inflammatory response of sarcoidosis may not have terminated, it has been reported that the proliferative capacity of CD4+ T cells in peripheral blood of patients with sarcoidosis is restored to that of healthy controls by blockade of the PD-1 pathway [23].
The ability of PD1+NK cells to reduce degranulation and expression of TNF-α and IFN-γ has been demonstrated [25]. However, in our cohort 0.39% of PD1+NK cells were identified in PBMC samples of sarcoidosis patients and none were identified in BAL and LLN samples of patients or in PBMC from controls.
Notably, the inhibitory receptors CTLA-4 and PD-1 act to constrain autoimmunity and their inhibition has been reported to initiate or exacerbate sarcoid-like inflammation [26], reinforcing the notion of altered immune activation and possibly an autoimmune component in sarcoidosis. Reduced expression of CTLA-4 on BAL fluid and mediastinal lymph node cells are seen in sarcoidosis, especially in patients with the non-Löfgren variant [27, 28]. Broos et al. found lower CTLA-4 expression on BAL T cells from patients with sarcoidosis than on those from healthy persons, suggesting a shift in the balance between immune activation and regulation that results in exacerbated T-cell activity and eventually in chronic inflammation and tissue damage [29]. Our study population showed higher CTLA-4 expression on CD4-positive and CD8-positive cells from LLN samples than on those from BAL of sarcoidosis patients, and lower expression in PBMC of controls. Moreover, peripheral percentages of CD4+CTLA4+ have been identified as best cluster variable to distinguish our sarcoidosis patients from healthy volunteers.
TIGIT belongs to the second wave of IC receptors and works in synergy with PD-1. It is reported to be highly expressed in tumour-infiltrating T lymphocytes in different tissues, especially in the lungs [30]. Suzuki and colleagues found high expression of TIGIT in T cells of BAL from patients with interstitial lung disease related to IC inhibitor therapy [31]. Our study revealed higher TIGIT expression in CD4+ cells and lower expression in CD8+ cells from BAL than in those from PBMC and LLN samples of sarcoidosis patients. TIGIT expression was also higher in CD4-positive cells of our sarcoidosis patients than in controls (but was not expressed in CD8+ cells of controls). Recently, Esen et al. investigated the role of TIGIT in NK cells from healthy donors and demonstrated that TIGIT+NK cells had reduced degranulation in the absence of stimulation, but their expression of cytotoxic granules remained unchanged [32]. Our study is the first to reveal higher expression of TIGIT in CD56-positive cells in LLN than in PBMC samples from sarcoidosis patients. Moreover, TIGIT+CD56+ cell percentages were higher in PBMC from sarcoidosis patients than controls.
Our study has some limitations; first of all, the monocentric nature of the study and the limited sample size of study population. Although our study demonstrated the level of IC expression on T and NK cells at the time of bronchoscopic examination, we were not able to enrol enough patients to establish a definite cut-off value, or for ethical reasons to do a comparative analysis on BAL and LLN samples from healthy donors. Moreover, due to technical reasons and ethical issues, we were forced to enrol in the study a specific phenotype of sarcoidosis patients, characterized by hilo-mediastinal lymph node enlargement and lung parenchymal involvement. Even though this can be considered the most common clinical manifestation of sarcoidosis, our findings may not be suitable for all disease phenotypes, also considering its wide heterogeneity in terms of clinical aspect and disease localizations.
5 Conclusion
We now know more about the pathogenesis of sarcoidosis and are entering a new era of personalized, and hopefully more effective, treatment of sarcoidosis.
Although the detailed mechanism of the present findings remains unclear, high expression of PD1 and TIGIT on T cells in BAL, as well as CTLA-4 and TIGIT on T cells in LLN, suggest that inhibition of these molecules could be a therapeutic strategy for avoiding the development of chronic inflammation and tissue damage in sarcoidosis patients.
References
Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers. 2019;5:45.
Ruiz SJ, Zhang Y, Mukhopadhyay S. CD4/CD8 ratio in mediastinal lymph nodes involved by sarcoidosis: analysis of flow cytometry data obtained by endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchology Interv Pulmonol. 2016;23:288–97.
Li B, Chan HL, Chen P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr Med Chem. 2019;26:3009–25.
Wahlström J, Dengjel J, Winqvist O, Targoff I, Persson B, Duyar H, et al. Autoimmune T cell responses to antigenic peptides presented by bronchoalveolar lavage cell HLA-DR molecules in sarcoidosis. Clin Immunol. 2009;133:353–63.
Kasagi S, Kawano S, Kumagai S. PD-1 and autoimmunity. Crit Rev Immunol. 2011;31:265–95.
Qu H-Q, Bradfield JP, Grant SFA, Hakonarson H, Polychronakos C, Type I Diabetes Genetics Consortium. Remapping the type I diabetes association of the CTLA4 locus. Genes Immun. 2009;10 Suppl 1:S27-32.
Zou W, Wolchok JD, Chen L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45:358–73.
Kotetsu Y, Yanagihara T, Suzuki K, Ando H, Eto D, Hata K, et al. Imaging changes and immune-checkpoint expression on T cells in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Biomedicines. 2021;9:1231.
D’alessandro M, Bergantini L, Carleo A, Cameli P, Perrone A, Fossi A, et al. Neutrophil-to-lymphocyte ratio in bronchoalveolar lavage from IPF patients: a novel prognostic biomarker? Minerva Med. 2020. https://doi.org/10.23736/S0026-4806.20.06614-8.
Bergantini L, Cameli P, d’Alessandro M, Vagaggini C, Refini RM, Landi C, et al. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin Exp Med. 2019;19:487–94.
Bergantini L, d’Alessandro M, Vietri L, Rana GD, Cameli P, Acerra S, et al. Utility of serological biomarker’ panels for diagnostic accuracy of interstitial lung diseases. Immunol Res. 2020;68:414–21.
d’Alessandro M, Carleo A, Cameli P, Bergantini L, Perrone A, Vietri L, et al. BAL biomarkers’ panel for differential diagnosis of interstitial lung diseases. Clin Exp Med. 2020;20:207–16.
Manika K, Domvri K, Kyriazis G, Kontakiotis T, Papakosta D. BALF and BLOOD NK-cells in different stages of pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2022;38: e2021039.
Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111:A3.B1-A3.B3.
d’Alessandro M, Bergantini L, Cameli P, Fanetti M, Alderighi L, Armati M, et al. Immunologic responses to antifibrotic treatment in IPF patients. Int Immunopharmacol. 2021;95: 107525.
d’Alessandro M, Bergantini L, Cameli P, Mezzasalma F, Refini RM, Pieroni M, et al. Adaptive immune system in pulmonary sarcoidosis-Comparison of peripheral and alveolar biomarkers. Clin Exp Immunol. 2021;205:406–16.
Human Th1/Th2/Th17 CBA Kit [Internet]. [cited 2021 Nov 18]. https://www.bdbiosciences.com/en-us/products/reagents/immunoassay-reagents/cba/cba-kits/human-th1-th2-th17-cba-kit.560484. Accessed 12 Feb 2022.
Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–7.
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70.
Gruber R, Pforte A, Beer B, Riethmüller G. Determination of gamma/delta and other T-lymphocyte subsets in bronchoalveolar lavage fluid and peripheral blood from patients with sarcoidosis and idiopathic fibrosis of the lung. APMIS. 1996;104:199–205.
Kurumagawa T, Seki S, Kobayashi H, Koike Y, Kanoh S, Hiraide H, et al. Characterization of bronchoalveolar lavage T cell subsets in sarcoidosis on the basis of CD57, CD4 and CD8. Clin Exp Immunol. 2003;133:438–47.
Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67.
Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med. 2014;190:560–71.
Kaiser Y, Lakshmikanth T, Chen Y, Mikes J, Eklund A, Brodin P, et al. Mass cytometry identifies distinct lung CD4+ T cell patterns in Löfgren’s syndrome and non-Löfgren’s syndrome sarcoidosis. Front Immunol. 2017;8:1130.
Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med. 2018;10(460):eaar8356. https://doi.org/10.1126/scitranslmed.aar8356.
Danlos F-X, Pagès C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:e133-136.
Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–50.
Broos CE, van Nimwegen M, In't Veen JC, Hoogsteden HC, Hendriks RW, van den Blink B, Kool M. Decreased cytotoxic T-lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in Sarcoidosis: double trouble? Am J Respir Crit Care Med 2015;192(6):763–5. https://doi.org/10.1164/rccm.201503-0635LE.
Broos CE, van Nimwegen M, In’t Veen JC, Hoogsteden HC, Hendriks RW, van den Blink B, et al. Decreased cytotoxic T-lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in sarcoidosis: double trouble? Am J Respir Crit Care Med. 2015;192(6):763–5. https://doi.org/10.1164/rccm.201503-0635LE.
Suzuki K, Yanagihara T, Matsumoto K, Kusaba H, Yamauchi T, Ikematsu Y, Tanaka K, Otsubo K, Inoue H, Yoneshima Y, Iwama E, Arimura-Omori M, Harada E, Hamada N, Okamoto I, Nakanishi Y. Immune-checkpoint profiles for T cells in bronchoalveolar lavage fluid of patients with immune-checkpoint inhibitor-related interstitial lung disease. Int Immunol. 2020;32(8):547–57. https://doi.org/10.1093/intimm/dxaa022.
Esen F, Deniz G, Aktas EC. PD-1, CTLA-4, LAG-3, and TIGIT: The roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol Lett. 2021;240:15–23. https://doi.org/10.1016/j.imlet.2021.09.009.
Cameli P, Gonnelli S, Bargagli E, d’Alessandro M, Bergantini L, Favetta V, Tomai Pitinca MD, Lisi E, Refini RM, Pieroni M, Sestini P, Caffarelli C. The role of urinary calcium and Chitotriosidase in a cohort of chronic Sarcoidosis patients. Respiration 2020;99(3):207–12. https://doi.org/10.1159/000505653.
Bennett D, Cameli P, Lanzarone N, Carobene L, Bianchi N, Fui A, et al. Chitotriosidase: a biomarker of activity and severity in patients with sarcoidosis. Respir Res 2020;21(1). https://doi.org/10.1186/s12931-019-1263-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Comitato Etico Area Vasta Sud Est, Tuscany (Markerlung, 17431). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Availability of data and material
The data presented in this study are available on request from the corresponding author.
Code availability
Not applicable.
Authors' contributions
Conceptualization: Md; Methodology: Md, LB, FM; Software: Md; Validation: Md, PC, SC, EB, LB; Formal analysis: Md; Investigation: PC, FM, EB; Resources: EB, LB, Md; Data curation: Md; Writing—original draft preparation: Md, LB, FM, PC, SC, EB; Writing—review and editing: all authors; visualization: Md, EB; Supervision: Md, LB, FM, PC, SC, EB. All authors have read and agreed to the published version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
d’Alessandro, M., Bergantini, L., Mezzasalma, F. et al. Immune-Checkpoint Expression on CD4, CD8 and NK Cells in Blood, Bronchoalveolar Lavage and Lymph Nodes of Sarcoidosis. Mol Diagn Ther 26, 437–449 (2022). https://doi.org/10.1007/s40291-022-00596-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00596-0