Abstract
Background
Molecular characterization of tumors could be a key to therapeutic decision-making with regards to targeted therapies in castration-resistant prostate cancer (CRPC). A convenient solution may be non-invasive liquid biopsy testing of circulating tumor cells (CTCs). For this reason, CTC-enriched samples obtained by immunomagnetic separation (AdnaTest®) were studied as a source material for high-throughput gene expression analysis using BioMark™.
Patients and Methods
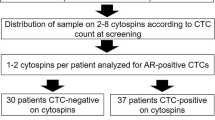
CTC-enriched samples from 41 CRPC patients previously determined to be CTC positive using the AdnaTest® were retrospectively re-analysed for androgen receptor (AR) messenger RNA (mRNA), using the updated AdnaTest®. Blood samples were drawn two times from each patient: at the time of CRPC diagnosis and after the third docetaxel cycle. A gene expression panel of 27 genes related to CRPC therapeutic decision-making, including AR full length (ARFL) and splice variant 7 (ARV7), was retrospectively analyzed on a BioMark™ platform in 29 of 41 patients.
Results
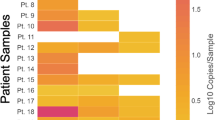
The AdnaTest® detected AR mRNA in three-quarters of CTC-positive samples taken at the time of CRPC diagnosis and after the third docetaxel cycle. AR detection was associated with a shorter disease-specific survival (45.0 vs. 20.4 months) at the time of CRPC diagnosis. ARFL expression at the time of CRPC diagnosis, measured on the BioMark™ platform, was associated with a lower decrease of serum level of prostate-specific antigen (sPSA) (p = 0.029), i.e., worse therapy response. ARV7 was found in 38% of the ARFL–-positive samples at both analyzed timepoints.
Conclusion
Detection of AR expression by AdnaTest® in CTC-enriched samples may help predict patients’ survival. These AdnaTest® CTC-enriched samples can be used in a high-throughput quantitative polymerase chain reaction (qPCR) analysis of gene expression, provided that the specificity of the assay for each individual gene is properly validated. The BioMark™ platform can be used for the simultaneous detection of ARFL and ARV7 and other genes in CTC-enriched samples from CRPC patients.




Similar content being viewed by others
References
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Bahl A, Oudard S, Tombal B, Ozgüroglu M, Hansen S, Kocak I, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24:2402–8.
Gulley JL, Mulders P, Albers P, Banchereau J, Bolla M, Pantel K, et al. Perspectives on sipuleucel-T: its role in the prostate cancer treatment paradigm. Oncoimmunology. 2016;5:e1107698.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Armstrong AJ, Eisenberger MA, Halabi S, Oudard S, Nanus DM, Petrylak DP, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol. 2012;61:549–59.
Lunardi A, Ala U, Epping MT, Salmena L, Clohessy JG, Webster KA, et al. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat Genet. 2013;45:747–55.
Mahon KL, Henshall SM, Sutherland RL, Horvath LG. Pathways of chemotherapy resistance in castration-resistant prostate cancer. Endocr Relat Cancer. 2011;18:R103–23.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38.
Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9.
Chen J-F, Lu Y-T, Cheng S, Tseng H-R, Figlin RA, Posadas EM. Circulating tumor cells in prostate cancer: beyond enumeration. Clin Adv Hematol Oncol. 2017;15:63–73.
Chen C-L, Mahalingam D, Osmulski P, Jadhav RR, Wang C-M, Leach RJ, et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate. 2013;73:813–26.
Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, Sleijfer S. The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2016;46:42–50.
Galletti G, Portella L, Tagawa ST, Kirby BJ, Giannakakou P, Nanus DM. Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential. Mol Diagn Ther. 2014;18:389–402.
Čapoun O, Mikulová V, Jančíková M, Honová H, Kološtová K, Sobotka R, et al. Prognosis of castration-resistant prostate cancer patients—use of the AdnaTest® system for detection of circulating tumor cells. Anticancer Res. 2016;36:2019–26.
Todenhöfer T, Hennenlotter J, Feyerabend S, Aufderklamm S, Mischinger J, Kühs U, et al. Preliminary experience on the use of the Adnatest® system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res. 2012;32:3507–13.
Hensler M, Vančurová I, Becht E, Palata O, Strnad P, Tesařová P, et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. Oncoimmunology. 2016;5:e1102827.
Škereňová M, Mikulová V, Čapoun O, Zima T. The characterization of four gene expression analysis in circulating tumor cells made by Multiplex-PCR from the AdnaTest kit on the lab-on-a-chip Agilent DNA 1000 platform. Biochem Medica. 2016;26:103–13.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of realtime quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034
Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–50.
Allan AL, Keeney M. Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol. 2010;2010:426218.
Sieuwerts AM, Kraan J, Bolt-de Vries J, van der Spoel P, Mostert B, Martens JWM, et al. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat. 2009;118:455–68.
Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56:1492–5.
Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, Eisenberger MA, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–65.
Sprenger C, Uo T, Plymate S. Androgen receptor splice variant V7 (AR-V7) in circulating tumor cells: a coming of age for AR splice variants? Ann Oncol. 2015;26:1805–7.
Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJM, Mathijssen RHJ, et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45.
Dijkstra S, Leyten GHJM, Jannink SA, de Jong H, Mulders PFA, van Oort IM, et al. KLK3, PCA3, and TMPRSS2-ERG expression in the peripheral blood mononuclear cell fraction from castration-resistant prostate cancer patients and response to docetaxel treatment. Prostate. 2014;74:1222–30.
Yao X, Choudhury AD, Yamanaka YJ, Adalsteinsson VA, Gierahn TM, Williamson CA, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr Biol. 2014;6:388–98.
Gorges TM, Riethdorf S, von Ahsen O, Nastał YP, Röck K, Boede M, et al. Heterogeneous PSMA expression on circulating tumor cells: a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget. 2016;7:34930–41.
Gao S, Ye H, Gerrin S, Wang H, Sharma A, Chen S, et al. ErbB2 signaling increases androgen receptor expression in abiraterone-resistant prostate cancer. Clin Cancer Res. 2016;22:3672–82.
Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105.
Olmos D, Arkenau H-T, Ang JE, Ledaki I, Attard G, Carden CP, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol. 2009;20:27–33.
Bitting RL, Healy P, Halabi S, George DJ, Goodin M, Armstrong AJ. Clinical phenotypes associated with circulating tumor cell enumeration in metastatic castration-resistant prostate cancer. Urol Oncol. 2015;33:110.e1–9.
Acknowledgements
We would like to thank our collaborators from the Laboratory of Gene Expression, Institute of Biotechnology, Czech Academy of Sciences (CAS) for their help with qPCR measurement and data analysis and Ing. Stanislav Kormunda for his help with the statistical evaluation of the results.
Author information
Authors and Affiliations
Ethics declarations
Funding
This work was supported by the Ministry of Health, Czech Republic, IGA NT 12205-5/201; institutional support of the General University Hospital RVO VFN 64165 and Progres Q25/LF1; research support of the First Faculty of Medicine SVV 260 263; BIOCEV CZ.1.05/1.1.00/02.0109 from the European Regional Development Fund (ERDF); and LQ1604 NPU II provided by Ministry of Education, Youth and Sports (MEYS).
Conflict of interest
Markéta Škereňová, Veronika Mikulová, Otakar Čapoun, David Švec, Katarína Kološtová, Viktor Soukup, Hana Honová, Tomáš Hanuš, and Tomáš Zima state that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Škereňová, M., Mikulová, V., Čapoun, O. et al. Gene Expression Analysis of Immunomagnetically Enriched Circulating Tumor Cell Fraction in Castration-Resistant Prostate Cancer. Mol Diagn Ther 22, 381–390 (2018). https://doi.org/10.1007/s40291-018-0333-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-018-0333-0