Abstract
Background
The cell surface glycoprotein mesothelin is highly expressed in several malignant diseases. Normal mesothelin expression is limited to mesothelial cells lining the pleura, peritoneum, and pericardium, making it a biomarker and an attractive target for cancer therapy.
Methods
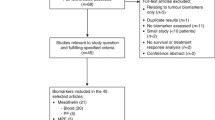
We investigated tumor mesothelin expression and serum mesothelin levels in patients with epithelial ovarian cancer or borderline tumors. In total, 161 patients selected from a previous prospective study were analyzed for tumor mesothelin expression using immunohistochemistry and serum mesothelin expression using enzyme-linked immunosorbent assay.
Results
Eighty-eight (68.8%) epithelial ovarian cancers and eight (24.2%) borderline tumors showed high mesothelin expression, which was associated with shorter progression-free and overall survival. The tumor mesothelin expression status was moderately correlated with serum mesothelin levels in epithelial ovarian cancer patients. Based on receiver operating characteristic analysis, a serum mesothelin level above 2.20 nM predicted high tumor mesothelin expression in epithelial ovarian cancer patients (area under the curve = 0.81). In 45 patients with recurrent epithelial ovarian cancer, we observed relatively lower levels of serum mesothelin, compared to the level at the primary diagnosis. We also tracked the change in the serum mesothelin level during the course of second-line chemotherapy and found a discrepancy between the clinical response and the serum mesothelin change in some patients, which suggested tumor heterogeneity among the tumor cells with or without mesothelin expression.
Conclusion
Serum mesothelin may be a useful noninvasive biomarker surrogate for tumor mesothelin expression in future clinical trials for mesothelin-targeted therapy.




Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166.
Gonzalez-Diego P, Lopez-Abente G, Pollan M, Ruiz M. Time trends in ovarian cancer mortality in Europe (1955–1993): effect of age, birth cohort and period of death. Eur J Cancer (Oxford, England: 1990). 2000;36(14):1816–24.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi:10.1056/NEJM199601043340101.
Ledermann JA, Kristeleit RS. Optimal treatment for relapsing ovarian cancer. Ann Oncol. 2010;21(Suppl 7):vii218–22. doi:10.1093/annonc/mdq377.
Schwab CL, English DP, Roque DM, Pasternak M, Santin AD. Past, present and future targets for immunotherapy in ovarian cancer. Immunotherapy. 2014;6(12):1279–93. doi:10.2217/imt.14.90.
Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16(24):6132–8. doi:10.1158/1078-0432.ccr-10-2275.
Hassan R, Kindler HL, Jahan T, Bazhenova L, Reck M, Thomas A, et al. Phase II clinical trial of amatuximab, a chimeric anti-mesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014. doi:10.1158/1078-0432.CCR-14-0804.
Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48. doi:10.1158/1535-7163.mct-13-0926.
Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2015. doi:10.1158/2159-8290.CD-15-0583.
Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer (Oxford, England: 1990). 2008;44(1):46–53. doi:10.1016/j.ejca.2007.08.028.
Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10(12 Pt 1):3937–42. doi:10.1158/1078-0432.ccr-03-0801.
Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120(21):3311–9. doi:10.1002/cncr.28875.
Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15(16):5274–9. doi:10.1158/1078-0432.ccr-09-0062.
Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13(17):5144–9. doi:10.1158/1078-0432.ccr-07-0869.
Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20.
Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–68. doi:10.1158/1078-0432.ccr-11-2121.
Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–33. doi:10.1200/jco.2014.57.4244.
Scales SJ, Gupta N, Pacheco G, Firestein R, French DM, Koeppen H, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther. 2014;13(11):2630–40. doi:10.1158/1535-7163.mct-14-0487-t.
Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1(1):43–53. doi:10.1158/2326-6066.cir-13-0008.
Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci. 1999;96(20):11531–6.
Hollevoet K, Nackaerts K, Thimpont J, Germonpre P, Bosquee L, De Vuyst P, et al. Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med. 2010;181(6):620–5. doi:10.1164/rccm.200907-1020OC.
Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet (London, England). 2003;362(9396):1612–6. doi:10.1016/s0140-6736(03)14794-0.
Wheatley-Price P, Yang B, Patsios D, Patel D, Ma C, Xu W, et al. Soluble mesothelin-related peptide and osteopontin as markers of response in malignant mesothelioma. J Clin Oncol. 2010;28(20):3316–22. doi:10.1200/jco.2009.26.9944.
Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12(2):447–53. doi:10.1158/1078-0432.CCR-05-1477.
McIntosh MW, Drescher C, Karlan B, Scholler N, Urban N, Hellstrom KE, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004;95(1):9–15. doi:10.1016/j.ygyno.2004.07.039.
Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110(2):196–201. doi:10.1016/j.ygyno.2008.04.002.
Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–8. doi:10.1016/j.ygyno.2007.10.017.
Shah CA, Lowe KA, Paley P, Wallace E, Anderson GL, McIntosh MW, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125. Cancer Epidemiol Biomark Prev. 2009;18(5):1365–72. doi:10.1158/1055-9965.EPI-08-1034.
Fritz-Rdzanek A, Grzybowski W, Beta J, Durczynski A, Jakimiuk A. HE4 protein and SMRP: potential novel biomarkers in ovarian cancer detection. Oncol Lett. 2012;4(3):385–9. doi:10.3892/ol.2012.757.
Saeki H, Hashizume A, Izumi H, Suzuki F, Ishi K, Nojima M, et al. The utility of serum N-ERC/mesothelin as a biomarker of ovarian carcinoma. Oncol Lett. 2012;4(4):637–41. doi:10.3892/ol.2012.796.
Ibrahim M, Bahaa A, Ibrahim A, El Hakem AA, Abo-El Noor A, El Tohamy U. Evaluation of serum mesothelin in malignant and benign ovarian masses. Arch Gynecol Obstet. 2014;290(1):107–13. doi:10.1007/s00404-014-3147-2.
Wu X, Li D, Liu L, Liu B, Liang H, Yang B. Serum soluble mesothelin-related peptide (SMRP): a potential diagnostic and monitoring marker for epithelial ovarian cancer. Arch Gynecol Obstet. 2014;289(6):1309–14. doi:10.1007/s00404-013-3128-x.
Lin J-Y, Qin J-B, Li X-Y, Dong P, Yin B-D. Diagnostic value of human epididymis protein 4 compared with mesothelin for ovarian cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2012;13(11):5427–32. doi:10.7314/apjcp.2012.13.11.5427.
Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):827–31. doi:10.1158/1078-0432.CCR-05-1397.
Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi:10.1371/journal.pmed.0050232.
Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13(3):243–7.
Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279(10):9190–8. doi:10.1074/jbc.M312372200.
Kurosaki A, Hasegawa K, Kato T, Abe K, Hanaoka T, Miyara A, et al. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138(8):1994–2002. doi:10.1002/ijc.29937.
Cristaudo A, Foddis R, Vivaldi A, Guglielmi G, Dipalma N, Filiberti R, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res. 2007;13(17):5076–81. doi:10.1158/1078-0432.ccr-07-0629.
Sharon E, Zhang J, Hollevoet K, Steinberg SM, Pastan I, Onda M, et al. Serum mesothelin and megakaryocyte potentiating factor in pancreatic and biliary cancers. Clin Chem Lab Med. 2012;50(4):721–5. doi:10.1515/cclm.2011.816.
Scholler N, Lowe KA, Bergan LA, Kampani AV, Ng V, Forrest RM, et al. Use of yeast-secreted in vivo biotinylated recombinant antibodies (Biobodies) in bead-based ELISA. Clin Cancer Res. 2008;14(9):2647–55. doi:10.1158/1078-0432.CCR-07-1442.
Badgwell D, Lu Z, Cole L, Fritsche H, Atkinson EN, Somers E, et al. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol Oncol. 2007;106(3):490–7. doi:10.1016/j.ygyno.2007.04.022.
Abdel-Azeez HA, Labib HA, Sharaf SM, Refai AN. HE4 and mesothelin: novel biomarkers of ovarian carcinoma in patients with pelvic masses. Asian Pac J Cancer Prev. 2010;11(1):111–6.
Chopra A. 111In-Labeled CHX-A’’-DTPA-conjugated MORAb-009, a chimeric monoclonal antibody directed against mesothelin. In: Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD): National Center for Biotechnology Information (US); 2004.
Ma J, Tang WK, Esser L, Pastan I, Xia D. Characterization of crystals of an antibody-recognition fragment of the cancer differentiation antigen mesothelin in complex with the therapeutic antibody MORAb-009. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68(Pt 8):950–3. doi:10.1107/s1744309112028229.
Fujisaka Y, Kurata T, Tanaka K, Kudo T, Okamoto K, Tsurutani J, et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs. 2015;33(2):380–8. doi:10.1007/s10637-014-0196-0.
Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74(11):2907–12. doi:10.1158/0008-5472.can-14-0337.
Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11(10):3814–20. doi:10.1158/1078-0432.ccr-04-2304.
Acknowledgements
We thank Dr. N. Iwasa and Dr. T. Nishikawa for their helpful support in sample collection during our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Hanaoka, Hasegawa, Kato, Sato, Kurosaki, Miyara, Nagao, Seki, Yasuda, and Fujiwara declare that they have no conflict of interest.
Funding
This study was partially supported by a grant from Hidaka Research Projects at Saitama Medical University International Medical Center (Saitama, Japan), a Saitama Medical University Internal Research Grant, and a grant from the Ochiai Memorial Award 2012.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40291_2017_255_MOESM3_ESM.docx
Supplementary material 3 (DOCX 115 kb) Fig. 1 Kaplan–Meier survival curves in ovarian cancer patients, based on the serum mesothelin level (a and c) and on CA125 level (b and d). Statistical analysis of prognostic survival was performed using the log-rank test. OS overall survival, PFS progression-free survival
Rights and permissions
About this article
Cite this article
Hanaoka, T., Hasegawa, K., Kato, T. et al. Correlation Between Tumor Mesothelin Expression and Serum Mesothelin in Patients with Epithelial Ovarian Carcinoma: A Potential Noninvasive Biomarker for Mesothelin-targeted Therapy. Mol Diagn Ther 21, 187–198 (2017). https://doi.org/10.1007/s40291-017-0255-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-017-0255-2