Abstract
Introduction
Spontaneous reporting of adverse events (AEs) is a mainstay of pharmacovigilance, and an ongoing challenge is how to ensure that more high-quality reports are collected for comprehensive information provision. The Med Safety App, a smartphone-based application, was launched in Nigeria in November 2020 to provide an electronic platform for users to seamlessly report AEs. There has been a paucity of evidence on the use of this application or other mobile applications for reporting adverse drug reactions/AEs following immunization in the Nigerian environment.
Objective
The aim of this study was to evaluate the trends in adverse event reporting before and after the introduction of the Med Safety App in Nigeria.
Methods
This was a retrospective, observational study using data from the VigiFlow database to compare adverse event reporting in Nigeria before and after the deployment of the Med Safety App. The baseline period was 1st April 2019 to 30th October 2020 and the comparison period was 1st November 2020 to 31st May 2022. We used Vigilance Hub, the back-end system for the Med Safety App, to extract data on App downloads and de-identified user statistics. Data were summarized using descriptive statistics, frequencies and proportions. Quality was assessed by assigning a completeness score to each individual case safety report. The Kruskal–Wallis test was used to test for differences in medians between groups.
Results
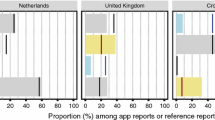
Following deployment of the App, the Nigerian National Pharmacovigilance Centre recorded an increase in the total number of adverse event reports received in VigiFlow, from 2051 in the baseline period to 18,995 following deployment of the App, with 81.7% of those reported via the Med Safety App. There was a reduction in the proportion of paper-based reporting from 98.4 to 15.7% post-deployment, and direct reporting by consumers increased from 2.7 to 17.6%. Of the 15,526 reports submitted via the App, 15,111 (97.3%) had a completeness score above 70% and 6993 (45%) had a completeness score of 100%. The median completeness score of adverse event reports on the Med Safety App was 6 out of 7. On bivariate analysis using the Kruskal–Wallis test, there was an association between means of reporting and completeness score, and this association was significant, with a p value of 0.0001, which may reflect the validation rules that are applied within the App.
Conclusion
Deployment of the Med Safety App increased both the number and quality of adverse event reports; however, more awareness and capacity building are needed to strengthen and sustain reporting on the tool by all categories of healthcare professionals and consumers/patients.



Similar content being viewed by others
References
World Health Organization. Safety of medicines. A guide to detecting and reporting adverse drug reactions (WHO/EDM/QSM/2002.2). Geneva: World Health Organization; 2002.
Federal Ministry of Health. Nigerian National Pharmacovigilance Policy and Implementation Framework. 2020. https://msh.org/wp-content/uploads/2021/03/nigerian_national_pharmacogilance_policy_implementation_framework.pdf. Accessed 10 July 2023.
World Health Organization. WHO pharmacovigilance indicators: a practical manual for the assessment of pharmacovigilance systems (ISBN 9789241508254). https://apps.who.int/iris/bitstream/handle/10665/186642/9789241508254_eng.pdf. Accessed 10 July 2023.
Fukushima A, Iessa N, Balakrishnan MR, et al. Smartphone-based mobile applications for adverse drug reactions reporting: global status and country experience. BMC Med Inform Decis Mak. 2022;22(1):118. https://doi.org/10.1186/s12911-022-01832-7.
Gahr M, Eller J, Connemann BJ, et al. Underreporting of adverse drug reactions: results from a survey among physicians. Eur Psychiatry. 2017;2017(41 Suppl):S369. https://doi.org/10.1016/j.eurpsy.2017.02.377.
National Pharmacovigilance Centre, National Agency for Food and Drug Administration and Control. Safety of medicines in Nigeria: a guide for detecting and reporting adverse drug reactions. Lagos: NAFDAC-NPC-NIG; 2004.
National Agency for Food and Drug Administration and Control Annual Reports 2002–2019. www.nafdac.gov.ng
Med Safety App | AUDA-NEPAD. https://www.nepad.org/content/med-safety-app Accessed 10 July 2022.
Rayna. Med Safety App | Pharmacovigilance. PV training updates. https://www.allaboutpharmacovigilance.org/umc-med-safety-app-an-international-mobile-tool-for-drug-safety/. Accessed 10 July 2022
Med Safety App—WEB-RADR. https://web-radr.eu/mobile-apps/med-safety/. Accessed 10 July 2023.
National Agency for Food and Drug Administration and Control. 2020 Med Safety Week. https://www.nafdac.gov.ng/2020-med-safety-week/. Accessed 10 July 2022.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 2003.
World Health Organization. Safety monitoring of medicinal products: reporting system for the general public. Geneva: World Health Organization; 2012.
Avong YK, Jatau B, Gurumnaan R, et al. Addressing the under-reporting of adverse drug reactions in public health programs controlling HIV/AIDS, Tuberculosis and Malaria: a prospective cohort study. PLoS ONE. 2018;13(8): e0200810.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Pierce CE, de Vries ST, Bodin-Parssinen S, et al. Recommendations on the use of mobile applications for the collection and communication of pharmaceutical product safety information: lessons from IMI WEB-RADR. Drug Saf. 2019;42:477–89. https://doi.org/10.1007/s40264-019-00813-6.
Prakash J, Joshi K, Malik D, et al. “ADR PvPI” Android mobile app: report adverse drug reaction at any time anywhere in India. Indian J Pharmacol. 2019;51(4):236–42. https://doi.org/10.4103/ijp.IJP_595_18.
Oosterhuis I, Taavola H, Tregunno PM, et al. Characteristics, quality and contribution to signal detection of spontaneous reports of adverse drug reactions via the WEB-RADR mobile application: a descriptive cross-sectional study. Drug Saf. 2018;41(10):969–78. https://doi.org/10.1007/s40264-018-0679-6.
Salvador MR, Monteiro C, Pereira L, Duarte AP. Quality of spontaneous reports of adverse drug reactions sent to a regional pharmacovigilance unit. Int J Environ Res Public Health. 2022;19(7):3754. https://doi.org/10.3390/ijerph19073754.
Niu R, Xiang Y, Wu T, Zhi Z, et al. The quality of spontaneous adverse drug reaction reports from the pharmacovigilance centre in western China. Expert Opin Drug Saf. 2019;18(1):51–8. https://doi.org/10.1080/14740338.2019.1559812.
Ezeuko AY, Ebenebe UE, Nnebue CC, Ugoji JO. Factors associated with the reporting of adverse drug reactions by health workers in Nnewi Nigeria. Int J Prev Med. 2015;6:25. https://doi.org/10.4103/2008-7802.153862.
National Primary Health Care Development Agency. The Nigerian Field Guide on Surveillance of Adverse Event Following Immunization and Response manual. National Primary Health Care Development Agency; 2018.
Oh I, Baek Y, Kim H, et al. Differential completeness of spontaneous adverse event reports among hospitals/clinics pharmacies, consumers and pharmaceutical companies in South Korea. PLoS ONE. 2019;14(2): e0212336. https://doi.org/10.1371/journal.pone.0212336.
Toki T, Ono S. Assessment of factors associated with completeness of spontaneous adverse event reporting in United States: a comparison between consumer reports and healthcare professional reports. J Clin Pharm Ther. 2020;45(3):462–9. https://doi.org/10.1111/jcpt.13086
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no funding for this study.
Conflict of interest
Uchenna Geraldine Elemuwa, Fraden Bitrus, Ibrahim Adekunle Oreagba, Adeline Ijeoma Osakwe, Abiola Sadikat Abiodun, Kenneth Onu, Asmau Abubakar, Angela E. Faniyi, Victoria Etuk, Daniel Yuah, Rametu Momodu, and Christiana Mojisola Adeyeye declare no conflicts of interest relating to this article.
Ethical approval
Ethical approval was not required for this study. Permission to carry out the study was duly obtained from the Director General of the NAFDAC
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
UGE conceived the study. UGE and FB developed the proposal for the study. IAO and CMA reviewed the proposal. DY extracted data from VigiFlow for analysis. UGE and VE analyzed the extracted data. UGE drafted the manuscript. FB, IAO, AIO, ASA, KO, AA, AEF, RM and CMA reviewed and approved the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elemuwa, U.G., Bitrus, F., Oreagba, I.A. et al. Trends in Adverse Event Reporting Before and After the Introduction of the Med Safety App in Nigeria. Pharm Med 38, 251–259 (2024). https://doi.org/10.1007/s40290-024-00524-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-024-00524-z