Abstract
Use of real-world data (RWD) is gaining wide attention. To bridge the gap between diverse healthcare stakeholders and to leverage the impact of Chinese real-world evidence (RWE) globally, a multi-stakeholder External Advisory Committee (EAC) and EAC meetings were initiated, aiming to elucidate the current and evolving RWD landscape in China, articulate the values of RWE in ensuring Chinese patients’ equitable access to affordable medicines and solutions, and identify strategic opportunities and partnerships for expansion of RWE generation in China. Chinese and international experts who are clinicians and academic researchers were selected as EAC members based on their professional background and familiarity with RWD/RWE. Three EAC meetings were held quarterly in 2023. Various topics were presented and discussed for insights and suggestions. Nine experts from China, one from South Korea, and two from Europe were selected as EAC members and attended these meetings. Experts’ presentations were summarized by theme, including the RWD landscape and RWE enablement in China, as well as global development of a patient-centric ecosystem. Experts’ insights and suggestions on maximizing the RWD/RWE value to accelerate healthcare transformation in China were collected. We concluded that though data access, sharing, and quality are still challenging, RWD is developing to support evidence generation in the medicinal product lifecycle, inform clinical practice, and empower patient management in China. RWD/RWE creates value, accelerates healthcare transformation, and improves patient outcomes. Fostering a patient-centric ecosystem across healthcare stakeholders and maintaining global partnerships and collaboration are essential for unlocking the power of RWD/RWE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Real-world data are in fast development to support evidence generation in the medicinal product lifecycle, inform clinical practice, and empower patient management in China. The data sources and types are diversified across the country. |
Fostering a patient-centric ecosystem across healthcare stakeholders and maintaining global partnership and collaboration are essential for unlocking the power of real-world data and real-world evidence in China. |
1 Introduction
1.1 Utilization of Real-World Data (RWD) in Healthcare
Real-world data (RWD) refers to patient health status and healthcare delivery information collected from various sources outside traditional clinical trials. The analysis of RWD generates real-world evidence (RWE) and offers clinical insights into a medicinal product’s potential benefits or risks [1].
In recent years, with incentives for developing comparative effectiveness research evidence to improve the care delivery, the use of RWD has been growing. The rapid generation of various types of digital data has provided valuable and diversified RWD sources [2]. Current RWD sources contain healthcare data, patient and/or disease registries, claims data, environmental data, and health-related data generated from mobile devices, social media, and patient platforms [3]. Through different study designs (e.g., retrospective/prospective cohort, case-control, or pragmatic clinical trial), these RWD sources have transformed values from post-marketing safety evaluation to broader applications in healthcare. RWE generated from RWD can support identifying unmet medical needs, optimizing drug development strategy, and assessing drug safety and effectiveness among diverse populations in the real-world setting [3]. Beyond therapeutic development, RWE can also provide insights into informing clinical guidelines and changing clinical practice, which are essential for patient experience optimization for better clinical outcomes [4].
Enhanced data quality promotes the utilization of RWD. Digitalization has been extensively integrated into clinical practice. The accumulation of digitalized data contributes to the generation and linkage of large electronic datasets containing comprehensive data elements in the real-world clinical setting. Currently, many RWD sources have the potential to ensure the performance of statistical and machine learning procedures and result reproducibility and replicability, leading to valid RWE generation with savings in both cost and time and enhancing the efficiency of medical and health-related research and decision making [2]. Enhanced RWD quality has also facilitated global collaboration by establishing a catalogue of RWD sources and harmonizing these data for cross-country collaborative research [5].
Real-world study (RWS) design and methodology have also been advanced, which facilitates the utilization of RWD. Based on advances in data quality and linkage, registry-based randomized control trials (RCTs), which are defined as pragmatic trials that use registry data for case records, data collection, randomization, and follow-up, are playing an important role in answering clinical questions pragmatically and cost effectively [6, 7]. One example is the national-level SWEDEHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies) registry database [8]. The advanced statistical techniques and structured process have also allowed the emulation of RCTs with high-quality RWD to agree on the conclusions [9]. With data suitable for study questions, design and analysis, the causal effects of therapies can be evaluated through both RCTs and observational RWSs. Therefore, RWD may offer causal insights when trial data are either unavailable or cannot be generated quickly or feasibly, which has enhanced the credibility of RWD in research [10].
RWD utilization has obtained regulatory recognition. In 2016, the United States Food and Drug Administration (US FDA) took a significant step by introducing the 21st Century Cures Act, aiming to assess the potential use of RWE in regulatory decision-making processes, including fulfilling post-approval study requirements, extending drug labels, and considering RWE as an external comparator for new drug submissions [11]. Following this milestone, the National Medical Products Administration (NMPA), the European Medicines Agency (EMA) and the Japan's Pharmaceuticals and Medical Devices Agency (PMDA) subsequently issued guidance on RWE application in drug development and addressing the methodological and operational aspects concerning the use of RWE [12,13,14]. These collective efforts reflect the growing acceptance of RWE as trustworthy information about the safety and effectiveness of drug therapies.
1.2 Importance of RWD/Real-World Evidence (RWE) in China’s Healthcare Context
In China, the release of “Healthy China 2030” has played a significant role in driving digitalization in healthcare facilities, to enhance the accessibility of medical big data nationwide [15]. The adoption of digital healthcare systems (e.g., Hospital Information System) across hospitals has offered a rich source of RWD. These RWD sources are being utilized to support robust analyses and enhance the generalizability of research findings in real-world clinical settings among the broad Chinese population [16].
RWE is gaining wide attention in China, and the number of published, peer-reviewed articles reporting RWS has continued to increase [17]. In China, RWE arose to compensate for the limitations of traditional clinical trials and provides additional evidence to inform clinical practice and policy decisions [17]. RWD/RWE gained regulatory recognition from 2019 onwards following the issue of relevant guidelines by NMPA [18]. The value of RWD/RWE is fostering collaboration and partnerships across healthcare stakeholders and initiating public–private partnership models or muti-stakeholder alliances to some extent in China.
1.3 External Advisory Committee (EAC) Meetings
A multi-stakeholder External Advisory Committee (EAC) was established to promote collaboration and partnership among diverse healthcare stakeholders and leverage the impact of China RWE globally [19]. Meetings were initiated among the EAC members. These meetings gathered experiences and insights from diverse healthcare stakeholders intending to enhance patient experience by applying RWD and RWE, particularly in the context of China. See the electronic supplementary material (ESM, including Table A1 and Table A2) for a detailed introduction on the EAC and its members.
Three EAC meetings with different focus topics were held quarterly in 2023 to collect insights and suggestions. In total, nine experts from China, one from South Korea, and two from Europe (Sweden and the Netherlands) were selected as EAC members and attended the series of meetings. In each meeting, Chinese experts shared their cases and thoughts encompassing the current and evolving RWD landscape and RWE enablement in driving clinical practice change in China, followed by presentations from international experts to introduce RWD development in other countries, and/or multistakeholder partnership and collaboration at the global level. Each presenter referred to facts in the literature/guidelines or their research published in peer-reviewed articles. Points presented in these meetings led to panel discussions. Panel discussion was conducted to identify challenges and opportunities for RWD quality enhancement and strategic partnership development to unlock RWD potential in China.
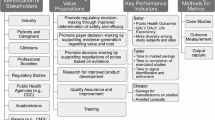
Experts’ presentations grouped by theme were summarized, including the RWD landscape in China, RWE enablement in China, and the patient-centric ecosystem to maximize the RWE/RWD value at a global level. Figure 1 shows an overview of the objectives of the three EAC meetings.
Based on the insights and experiences obtained from the EAC meetings, we aim to:
-
1.
Understand the current and evolving RWD landscape in China.
-
2.
Articulate the values of RWE in ensuring Chinese patients’ equitable access to affordable medicines and solutions.
-
3.
Identify strategic opportunities and partnerships for expansion of RWE generation in China.
2 RWD Landscape in China (First EAC Meeting)
2.1 Overview of RWD Development
RWD source types are diversified in China. The typical data source types that have evolved in recent years include product and disease registries, administrative claims, electronic health records (EHRs), patient-generated data, and data gathered from mobile devices.
Particularly, EHRs are developing rapidly in China following the release of “Healthy China 2030”, which aims to set up a national health record for each Chinese citizen [15]. One study in 2018 suggested that over 90% of Chinese hospitals have employed EHRs [20]. With the accumulation of digitalized healthcare data, in partnership with research institutes and health technology and pharmaceutical companies, a few regional governments in China have initiated the generation and linkage of healthcare data containing comprehensive data elements in the real-world clinical setting, leading to the generation of regional EHRs, that is, integrated closed-loop data of multi-source health records in a specific region [21].
The regional EHR databases are longitudinal, covering the regional population and containing patients’ healthcare records over the years across various hospitals and community healthcare service centers. There have been some well-established regional EHR databases. One example is the Yinzhou Integrated Health Big Data Platform (Yinzhou regional EHR). The Yinzhou regional EHR is a district-level database covering about 1.65 million residents in Yinzhou District, Ningbo City, China, since 2006. Pooled patient-level data are extracted from this district’s 10 hospitals, 22 community health service centers and 256 health service stations. The database contains integrated data on inpatient and outpatient care, public health management, mortality, census registration, and others. Data are extracted from various systems such as the public health information system, hospital information system, or community health information system [22]. Although the application of regional EHR databases is still preliminary, China has tremendous potential to develop more regional EHR databases with widespread digitalized data sources and partnerships among stakeholders.
2.2 Global Data Standardization of Chinese RWD
Global data standardization promotes Chinese data quality enhancement and sharing facilitation, providing a reasonable basis for scientific information sharing and cooperation across institutions and regions. Global data standardization is usually based on a unique platform to systematically merge data and generate standardized information internationally, yielding aggregated results for interpretation.
The global RWD community Observational Health Data Sciences and Informatics (OHDSI) has established an international network of researchers and observational health databases. OHDSI is a multi-stakeholder, interdisciplinary collaborative that brings out the value of health data through large-scale analytics [23]. So far, OHDSI has integrated the Yinzhou regional EHR database and four single-center EHR databases from China into diverse health databases drawn from various sources and countries through the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM). The OMOP CDM converts data from its local format to a designated structure and vocabulary containing all relevant coding dictionaries as a standardized mechanism [24]. Leveraging databases from the OHDSI, one published protocol aimed to investigate the relative effects of type 2 diabetes mellitus (T2DM) agents on cardiovascular risks by involving about 240 million patients across countries, including China [25]. This study will provide valuable insights into the comparative effectiveness and safety of the second-line T2DM agents, thereby guiding clinical decision making in T2DM management.
For future application and development of global data standardization, China is expected to make further efforts on human resource and talent development, localization of clinical research data models, generation of organized output of Chinese evidence, and collaboration with other international agencies to ensure a more coordinated RWD environment worldwide.
3 RWE Enablement in China (Second EAC Meeting)
3.1 RWE Supports the Medicinal Product Lifecycle in China
The RWE regulatory landscape in China has been dynamic and rapidly evolving. The NMPA published eight comprehensive guidelines related to RWD and RWE from different aspects since 2019 (Table A3 in the ESM). These guidelines cover a range of essential elements, including clarification on the utilization of RWE in drug development and registration; guidance on the collection, quality control and application of RWD; considerations for the design of RWS; and effective communication of RWE with the authority to support drug registration applications [18]. One guideline also explicitly explained how RWE can support the medicinal product lifecycle in China [13]. It is promising that RWE has obtained regulatory recognition and is in fast development to support medicinal product lifecycle development and monitoring in China.
RWE can be applied to address fundamental research questions across the medicinal product lifecycle in China. RWE can inform clinical trial strategy and design in the research and development stage by generating hypotheses and predicting free analysis. In China, specific utilization involves describing disease epidemiology and treatment patterns, assessing comparative effectiveness, safety, disease progression, and healthcare resource utilization, and estimating treatment costs [26]. In the launch and post-launch stages, RWE can be combined with evidence from clinical trials through conducting hybrid studies in safety monitoring and pharmacovigilance to produce generalizable results for supporting regulatory applications. RWE has also provided values for post-launch comparative effectiveness evaluation, treatment individualization and outcome prediction, and exploration of new indications [27]. Further, in the commercialization stage, through leveraging the global data standardization (e.g., OMOP CDM) and partnerships across multi-stakeholders, RWE can be applied to monitor the quality of care and drive practice change in China (Fig. 2).
3.2 RWE Informs Clinical Practice in China
In China, the concept of RWE arose from awareness of the limitations of traditional clinical trials and the need for additional evidence to inform clinical practice and policy decisions [17]. Compared with Europe and the US, which have applied RWE to inform the national clinical guidelines of the National Institute for Health and Care Excellence (NICE) [28], American College of Cardiology (ACC) [29], and American Heart Association (AHA) [29], applying RWE to inform clinical practice in China is still in the preliminary stage. However, compelling instances exist.
For example, RWE generated from the China Heart Failure (HF) center database helped to standardize HF diagnosis and treatment at the HF center, elevate the utilization of echocardiography and B-type natriuretic peptide (BNP) for HF diagnosis and prognosis-enhancing drugs for HF with reduced ejection fraction, and improve post-discharge mortality and re-hospitalization outcomes, leading to clinical guideline improvements for chronic HF exacerbation in China. Based on clinical insights from this database, healthcare professionals also developed a guideline for preventing, treating and managing chronic HF exacerbation in China [30]. Furthermore, RWE contributed to the development of hierarchical diagnosis and treatment of HF in primary and community hospitals in China, ensuring patients’ equitable access to affordable diagnoses and treatments of HF [31].
The frequency of applying RWE to influence clinical practice in China will increase within the context of “Big Health Evolution” in China, which indicates a high demand for rapid evidence on predictive, preventive, participatory, and personalized healthcare [32]. To accommodate this increasing demand, improved access and sharing of RWD is needed to ensure continuous and hyperlocal analysis of healthcare data. Organizations from academia and industry and across the patient-centric healthcare ecosystem need to work together to improve data access, sharing and quality, undertake translational and relevant research, and establish RWD sources to generate reliable RWE [28].
3.3 RWE Empowers Patient Management in China
Apart from informing the clinical guidelines, RWE empowers patient management in China by elucidating the most effective treatment for a disease/condition in real-world clinical practice, identifying diagnosis and treatment gaps between real-world clinical settings and guidelines, and benchmarking the current strategies for disease monitoring and treatment against the guidance for patient care optimization.
Relevant cases are also abundant in China. For example, using the Yinzhou regional EHR data, the protocol can identify the most effective treatment with the most minor safety signals for Chinese patients with T2DM, helping to inform critical decisions on T2DM treatment facing patients themselves, their caregivers and clinicians, and even policy-makers of healthcare systems in China [25]. RWE generated from the HF center database also supported monitoring the utilization rates of echocardiography, BNP, and guideline-directed medical therapy for HF over the years in both tertiary and secondary hospitals in China. The utilization gaps were therefore identified, and strategies for utilization advancements were developed in real time, contributing to increased utilization of these tests and treatments, which ensured diagnosis accuracy and distinct decreases in re-hospitalization and mortality outcomes [33].
4 Patient-Centric Ecosystem to Maximize the Value of RWD/RWE (Third EAC Meeting)
A patient-centric ecosystem can maximize the value of RWD/RWE to support the medicinal product lifecycle, inform clinical practice and empower patient management. The patient-centric ecosystem is based on building strategic partnerships among stakeholders, including government authority, industry, academia, and the healthcare system, to improve patient outcomes [19]. By leveraging each stakeholder’s expertise, experience, and connection, the patient-centric ecosystem enables all stakeholders to align values and objectives to advance equitable access to affordable, high-quality, and innovative healthcare solutions. This approach puts patients at the center of healthcare, moving them from passive players to active participants in their healthcare journey [19].
Two landmark cases of delivering innovative solutions to drive clinical practice change through a patient-centric ecosystem are the Optimised Pathway for Early Identification of Heart Failure in the Community (OPERA) project in Scotland [34] and the Federated E-Health Big Data for Evidence Renovation Network (FeederNet) in South Korea [35]. Both projects are well established and facilitated based on close collaboration among the government authority, healthcare, academia, and industry. Through solid multi-stakeholder partnership and collaboration, OPERA evaluated the new patient management pathways to enable earlier diagnosis and treatment on a large scale for the National Health Service transformation. Results of the OPERA pilot study have suggested a significant reduction in waiting time for patients’ HF diagnostic tests from 12 months to 6 weeks [34]. The OPERA project’s expected outcome is to facilitate the provision of equitable and more timely access to HF diagnostics and HF care in Scotland. OPERA has been an example of how partnership working can deliver innovative solutions to drive clinical practice change and provide equitable access to high-quality care [36]. FeederNet developed a national data network involving 66 hospitals (73 million patients) and three national claims databases, and data from the FeederNet platform showed over 3500 analysis activities in 2021, promoting collaborative research between hospitals and academia by using data from multiple hospitals [35]. FeederNet also joined the OHDSI and enabled researchers to access a network of billions of patients to generate evidence on all aspects of healthcare [37]. Therefore, these partnerships and collaborations have a great potential to improve patient care and ensure patients’ equitable access to affordable medicines and solutions.
5 Discussion
Since the EAC members have various backgrounds and all of them are key opinion leaders in their professional field, the viewpoints collected during EAC meetings in 2023, especially on challenges, opportunities, and potential solutions, may have long-term impact and initiate further discussion on how to maximize the value of RWD and RWE to accelerate healthcare transformation in China. However, there has yet to be any regulatory communication between the EAC and NMPA. The following sections summarized EAC members’ opinions/perspectives in the panel discussions.
5.1 Challenges of RWD Utilization in China
Currently, many RWD sources in China are available only to academic researchers upon request, exacerbating collaboration constraints across organizations. The literature also highlights this observation [17, 38]. Issues of data ownership, access processes, and compliance are substantive in China. The national or local policies to guide Chinese hospitals on approval of data access and sharing for research need to be improved. The payment structure for the data user fee and the distribution of scientific achievement and intellectual property across different functions should also be clarified [39]. Moreover, there are concerns about data confidentiality and privacy protection.
Although many Chinese hospitals have adopted data management systems such as the Hospital Information System or the picture archiving and communication system, integrating information across these systems for real-world database generation is still tricky due to distinct heterogeneity in data entries and formats [40]. Concerns about coding accuracy, consistency, and data completeness for longitudinal follow-up generally exist. The quality of RWD in China has generally improved, but high-quality RWD is still lacking [17, 38]. A unified and standardized data governance process and quality improvement based on China’s existing data management system is needed.
5.2 Opportunities for Chinese RWD to Support Healthcare
Fostering collaboration and sustainable partnerships across healthcare stakeholders is an opportunity to enhance RWD accessibility and quality. Through facilitating multi-stakeholder cooperation and building partnerships among government authorities, healthcare systems, academic researchers, and industry, the credibility of Chinese RWD can be improved distinctly as collaboration and partnership can provide the necessary infrastructural, technical, and scientific support and unique capabilities through a joint effort. The more players that join in the use of RWD, the better RWD will become, with high quality and accessibility.
Furthermore, to maximize RWE values and achieve patient empowerment in China, Chinese RWD/RWE implementers are encouraged to partner with international researchers and agencies and leverage their unique capabilities, experiences, and connections on the advanced methodology and technology of RWD/RWE. Maintaining global partnerships and collaboration is essential for transforming the future of healthcare and unlocking the power of RWD/RWE to improve patient outcomes in China. See Fig. 3 for an overview of Chinese RWD opportunities to tackle challenges.
5.3 Potential of Patient-Centric Ecosystem Development in China
Healthcare forms a complex ecosystem that includes stakeholders from hospitals, payers, academic institutions, and industry. In the past they were only loosely tied together, working with siloed systems and data. In recent years, healthcare stakeholders are making efforts to develop a patient-centric ecosystem in China. For instance, AstraZeneca and the Chengdu Wuhou District Health Bureau formed a strategic partnership to support chronic disease management through RWS. This collaboration focuses on applying RWD to provide solid RWE in managing high-prevalence chronic diseases, including respiratory, kidney and cardiovascular diseases. Stakeholders are confident that this private–public partnership and collaboration will aid in advancing the digitalization of chronic disease management and the standardization of clinical diagnosis and treatment, ultimately improving the health outcomes and quality of life of Chinese patients in this region [41]. China has also taken the initiative to set up the Boao Lecheng Pilot Zone in Hainan, China, to enable patients’ early access to medical products and to generate early patient-level RWD [42]. This pilot zone and related policies is another example that could reflect joint efforts from policy makers, hospitals, and industry to facilitate a partner-based patient-centric ecosystem in China.
Building upon widespread digitalized Chinese data sources and a shared vision to transform the future of healthcare and unlock the power of RWD to improve patient outcomes among multi-stakeholders, China has great potential to establish and maintain a data-driven, fully integrated and partner-based patient-centric ecosystem. Relying on this ecosystem, partners across various domains will be able to drive changes in clinical practice through high-quality RWD and RWE, maximize patient benefits, and help increase the global impact of Chinese RWD and RWE. A conceptual framework was developed to inform RWD opportunities, RWE enablement, and RWD/RWE maximization via the patient-centric ecosystem in China (see Fig. 4).
6 Conclusions
Chinese RWD source types are diverse. Though issues around data access, sharing, and quality are still challenging, RWD is developing to support evidence generation through the medical product lifecycle, inform clinical practice, and empower patient management in China. RWD/RWE have shown value to accelerate healthcare transformation and improve patient outcomes in China. Fostering a patient-centric ecosystem across healthcare stakeholders and maintaining global partnership and collaboration are essential for unlocking the power of RWD/RWE.
References
U.S. Food and Drug Administration. Real-World Evidence 2023. Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed on 30 Jun 2023
Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol. 2022;22(1):287.
Dang A. Real-world evidence: a primer. Pharmaceut Med. 2023;37(1):25–36.
Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295–304.
Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153(9):600–6.
Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, et al. Registry-based randomized controlled trials—What are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16–24.
Doherty DA, Tong SYC, Reilly J, Shrapnel J, McDonald S, Ahern S, et al. Registry randomised trials: a methodological perspective. BMJ Open. 2023;13(3): e068057.
Omerovic E, Erlinge D, Koul S, Frobert O, Andersson J, Ponten J, et al. Rationale and design of switch Swedeheart: a registry-based, stepped-wedge, cluster-randomized, open-label multicenter trial to compare prasugrel and ticagrelor for treatment of patients with acute coronary syndrome. Am Heart J. 2022;251:70–7.
Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–64.
Franklin JM, Patorno E, Desai RJ, Glynn RJ, Martin D, Quinto K, et al. Emulating randomized clinical trials with nonrandomized real-world evidence studies: first results from the RCT DUPLICATE initiative. Circulation. 2021;143(10):1002–13.
National Archives. Developing a framework for regulatory use of real-world evidence; Public Workshop 2017. Available from: https://www.federalregister.gov/documents/2017/07/31/2017-16021/developing-a-framework-for-regulatory-use-of-real-world-evidence-public-workshop. Accessed on 30 June 2023
Nishioka K, Makimura T, Ishiguro A, Nonaka T, Yamaguchi M, Uyama Y. Evolving acceptance and use of RWE for regulatory decision making on the benefit/risk assessment of a drug in Japan. Clin Pharmacol Ther. 2022;111(1):35–43.
NMPA. Issued the announcement on the guidelines for real-world evidence to support drug development and regulatory review (final) 2020. Available from: http://english.nmpa.gov.cn/2020-01/07/c_456245.htm. Accessed on 30 Jun 2023.
European Medicines Agency. Guideline on registry-based studies 2021. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-registry-based-studies_en-0.pdf. Accessed on 30 Jun 2023.
Tan X, Liu X, Shao H. Healthy China 2030: a vision for health care. Value Health Reg Issues. 2017;12:112–4.
Xie J, Wu EQ, Wang S, Cheng T, Zhou Z, Zhong J, et al. Real-world data for healthcare research in China: call for actions. Value Health Reg Issues. 2022;27:72–81.
Sun X, Tan J, Tang L, Guo JJ, Li X. Real world evidence: experience and lessons from China. BMJ. 2018;360: j5262.
Nie X, Wang S, Yao C, Peng X, Liu Z, Meng R, et al. Comparative study of domestic and foreign regulatory policies and guidelines on real-world data/evidence. Chin J Pharmacoepidemiol. 2022;31(01):5–12.
Bedenkov A, Walton R, Moreno C, Geldard T. New collaborative approaches to real-world data must put patients at the centre of healthcare. 2023. Available from: https://blogs.lse.ac.uk/businessreview/2023/09/19/new-collaborative-approaches-to-real-world-data-must-put-patients-at-the-centre-of-healthcare/. Accessed on 30 Nov 2023.
Zhang L, Wang H, Li Q, Zhao MH, Zhan QM. Big data and medical research in China. BMJ. 2018;360: j5910.
Wonders. Construction and application of regional population health big data platform. 2023. Available from: https://www.wondersgroup.com/07180942288353.html. Accessed on 30 Nov 2023.
Yexiang S, Jun L, Peng S, Siyan Z, Pei G, Luxia Z, et al. A new model for disease control and prevention driven by big data in healthcare. Chin J Epidemiol. 2021;42(8):1325–9.
OHDSI. Observational health data sciences and informatics. 2023. Available from: https://www.ohdsi.org/. Accessed on 30 Nov 2023.
Zhang M, Chai S, Long Z, Liu T, Chen X, Liu Z, et al. Establishment of evidence sharing network through common data model for Chinese Clinical Research: an Overview: OHDSI; 2023. Available from: https://ohdsi.org/2023apacsymposium/. Accessed on 30 Nov. 2023.
Khera R, Schuemie MJ, Lu Y, Ostropolets A, Chen R, Hripcsak G, et al. Large-scale evidence generation and evaluation across a network of databases for type 2 diabetes mellitus (LEGEND-T2DM): a protocol for a series of multinational, real-world comparative cardiovascular effectiveness and safety studies. BMJ Open. 2022;12(6): e057977.
Wang W, Tan J, Wu J, Huang S, Huang Y, Xie F, et al. Use of real world data to improve drug coverage decisions in China. BMJ. 2023;381: e068911.
Sun X, Tan J, Tang L, Li L, Wang W, Xiong W, et al. Technical framework system for post-marketing drug evaluation based on real-world evidence: considerations and suggestions. Chin J Evid Based Med. 2018;18:277–83.
Oyinlola JO, Campbell J, Kousoulis AA. Is real world evidence influencing practice? A systematic review of CPRD research in NICE guidances. BMC Health Serv Res. 2016;16:299.
Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301(8):831–41.
Electrophysiology and Cardiac Function Branch of Chinese Society of Geriatrics, Cardiovascular Medicine Branch of Chinese Physicians Association, Expert Committee of China Heart Failure Center. Chinese Expert Consensus on Comprehensive Management of Patients With Worsening Heart Failure. Chin Circ J. 2022;37:215–25.
Department of Medical Administration. Notice on printing and issuing technical plan for hierarchical diagnosis and treatment of heart failure. 2019. Available from: http://www.nhc.gov.cn/yzygj/s3593g/201905/bcc9cf90e44a4040bdf55b0d6d20857f.shtml. Accessed on 30 Nov 2023.
Lee A. GF Viewpiont | China’s Fast Evolving “Big Health” Landscape. 2019. Available from: http://www.edwardtseblog.com/lateest-posts/gf-viewpiont-chinas-fast-evolving-big-health-landscape/. Accessed on 30 Nov 2023.
EaCFBoCSoG CCA, Committee CHFCE. Report of Consortium of China Heart Failure Centers (2022): clinical performance and quality measures for adults with heart failure in China. Chin J Gen Pract. 2023;22(6):557–68.
University of Glasgow. Landmark partnership aims to improve Scotland’s health. 2022. Available from: https://www.gla.ac.uk/news/headline_876209_en.html. Accessed on 30 Nov 2023.
You SC, Lee S, Choi B, Park RW. Establishment of an international evidence sharing network through common data model for cardiovascular research. Korean Circ J. 2022;52(12):853–64.
NHS West of Scotland Innovation Hub. OPERA heart failure innovation shortlisted for top award. 2022. Available from: https://www.woshealthinnovation.scot/latest-updates/opera-heart-failure-innovation-shortlisted-for-top-award/. Accessed on 30 Nov 2023.
Woong PR. A clinical real-world evidence sharing platform over the globe. J Acupunct Meridian Stud. 2020;13(2):69.
Zhong J, Zhang J, Fang H, Liu L, Xie J, Wu E. Advancing the development of real-world data for healthcare research in China: challenges and opportunities. BMJ Open. 2022;12(7): e063139.
People's Daily Online. It is recommended to encourage multiple subjects to participate in promoting health care data sharing. 2023. Available from: http://health.people.com.cn/n1/2023/0305/c14739-32637144.html. Accessed on 30 Nov 2023.
Wang Z. Data integration of electronic medical record under administrative decentralization of medical insurance and healthcare in China: a case study. Isr J Health Policy Res. 2019;8(1):24.
Chinanews. AstraZeneca signs strategic cooperation with Chengdu Wuhou District Health Bureau. 2023. Available from: https://www.sh.chinanews.com.cn/yljk/2023-06-14/112902.shtml. Accessed on 30 Nov 2023.
Li J, Liu L, Cao H, Yang M, Sun X. Use of real-world evidence to support regulatory decisions on medical devices in China and a unique opportunity to gain accelerated approval in “Boao Lecheng Pilot Zone.” Cost Eff Resour Alloc. 2023;21(1):7.
Acknowledgements
EAC members: Feng Sun, Bi-Cheng Liu, Jiefu Yang, Jin-fu Xu, Linong Ji, Min Zhou, Xinli Li, Yuanlin Song, Pingyan Chen. AstraZeneca GEP China team: Carmen Moreno, Claudia Cabrera, Samuel Chen, Shaosen Zhang, Shirley Xiao, Mandy Ma. AstraZeneca China Medical team: Celine Yang, Filip Surmont, Viola Zhang, Joe Li, Benley Wu, Alyssa Xu, and other medical colleagues who supported the EAC meetings. IQVIA Greater China: Zheng Yin, Shuo Yang, Long Pang, Jing Li, Jenny Cui, Aimee Chiu, Wentian Lu, Lechun Huo, Junli Zhu. AstraZeneca and the authors thank Prof. David Erlinge from the Department of Cardiology, Lund University, Prof. Janwillem Kocks from the Groningen Research Institute for Asthma and COPD, University of Groningen, and Prof. Rae Woong Park from the Department of Biomedical Informatics, Ajou University, for presenting their work at the EAC meetings. Medical writing support was provided by IQVIA Real World Solutions Greater China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by AstraZeneca, who established the Global Evidence Powerhub (GEP), and organized the External Advisory Committee (EAC) and the series meetings. All authors participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
FS, PYC, AB, SSZ and CM designed the concept and drafted the outline of this manuscript. All EAC members contributed their insights to the content. All authors contributed to the drafting and critical review of the manuscript content and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sun, F., Bedenkov, A., Liu, BC. et al. Maximizing the Value of Real-World Data and Real-World Evidence to Accelerate Healthcare Transformation in China: Summary of External Advisory Committee Meetings. Pharm Med 38, 157–166 (2024). https://doi.org/10.1007/s40290-024-00520-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-024-00520-3