Abstract
European pharmaceutical companies have a legal requirement to provide non-promotional medical information (MI) services to support healthcare professionals (HCPs) using their medicinal products. While we are seeing an increased HCP preference and expectation towards digital channels, the lack of a compliance framework relating to the provision of non-promotional MI services is a key factor complicating and compromising the ability for companies to meet this need. Meanwhile, the internet is dominated by a large volume of unregulated, easy access, and potentially low-quality information from diverse sources. The Medical Information Leaders in Europe (MILE) association is therefore proposing a framework of principles to support pharmaceutical companies with the digital provision of MI services; these relate to digital access to non-promotional, medicinal product information to support clinical decision making and patient care. The three established European national MI associations have already considered the framework and expressed their endorsement. MILE continues to invite stakeholders, including pharmaceutical companies, regulators, national industry associations and healthcare professional bodies to engage and help refine and implement this framework. This publication does not constitute legal advice; decision making and accountability remains with each pharmaceutical company.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
The absence of a specific framework governing the provision of online medical information (MI) by the pharmaceutical industry for healthcare professionals (HCPs) in Europe is compromising effective digital MI to support clinical decision making and patient care. |
Medical Information Leaders in Europe (MILE) members have collaborated to develop and publish a set of practical guiding principles to support MI services to better meet HCPs’ online information expectations and needs. |
MILE seeks further feedback and discussion with regulators, industry associations, HCPs and non-member companies to help refine and endorse these principles as part of a clear and practical model for implementation by pharmaceutical companies. |
1 Introduction
The European Directive 2001/83/EC relating to the regulation of medicinal products for human use mandates pharmaceutical companies to establish a scientific service in control of the medicinal product information [1]. Most companies have established medical information (MI) services to fulfil this requirement and support clinical decision making and patient care. The channels of communication are not detailed in the Directive, however the expectancy and benefits of online information have clearly escalated over the past 20 years. At a multi-national level, the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and European Federation of Pharmaceutical Industries and Associations (EFPIA) detail standards for the ethical promotion of pharmaceutical products to healthcare professionals (HCPs). However, it is notable that the IFPMA and EFPIA Codes of Practice confirm that responses to specific questions about medicinal products are out of scope and the IFPMA/EFPIA Joint Note for Guidance on Social Media and Digital Channels does not provide standards for the publication of, or access to, non-promotional MI online [2,3,4].
Country-specific MI guidelines have been published in a few European countries [5,6,7]. These guidelines include similar criteria to ensure standards are maintained. They apply across all channels and are adopted by most companies across all countries. They include the need for verbal and written responses to HCPs to always be:
-
reactive to unsolicited enquiries;
-
non-promotional in style and content;
-
avoiding disguised promotion (limited judicious use of brand name permitted where essential for the understanding of the content);
-
accurate, balanced and not misleading;
-
up-to-date;
-
referenced to appropriate sources (whether published or high quality internal resources);
-
specific to the question only.
It is important to also highlight that the national MI guidelines and established industry practice also permit off-label information to be included in the response when required to answer unsolicited requests for information.
While pharmaceutical industry MI services are highly committed to supporting clinical decision making through the provision of quality MI content, they have not been able to effectively evolve from traditional channels (e.g., phone, letter, email) to self-service digital solutions. A relatively small number of companies offer some information online; however, this is limited in both the scale and scope of content; there is a significant mismatch between the service offering and needs [8].
MILE (Medical Information Leaders in Europe) is an industry association founded in 2018. It is open to all European pharmaceutical companies that hold marketing authorisations for the use of human medicinal products in the continent of Europe. MILE works to share knowledge and best practice around the function of MI in Europe, with an overall goal to continuously improve access to information about medicinal products for HCPs and patients, and thereby contribute to a safer and more appropriate use of such products.
As online access to quality information is commonplace across all sectors of society, including the healthcare sector, pharmaceutical companies across Europe have started to operate digital MI services. Studies have shown that HCPs are making use of online resources to support clinical decision making [8]. Benchmarking surveys conducted by MILE in 2019 and 2021 demonstrated the limited availability of these digital services across European countries, with limited progression over the 2 years [8, 10]. MILE also recognised that some pharmaceutical companies are actively avoiding operating MI services in the digital space due to the risk and complexity of working in the absence of a clear framework, and perceived regulatory constraints; the lack of a clear governance model is an important barrier to omnichannel development, as the industry defaults to the established but restrictive promotional regulations and codes of promotional practice which are not suitable for this non-promotional function [8]. Pharmaceutical companies would therefore benefit from clear guidelines relating to MI provision in the digital space.
MILE members have already collaborated to publish clear guidance regarding the responsible sharing of safety information via the MI channel [9] and now propose new principles to ensure high-quality digital MI resources for HCPs. Similar guidelines have been developed by phactMI (Pharma Collaboration for Transparent Medical Information) for the United States and shared within their membership. MILE recognise that there are differences to the medicine legislation in Europe, and these need to be considered while building a European framework.
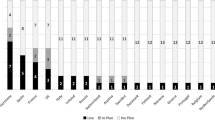
Three European national MI associations have already considered the framework of principles proposed in this paper and expressed their endorsement (Fig. 1).
The aim of this guidance is to support navigation of the legal and compliance aspects, and provide a framework in which MI functions can develop digital resources for HCPs. Expanded access to a trusted source of up-to-date information will reduce the need for HCPs to consult unauthoritative sources for information.
2 Guiding Principles
This framework focuses on four guiding principles, as shown in Fig. 2 and expanded in the following four sections.
While the guiding principles are shared for consideration, they are not meant as directives. Rather, they may help provide direction to companies as they develop and deploy their digital solutions and engage with stakeholders.
While MILE has taken every effort to provide informed and professional guidance, individual companies must make their own decisions and MILE is not accountable for the interpretation or implementation of this guidance.
In proposing this framework of principles, MILE maintains the importance for all pharmaceutical companies to continue complying with the legal requirements (e.g., laws and regulations applicable to our industry such as pharmaceutical, competition, intellectual property and data protection laws as well as anti-bribery and anti-corruption legislation), and integrity commitments.
The framework applies a range of standard definitions which have been agreed across the MILE membership (Table 1).
2.1 Optimal User Experience
Modern technology has the ability to vastly improve the user experience of MI services. For MI, it is important to consider the accessibility of the services, the discoverability of the content and also it’s suitability for the digital channel. MILE recommend that the design principles shared in Table 2 should be considered to optimise the HCP experience.
2.2 Healthcare Professional Authentication
MILE recognises that pharmaceutical companies should put guardrails in place to limit access to content to the intended audience. However, there is also a personal responsibility of the user to correctly identify themselves. In the context of providing online MI (non-promotional and unsolicited), MILE considers self-validation an appropriate measure to limit access to HCP resources by the general public or patients. This approach is effectively the same as telephone calls or emails, where the customer simply confirms that they are an HCP, without the need for additional confirmation.
Companies should also provide other MI services and/or relevant resources for the general public and patients, which further reduces the risk of inappropriate access of online HCP resources.
Companies should always clearly indicate the intended audience for the content. For example, pages for HCPs should include wording along the lines of ‘This information is intended for healthcare professionals only’ and indicate the country. Patients, consumers and other non-HCPs should be directed away from the HCP-only pages.
There are many advantages for customers and companies in implementing self-validation on MI websites, including:
-
avoiding significant access hurdles caused by time-consuming, cumbersome registration processes, thereby offering HCPs straightforward access to MI content. This avoids the need to turn to other online, potentially unauthoritative, but easy to access resources;
-
aligning with customers’ expectations in terms of easy access to information in a digital environment;
-
supporting discoverability of MI websites through search engines, which is often limited by registration mechanisms;
-
facilitating access and improving legitimate transparency for HCPs who require the information for their clinical decision making.
Where central HCP authentication systems already exist and are widely adopted, such as DocCheck in Germany, these could also serve as a straightforward HCP authentication mechanism. However, these should not be a prerequisite for HCPs to access MI content.
2.3 Surfacing Scientific Content
MILE proposes that MI websites should be able to compliantly surface scientific content online and deliver a positive user experience.
Information which is on-label could be retrieved through a free-text search tool, guided searching, site navigation (e.g., using a product list) or internet-wide search engine. However, information which is off-label should only be accessible behind a free-text search tool on the MI website.
Search algorithms and tools should be sophisticated enough to ensure appropriate levels of specificity. Ideally, such search algorithms should deal with word stemming, medical synonyms and abbreviations.
2.3.1 Providing Information Supporting the Label
On-label non-promotional MI content may also be published without the need for specific search logic. It could be retrievable by search engines. MI websites may also offer sections that contain information that supports the Product Label (e.g., through product-related ‘Frequently Asked Questions [FAQs]’ or chatbots which guide HCPs through fixed menu options to obtain the response to their enquiry). The interest in these topics has been demonstrated (e.g., through high enquiry volumes on other channels), making MI responses appropriate for inclusion on these tools. Other topics could also be included based on their importance (with referral to the Product Label or adverse event reporting information).
In these scenarios, the HCP remains in control of the information they click on. When providing scientific content, companies should ensure that upfront displays of information only include relevant details for the purpose of navigating, and there must be a proactive click from the HCP to retrieve the full information. To obtain scientific content relating to a specific product, the HCP must always indicate which product their enquiry relates to.
It is MILE’s position that, if HCPs are required to proactively click to obtain the information, MI websites housing FAQs or chatbots adhere to the MI standards for enquiries to be unsolicited.
2.3.2 Free-Text Search Functionality
Free-text searching typically works by indexing specific attributes of the scientific content (title, keywords) or full-text indexing of the entire document. Specific attributes can then be weighted or mandated to ensure only relevant content is returned. This functionality offers an appropriate method to ensure that results are only surfaced if specifically requested by the appropriate search terms used by an HCP. Therefore, it is MILE’s proposal that both on- and off-label content should be accessible through free-text search to the extent necessary to answer a specific question about a particular medicinal product.
2.3.2.1 Unsolicited Nature
If online MI is surfaced within the below framework, MILE considers online provision of MI unsolicited in nature. HCPs must proactively initiate the search for scientific content, based on their information need.
Companies should demonstrate that they have put strategies in place to ensure specificity (see below). If Scientific/Standard Response Documents (SRDs) or other MI resources are being provided (e.g., mode-of-action videos, administration guidelines, infographics), companies should limit search results to only display the material titles or succinct summaries that support HCPs in identifying content relevant to their need. HCPs should be required to perform another proactive step where they select content that matches their information need, demonstrating the request for information was intrinsically motivated.
2.3.2.2 Ensuring Specificity
Where search functionality is included, companies should put strategies in place to ensure specificity so that the HCP viewer is most efficiently directed to the content they require. Depending on the type of content provided, a range of different tactics may be deployed. For example:
-
requiring a product name to be provided in order to retrieve scientific content aligned to that product;
-
requiring a minimum number of words to be entered for a valid search (excluding connecting words);
-
ordering and limiting the search results based on their relevance to the search terms;
-
developing search algorithms that are systematic, logical, non-arbitrary, objective and support surfacing content with high relevancy;
-
enhancing the search methodology over time. For example, through periodic reviews of search terms and search results to ensure relevancy.
2.3.2.3 Adherence to MI Standards
In the digital context, it is important to recognise that search algorithms cannot guarantee 100% specificity. It is MILE’s position that, if companies deploy the above-mentioned strategies coupled with proactive searching by HCPs, this supports adherence to MI standards.
2.4 Content
To ensure compliance to MI standards, content surfaced on digital MI platforms should always be up-to-date, referenced, accurate and balanced, unsolicited, non-promotional, specific and relevant to the question. In line with other MI response channels, the content offered should contain clearly visible and appropriate statements to inform HCPs of potential off-label information.
It would be good practice for the approved Product Label to be referenced and easy to access. Disclaimers may also be used to indicate that data provided is intended to inform HCPs in their clinical decision-making and that it does not constitute medical advice.
Digital resources must also include clear signposting for adverse event reporting and product quality concerns.
MILE recognises the sensitivity of providing access to information relating to off-label use through online MI resources and so, to ensure appropriate provision of off-label information to the extent necessary to answer a specific question, recommends that such information should only be accessible behind a robust search functionality.
MILE also recognises there are topics that are not specifically described within the Product Label which could be important for the safe and effective use of medicines (e.g., safety or allergen information). It is MILE’s position that these do not contradict the Product Label and are non-promotional and therefore should be easier to access than off-label information.
3 Conclusion
This position paper proposes a principles framework for the online provision of medical information for HCPs.
Improved online access to information contributes to the safe and effective use of medicines. MILE hopes this proposed guidance will facilitate discussions with key stakeholders to shape clear guidelines for the provision of online services. While MILE also recognises the need for guidance on the provision of information for patients and members of the general public, as well as guidance on utilisation of social media, these are out of scope for this specific guidance.
This framework is endorsed by MILE, and may be updated in light of further stakeholder input, technology and experience evolution. This publication does not constitute legal advice; decision making and accountability remains with each pharmaceutical company.
References
European Parliament and the Council of the European Union. Directive 2001/83/EC. 2001. https://www.legislation.gov.uk/eudr/2001/83/2019-07-26. Accessed 15 Feb 2023.
European Federation of Pharmaceutical Industries and Associations (EFPIA) Code of Practice—2019. 2019. https://www.efpia.eu/media/676434/220718-efpia-code.pdf. Accessed 15 Feb 2023.
International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Code of Practice—2019. 2019. https://ifpma.org/publications/ifpma-code-of-practice-2019/. Accessed 15 Feb 2023.
International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) Joint Note for Guidance on social media and digital channel. 2022. https://ifpma.org/publications/joint-note-for-guidance-on-social-media-and-digital-channels/. Accessed 15 Feb 2023.
Associación de Medicina de la Industria Farmacéutica Medical Information Forum. ‘Good Medical Information Practice Guidelines in the Spanish Pharmaceutical Industry’, 2017. Guía de buenas prácticas de Información Médica en la industria farmacéutica española. Documenta Universitaria. 2023. https://www.documentauniversitaria.com/en/products/gua-de-buenas-prcticas-de-informacin-mdica-en-la-industria-farmacutica-espaola/. Accessed 15 Feb 2023.
Die forschenden Pharma-Unternehmen (vfa), the German Association of Research-Based Pharmaceutical Companies. ‘Positionspapier - Medizinische Information durch die forschenden Pharma-Unternehmen in Deutschland‘. 2020. https://www.vfa.de/download/positionspapier-medizinische-information.pdf. Accessed 15 Feb 2023.
Pharmaceutical Information and Pharmacovigilance Association. ‘Guidelines on Standards in Medical Information’. 2022. https://pipaonline.org/wp-content/uploads/2022/02/Pipa-MI-Guideline-Document_Jan-2022_final.pdf. Accessed 15 Feb 2023.
Pienaar S, Dunnett S, Flores A, et al. The critical need to build a european governance model for online access to medical information services: A position paper. Pharm Med. 2021;35:147–55.
Hristoskova S, Milligan J, De Wit J, et al. Principles and considerations for responsible sharing of safety information via the medical information channel. Ther Innov Regul Sci. 2020;54:939–46.
MILE (Medical Information Leaders in Europe). Technology Benchmarking Survey—2021. 2022. https://www.mile-association.org/wp-content/uploads/2022/04/MILE-2021-technology-benchmarking-survey-summary-for-website.pdf. Accessed 15 Feb 2023.
Acknowledgements
The authors would like to thank Andrew Finlay, Medical Information Director (EU and Canada), AstraZeneca, for his valuable contribution to the working group developing the principles. The views expressed in this publication are those of the MILE (Medical Information Leaders in Europe) association and are not necessarily the views of each of the authors’ companies or other MILE member companies.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
MILE (Medical Information Leaders in Europe) covered the costs associated with publication of this article, including engaging research and author services from Sarah Dunnett. No other financial support was received.
Conflicts of interest
MILE is an industry association that works to share knowledge and best practice around the function of Medical Information in Europe, with an overall goal to continuously improve access to information about medicinal products for healthcare providers and patients. Susan Mohamed is an employee of Pfizer, Angela Flores is an employee and shareholder of Lilly, Eva Loew is an employee of Novartis Pharma GmbH and shareholder of Novartis AG and Alcon AG, Stefne Pienaar is an employee and shareholder of Pfizer, Sarah Dunnett is Director and shareholder of Sarah Dunnett Consulting Limited and shareholder of Baxter and Takeda.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
From Corresponding Author on reasonable request.
Code availability
Not applicable.
Authors' contributions
All authors were involved in researching, authoring and reviewing the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mohamed, S., Dunnett, S., Flores, A. et al. A Principles Framework for Digital Provision of Medical Information for Healthcare Professionals. Pharm Med 37, 103–109 (2023). https://doi.org/10.1007/s40290-023-00464-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-023-00464-0