Abstract
Introduction
Artificial intelligence through machine learning uses algorithms and prior learnings to make predictions. Recently, there has been interest to include more artificial intelligence in pharmacovigilance of products already in the market and pharmaceuticals in development.
Objective
The aim of this study was to identify and describe the uses of artificial intelligence in pharmacovigilance through a systematic literature review.
Methods
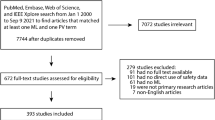
Embase and MEDLINE database searches were conducted for articles published from January 1, 2015 to July 9, 2021 using search terms such as ‘pharmacovigilance,’ ‘patient safety,’ ‘artificial intelligence,’ and ‘machine learning’ in the title or abstract. Scientific articles that contained information on the use of artificial intelligence in all modalities of patient safety or pharmacovigilance were reviewed and synthesized using a pre-specified data extraction template. Articles with incomplete information and letters to editor, notes, and commentaries were excluded.
Results
Sixty-six articles were identified for evaluation. Most relevant articles on artificial intelligence focused on machine learning, and it was used in patient safety in the identification of adverse drug events (ADEs) and adverse drug reactions (ADRs) (57.6%), processing safety reports (21.2%), extraction of drug–drug interactions (7.6%), identification of populations at high risk for drug toxicity or guidance for personalized care (7.6%), prediction of side effects (3.0%), simulation of clinical trials (1.5%), and integration of prediction uncertainties into diagnostic classifiers to increase patient safety (1.5%). Artificial intelligence has been used to identify safety signals through automated processes and training with machine learning models; however, the findings may not be generalizable given that there were different types of data included in each source.
Conclusion
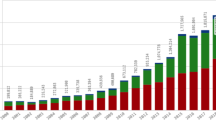
Artificial intelligence allows for the processing and analysis of large amounts of data and can be applied to various disease states. The automation and machine learning models can optimize pharmacovigilance processes and provide a more efficient way to analyze information relevant to safety, although more research is needed to identify if this optimization has an impact on the quality of safety analyses. It is expected that its use will increase in the near future, particularly with its role in the prediction of side effects and ADRs.

Similar content being viewed by others
References
Das S, dey A, Pal A, Roy N. Applications of artificial intelligence in machine learning: review and prospect. Int J Comput Appl. 2015;115:31–41.
Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Ann Surg. 2018;268(1):70–6.
Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–9.
Macrae C. Governing the safety of artificial intelligence in healthcare. BMJ Qual Saf. 2019;28(6):495–8.
Grossman LV, Choi SW, Collins S, Dykes PC, O’Leary KJ, Rizer M, et al. Implementation of acute care patient portals: recommendations on utility and use from six early adopters. J Am Med Inf Assoc. 2018;25(4):370–9.
Bates DW, Levine D, Syrowatka A, Kuznetsova M, Craig KJT, Rui A, et al. The potential of artificial intelligence to improve patient safety: a scoping review. NPJ Digit Med. 2021;4(1):54.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Abatemarco D, Perera S, Bao SH, Desai S, Assuncao B, Tetarenko N, et al. Training augmented intelligent capabilities for pharmacovigilance: applying deep-learning approaches to individual case safety report processing. Pharmaceut Med. 2018;32(6):391–401.
Alvaro N, Conway M, Doan S, Lofi C, Overington J, Collier N. Crowdsourcing Twitter annotations to identify first-hand experiences of prescription drug use. J Biomed Inform. 2015;58:280–7.
Basile AO, Yahi A, Tatonetti NP. Artificial intelligence for drug toxicity and safety. Trends Pharmacol Sci. 2019;40(9):624–35.
Baumgartner M, Eggerth A, Ziegl A, Hayn D, Schreier G. Experimenting with generative adversarial networks to expand sparse physiological time-series data. Stud Health Technol Inform. 2020;271:248–55.
Bean DM, Wu H, Iqbal E, Dzahini O, Ibrahim ZM, Broadbent M, et al. Knowledge graph prediction of unknown adverse drug reactions and validation in electronic health records. Sci Rep. 2017;7(1):16416.
Ben Abacha A, Chowdhury MFM, Karanasiou A, Mrabet Y, Lavelli A, Zweigenbaum P. Text mining for pharmacovigilance: using machine learning for drug name recognition and drug-drug interaction extraction and classification. J Biomed Inf. 2015;58:122–32.
Benin AL, Fodeh SJ, Lee K, Koss M, Miller P, Brandt C. Electronic approaches to making sense of the text in the adverse event reporting system. J Healthc Risk Manag. 2016;36(2):10–20.
Bouzillé G, Morival C, Westerlynck R, Lemordant P, Chazard E, Lecorre P, et al. An Automated detection system of drug-drug interactions from electronic patient records using big data analytics. Stud Health Technol Inf. 2019;264:45–9.
Calix RA, Gupta R, Gupta M, Jiang K, editors. Deep gramulator: improving precision in the classification of personal health-experience tweets with deep learning. In: 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); 2017;1154–1159.
Chandak P, Tatonetti NP. Using machine learning to identify adverse drug effects posing increased risk to women. Patterns (N Y). 2020;1(7):100108.
Chauvet R, Bousquet C, Lillo-Lelouet A, Zana I, Ben Kimoun I, Jaulent MC. Classification of the severity of adverse drugs reactions. Stud Health Technol Inf. 2020;270:1227–8.
Chen J, Lalor J, Liu W, Druhl E, Granillo E, Vimalananda V, et al. Detecting Hypoglycemia Incidents Reported in Patients’ Secure Messages: Using Cost-sensitive Learning and Oversampling to Reduce Data Imbalance (Preprint). J Med Internet Res. 2019;21(3):e11990.
Chen Z, Zhang H, George T, Prosperi M, Guo Y, Braithwaite D, et al. Abstract PO-071: simulation of colorectal cancer clinical trials using real-world data and machine learning. Clin Cancer Res. 2021;27(5 Supplement):PO-071.
Cocos A, Fiks AG, Masino AJ. Deep learning for pharmacovigilance: recurrent neural network architectures for labeling adverse drug reactions in Twitter posts. J Am Med Inf Assoc. 2017;24(4):813–21.
Colón-Ruiz C, Segura-Bedmar I. Comparing deep learning architectures for sentiment analysis on drug reviews. J Biomed Inf. 2020;110:103539.
Comfort S, Perera S, Hudson Z, Dorrell D, Meireis S, Nagarajan M, et al. Sorting through the safety data haystack: using machine learning to identify individual case safety reports in social-digital media. Drug Saf. 2018;41(6):579–90.
Correia Pinheiro L, Durand J, Dogné JM. An application of machine learning in pharmacovigilance: estimating likely patient genotype from phenotypical manifestations of fluoropyrimidine toxicity. Clin Pharmacol Ther. 2020;107(4):944–7.
Courtois É, Pariente A, Salvo F, Volatier É, Tubert-Bitter P, Ahmed I. Propensity score-based approaches in high dimension for pharmacovigilance signal detection: an empirical comparison on the French spontaneous reporting database. Front Pharmacol. 2018;9:1010.
Dandala B, Joopudi V, Devarakonda M. Adverse drug events detection in clinical notes by jointly modeling entities and relations using neural networks. Drug Saf. 2019;42(1):135–46.
Daniel C, Kalra D. Section editors for the IYSoCRI. Clinical research informatics. Yearb Med Inf. 2020;29(1):203–7.
Davazdahemami B, Delen D. A chronological pharmacovigilance network analytics approach for predicting adverse drug events. J Am Med Inform Assoc. 2018;25(10):1311–21.
De Pretis F, Landes J, Peden W. Artificial intelligence methods for a Bayesian epistemology-powered evidence evaluation. J Eval Clin Pract. 2021;27(3):504–12.
Desai S, Chan K, Bannout K, Mingle E, Freeman J, Parikh U, et al. A novel approach to standardizing data & detecting duplicates across adverse event data sources using machine learning. Drug Saf. 2018;41(11):1246–7.
Desai SME, Egan B, Gulati R, Freeman J. A framework for leveraging emerging technologies in pharmacovigilance. Drug Saf. 2018;41(11):1223–4.
Dewulf P, Stock M, De Baets B. Cold-start problems in data-driven prediction of drug-drug interaction effects. Pharmaceuticals. 2021;14(5):429.
El-Allaly ED, Sarrouti M, En-Nahnahi N, Ouatik El Alaoui S. An adverse drug effect mentions extraction method based on weighted online recurrent extreme learning machine. Comput Methods Programs Biomed. 2019;176:33–41.
Eshleman R, Singh R. Leveraging graph topology and semantic context for pharmacovigilance through twitter-streams. BMC Bioinform. 2016;17(13):335.
Evans HP, Anastasiou A, Edwards A, Hibbert P, Makeham M, Luz S, et al. Automated classification of primary care patient safety incident report content and severity using supervised machine learning (ML) approaches. Health Inf J. 2019;26(4):3123–39.
Fan Y, He L, Zhang R. Evaluating automatic methods to extract patients' supplement use from clinical reports. Proceedings IEEE International Conference on Bioinformatics and Biomedicine. 2017;2017:1258-61
Fan Y, Zhang R. Using natural language processing methods to classify use status of dietary supplements in clinical notes. BMC Med Inf Decis Mak. 2018;18(2):51.
Fong A, Behzad S, Pruitt Z, Ratwani RM. A machine learning approach to reclassifying miscellaneous patient safety event reports. J Patient Saf. 2021;17(8):e829–33.
Fong A, Harriott N, Walters DM, Foley H, Morrissey R, Ratwani RR. Integrating natural language processing expertise with patient safety event review committees to improve the analysis of medication events. Int J Med Inf. 2017;104:120–5.
Foufi V, Ing Lorenzini K, Goldman JP, Gaudet-Blavignac C, Lovis C, Samer C. Automatic classification of discharge letters to detect adverse drug reactions. Stud Health Technol Inf. 2020;270:48–52.
Gartland A, Bate A, Painter JL, Casperson TA, Powell GE. Developing crowdsourced training data sets for pharmacovigilance intelligent automation. Drug Saf. 2021;44(3):373–82.
Gavrielov-Yusim N, Kürzinger ML, Nishikawa C, Pan C, Pouget J, Epstein LB, et al. Comparison of text processing methods in social media-based signal detection. Pharmacoepidemiol Drug Saf. 2019;28(10):1309–17.
Gupta J, Patrick J, Poon S. Clinical safety incident taxonomy performance on C4.5 decision tree and random forest. Stud Health Technol Inform. 2019;266:83–8.
Gupta S, Pawar S, Ramrakhiyani N, Palshikar GK, Varma V. Semi-supervised recurrent neural network for adverse drug reaction mention extraction. BMC Bioinform. 2018;19(8):212.
Henriksson A, Zhao J, Dalianis H, Boström H. Ensembles of randomized trees using diverse distributed representations of clinical events. BMC Med Inf Decis Mak. 2016;16(2):69.
Jagannatha AN, Yu H. Bidirectional RNN for medical event detection in electronic health records. Proc Conf. 2016;2016:473–82.
Kalaiselvan V, Sharma A, Gupta SK. “Feasibility test and application of AI in healthcare”—with special emphasis in clinical, pharmacovigilance, and regulatory practices. Health Technol. 2021;11(1):1–15.
Kreimeyer K, Dang O, Spiker J, Muñoz MA, Rosner G, Ball R, et al. Feature engineering and machine learning for causality assessment in pharmacovigilance: Lessons learned from application to the FDA Adverse Event Reporting System. Comput Biol Med. 2021;135: 104517.
Laves M-H, Ihler S, Ortmaier T, Kahrs LA. Quantifying the uncertainty of deep learning-based computer-aided diagnosis for patient safety. Curr Dir Biomed Eng. 2019;5:223–6.
Letinier LJJ, Miremont G, Bel-Letoile A, Salvo F, Rouby F, et al. Machine learning and semantic information for unstructured healthcare data: Comparison of methods through the automatic analysis of adverse drug reaction reports. MAI TAI study. Fundam Clin Pharmacol. 2021;35(SUPPL 1):20.
Li F, Liu W, Yu H. Extraction of information related to adverse drug events from electronic health record notes: design of an end-to-end model based on deep learning. JMIR Med Inf. 2018;6(4): e12159.
Li H, Yang M, Chen Q, Tang B, Wang X, Yan J. Chemical-induced disease extraction via recurrent piecewise convolutional neural networks. BMC Med Inf Decis Mak. 2018;18(2):60.
Liang C, Gong Y. Enhancing patient safety event reporting by K-nearest neighbor classifier. Stud Health Technol Inf. 2015;218:93–9.
Lien F, Wang HY, Lu JJ, Wen YH, Chiueh TS. Predicting 2-day mortality of thrombocytopenic patients based on clinical laboratory data using machine learning. Med Care. 2021;59(3):245–50.
Liu F, Pradhan R, Druhl E, Freund E, Liu W, Sauer BC, et al. Learning to detect and understand drug discontinuation events from clinical narratives. J Am Med Inf Assoc. 2019;26(10):943–51.
Marella WM, Sparnon E, Finley E. Screening electronic health record-related patient safety reports using machine learning. J Patient Saf. 2017;13(1):31–6.
Ménard T, Barmaz Y, Oettinghaus B, Bowling R, Popko L. Enabling data-driven clinical quality assurance: predicting adverse event reporting in clinical trials using machine learning. Drug Saf. 2019;42(9):1045–53.
Mockute R, Desai S, Perera S, Assuncao B, Danysz K, Tetarenko N, et al. Artificial intelligence within pharmacovigilance: a means to identify cognitive services and the framework for their validation. Pharmaceut Med. 2019;33(2):109–20.
Mower J, Cohen T, Subramanian D. Complementing observational signals with literature-derived distributed representations for post-marketing drug surveillance. Drug Saf. 2020;43(1):67–77.
Mower J, Subramanian D, Cohen T. Learning predictive models of drug side-effect relationships from distributed representations of literature-derived semantic predications. J Am Med Inf Assoc. 2018;25(10):1339–50.
Negi K, Pavuri A, Patel L, Jain C. A novel method for drug-adverse event extraction using machine learning. Inf Med Unlocked. 2019;17: 100190.
Nikfarjam A, Sarker A, O’Connor K, Ginn R, Gonzalez G. Pharmacovigilance from social media: mining adverse drug reaction mentions using sequence labeling with word embedding cluster features. J Am Med Inf Assoc. 2015;22(3):671–81.
Noguchi Y, Tachi T, Teramachi H. Subset analysis for screening drug-drug interaction signal using pharmacovigilance database. Pharmaceutics. 2020;12(8):762.
Qiu Y, Zhang Y, Deng Y, Liu S, Zhang W. A comprehensive review of computational methods for drug-drug interaction detection. IEEE/ACM Trans Comput Biol Bioinform. 2021;3:7487.
Salathé M. Digital pharmacovigilance and disease surveillance: combining traditional and big-data systems for better public health. J Infect Dis. 2016;214(suppl_4):S399–403.
Sarker A, Gonzalez G. Portable automatic text classification for adverse drug reaction detection via multi-corpus training. J Biomed Inform. 2015;53:196–207.
Schmider J, Kumar K, LaForest C, Swankoski B, Naim K, Caubel PM. Innovation in pharmacovigilance: use of artificial intelligence in adverse event case processing. Clin Pharmacol Ther. 2019;105(4):954–61.
Simon ST, Mandair D, Tiwari P, Rosenberg MA. Prediction of drug-induced long QT syndrome using machine learning applied to harmonized electronic health record data. J Cardiovasc Pharmacol Ther. 2021;26(4):335–40.
Thompson P, Daikou S, Ueno K, Batista-Navarro R, Ji T, Ananiadou S. Annotation and detection of drug effects in text for pharmacovigilance. J Cheminform. 2018;10(1):37.
Wang M, Ma X, Si J, Tang H, Wang H, Li T, et al. Adverse drug reaction discovery using a tumor-biomarker knowledge graph. Front Genet. 2021;11:625659.
Yang J, Wang L, Phadke NA, Wickner PG, Mancini CM, Blumenthal KG, et al. Development and validation of a deep learning model for detection of allergic reactions using safety event reports across hospitals. JAMA. 2020;3(11):e2022836-e.
Yang X, Bian J, Gong Y, Hogan WR, Wu Y. MADEx: a system for detecting medications, adverse drug events, and their relations from clinical notes. Drug Saf. 2019;42(1):123–33.
Zhao J, Henriksson A, Asker L, Boström H. Predictive modeling of structured electronic health records for adverse drug event detection. BMC Med Inf Decis Mak. 2015;15 Suppl 4(Suppl 4):S1.
Sujan MBC, Salmon P, Pool R, Chozos N. Human factors and ergonomics in healthcare AI. Chartered Institute of Ergonomics and Human Factors; 2021.
Lewis DJ, McCallum JF. Utilizing advanced technologies to augment pharmacovigilance systems: challenges and opportunities. Ther Innov Regul Sci. 2020;54(4):888–99.
Artificial Intelligence in Health Care: Benefits and Challenges of Technologies to Augment Patient Care. US Government Accountability Office. 2020. https://www.gao.gov/products/gao-21-7sp. Accessed 10 June 2022.
Laying down harmonised rules on artificial intelligence (Artificial Intelligence Act) and amending certain union legislative acts. EUR-LEX. 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52021PC0206. Accessed 10 June 2022.
Kassekert R, Grabowski N, Lorenz D, Schaffer C, Kempf D, Roy P, et al. Industry perspective on artificial intelligence/machine learning in pharmacovigilance. Drug Saf. 2022;45(5):439–48.
Lee J-Y, Lee Y-S, Kim DH, Lee HS, Yang BR, Kim MG. The use of social media in detecting drug safety-related new black box warnings, labeling changes, or withdrawals: scoping review. JMIR Public Health Surveill. 2021;7(6): e30137.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received. All authors are members of the North America Chapter of the International Society of Pharmacovigilance (NASoP), which is a non-profit scientific organization.
Conflicts of interest
Maribel Salas and Priyanka Yalamanchili are employees of Daiichi Sankyo, Inc. Jan Petracek is the founder and director of the Institute of Pharmacovigilance. Omar Aimer is an employee of Innovigilance. Dinesh Kasthuril is an employee of Labcorp Drug Development. Sameer Dhingra is Associate Professor and Head of Department of Pharmacy Practice at National Institute of Pharmaceutical Education and Research (NIPER), Hajipur. Toluwalope Junaid is an employee of Syneos Health. Tina Bostic is an employee of PPD, part of Thermo Fisher Scientific. The opinions and positions taken in this article are personal to the authors and not their employer/affiliated institutions.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
MS: study design, study implementation (title/abstract screening, quality control), preparation of draft manuscript, review of manuscript draft, and interpretation of results. JP: study design, study implementation (title/abstract screening), review of manuscript draft, and interpretation of results. PY: study design, study implementation (full article screening, quality control), preparation of draft manuscript, review of manuscript draft, and interpretation of results. OA: study design, study implementation (title/abstract screening, full article screening), review of manuscript draft, and interpretation of results. DK: study design, study implementation (full article screening), review of manuscript draft, and interpretation of results. SD: review of manuscript draft and interpretation of results. TJ: study implementation (quality control), review of manuscript draft, and interpretation of results. TB: study design, study implementation (title/abstract screening, quality control), review of manuscript draft, interpretation of results, quality control of summary table. All authors have read and approved the final version of the manuscript and agree to be accountable for the work presented.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salas, M., Petracek, J., Yalamanchili, P. et al. The Use of Artificial Intelligence in Pharmacovigilance: A Systematic Review of the Literature. Pharm Med 36, 295–306 (2022). https://doi.org/10.1007/s40290-022-00441-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-022-00441-z