Abstract
Background
Legislative requirements for Marketing Authorisation Holders (MAHs) to maintain a Pharmacovigilance System Master File (PSMF) were introduced in the European Union (EU) in 2010, operationalised in 2012 and subsequently introduced in other territories. There are no internationally agreed standards for the PSMF and country/regional requirements vary, leaving room for interpretation. This creates complexities for MAHs in implementing and maintaining multiple PSMFs.
Objectives
The approaches taken towards the creation and maintenance of PSMFs in a global environment were investigated using a survey in order to gain a better understanding of the impact of the PSMF for MAHs.
Methods
A structured benchmarking survey was conducted during September and October of 2019 and the responses were analysed. A questionnaire with open-ended questions was designed to elicit detailed information on PSMF management and provide insights into company experiences. Companies affiliated to the EU Federation of Pharmaceutical Industries and Associations (EFPIA) and industry stakeholders with experience of PSMFs were contacted ensuring a broad representation including small, medium and large pharmaceutical companies, contract organisations/consultants and research-driven and generic organisations.
Results
Thirty companies responded; of these, 29 provided information relating to their PSMF practices. Respondents acknowledged that the PSMF is a valuable document that has helped to create greater awareness of pharmacovigilance within companies. Complex and varying international requirements were recognised as burdensome, especially in the context of consistent development and maintenance of multiple PSMFs. The respondents indicated that companies use the EU PSMF to manage requirements in other territories. Similar areas for standardisation were identified across respondents.
Conclusion
The survey results highlight both the value of the PSMF and the challenges in maintaining it. Building on these responses, the paper offers pragmatic solutions to the challenges faced by MAHs and proposes a continued dialogue with key stakeholders in industry and national regulatory authorities about PSMF globalisation, harmonisation and simplification of requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The value of the Pharmacovigilance System Master File (PSMF) and the commitment within industry to comply with the requirements are recognised and reflected in survey feedback received from Marketing Authorisation Holders. |
The responses also showed that complex and varying international requirements are burdensome. |
The authors would welcome dialogue between industry and national regulatory authorities with respect to globalisation of PSMF requirements, including their simplification and harmonisation. |
1 Introduction
The concept of the Pharmacovigilance System Master File (PSMF) was first introduced in European Union (EU) legislation in 2010 [1, 2] with the Commission Implementing Regulation No. 520/2012 [3] and GVP Module II [4] providing requirements and guidance, respectively, in 2012 for EU Marketing Authorisation Holders (MAHs) on the operational aspects of the legislation. The PSMF replaced the earlier requirement for a Detailed Description of the Pharmacovigilance System (DDPS) [5].
Since the introduction of the PSMF in the EU, national regulatory authorities (NRAs) in other territories have introduced similar legislation and/or local Good Pharmacovigilance (GVP) guidelines that include PSMF requirements (see Section 1.1.2. Other Territories).
Whilst it is recognised that the PSMF has multiple benefits (e.g., internal/external oversight of the pharmacovigilance [PV] system, a central place for PV information, internal training purposes), pharmaceutical companies have experienced practical challenges in maintenance and implementation due to the varying requirements, their different interpretation and lack of global legislative harmonisation.
To discuss the topic of managing the PSMF in the evolving PV landscape and to exchange best practices, the authors (members of the PSMF Networking Group,Footnote 1 hereafter referred to as the PSMF NG) have met regularly since 2017 to evaluate and discuss the implementation of global PSMF requirements, new and developing regulations and to check the mutual understanding of NRAs’ expectations. They conducted an industry survey in 2019 to better understand benefits and challenges, in particular when facing the globalisation of PSMF legislation. The results of this survey are reflected and evaluated in this paper, which
-
summarises the findings of the survey and the PSMF NG experience with regard to the construction and use of the PSMF globally;
-
aims to create awareness amongst stakeholders of the practical challenges for industry in addressing the global requirements;
-
aims to open up discussion with all stakeholders including NRAs and share future considerations to drive towards a global PSMF concept through harmonisation, simplification and reduced duplication of effort.
1.1 Global Pharmacovigilance System Master File (PSMF) Legislation and Requirements
1.1.1 The European Union (EU)
The PSMF provides an overview of a company’s global PV system to contribute to the EU Qualified Person for Pharmacovigilance’s (QPPV) oversight and to the planning and conduct of internal PV audits. It is registered in the Extended EudraVigilance Medicinal Product Dictionary (XEVMPD) and provided upon request to support PV inspections by NRAs. The PSMF “should reflect global availability of safety information for medicinal products authorised in the EU, presenting information on the pharmacovigilance system applied at global, regional and local levels” [4]. Key aspects relating to the PSMF content are provided in Table 1 [4].
The Implementing Regulation [3] and EU GVP Module II [4] provide a framework that allows flexibility to accommodate the different organisational structures of MAHs. However, the experience of the PSMF NG is of differing interpretations by EU NRAs on the scope of the PV system and information to be presented in the PSMF; for example, requests for study data or vendor presentation may vary, wet ink signature requirements for PSMF-related documents, and NRAs may request a supplementary, ‘local’ version of the PSMF.
One of the intended objectives for the PSMF, outlined in EU GVP Module II, was to reflect the PV system in place and act as an oversight tool for the EU QPPV and NRAs. The experience of the PSMF NG is that through the evolution of MAH PV and quality systems in the time since 2012, including enhanced processes and mechanisms to assess and share feedback on system performance, there may now be additional ways in which oversight is provided to the EU QPPV/MAH Senior Management (e.g., participation in regular PV governance/compliance forums). However, the PSMF has undoubtedly contributed to improvements in oversight over MAH's PV and quality systems. It continues to serve as a tool that offers systematic and holistic oversight of the PV and quality systems and their performance, and enables greater connectivity with other departments contributing to the PV system, thereby indirectly raising the profile of PV and PV compliance within companies.
1.1.2 Other Territories
The PSMF concept continues to evolve in other territories with the introduction of requirements and/or expectations for a PSMF or equivalent document; examples include Arab League, Eurasia and India [6,7,8]. Requirements for a PSMF in the different countries follow the principles of oversight, PV system reflection and audit planning as introduced in the EU, but they also frequently focus on country-specific information (e.g., inclusion of country organisational charts, country-specific lists for approved products, studies and local PV service providers).
The PV regulatory landscape also continues to develop at different rates across territories. In some regions the requirements may be driven by well-established PV departments of a ‘lead’ NRA with other countries in that region just starting their development of a PV infrastructure, as described by Alshammari et al. [9]. Even where requirements have been set at a regional level, individual NRAs within the region may insist on additional documentation such as country-specific annexes, supplementary documentation, or translations in local language.
The aim of this paper is not to provide a detailed overview of the requirements in these territories. However, the PSMF NG’s collective experience of documents provided to comply with regulatory requirements are summarised in Table 2.
From an industry perspective, an additional complexity is related to the varying document submission requirements across the countries/regions. These may include a PSMF kept on file and submitted only on NRA request, or submitted as part of a regulatory dossier (e.g., marketing authorisation application [MAA], renewal), or submitted after a major PV system change, or submitted annually, and/or a combination of any of these scenarios. It has also been noted that regulatory requirements in certain regions appear to be aligned with previous versions of EU GVP Module II, for example the Eurasian Economic Region and Arabic GVP Modules. The authors also note that, after the completion of the survey, further UK-specific requirements came into force in 2021 [10].
1.2 Summary
Both in and outside of the EU, it appears PSMF requirements vary because of interpretation, implementation status or actual country/regional requirements. Multinational companies are challenged with the task of complying with these requirements and interpretations. Manual adaptation and customisation of data reflected in the PSMF to address the requirements/interpretations are challenging, time consuming and duplicative and require quality control steps to ensure the delivery of high-quality data. Harmonisation and simplification have the potential to reduce the burden of PSMF maintenance and to create efficiencies in the process and therefore support and facilitate high-quality outputs.
2 Objectives
The approaches taken towards the creation and maintenance of PSMFs in a global environment were investigated using a survey in order to gain a better understanding of the impact of the PSMF for MAHs.
3 Methods
Informal exchange within the PSMF NG revealed that companies aim to comply with the various expectations and requirements, however they have different approaches to achieve this. An industry benchmarking survey was conducted during September and October of 2019 by the PSMF NG to better understand the various approaches taken across industry towards PSMF maintenance, as well as the influence of the EU PSMF model on the production and maintenance of PSMFs outside the EU.
For this paper, a qualitative approach was taken; a targeted questionnaire (survey) was used to obtain information about the PSMF experience (primary information related to experience of industry subject matter experts). Open-ended questions were developed allowing free-text responses.
Survey responses were solicited for EU and other territories’ perspectives; questions were included on the following:
-
Frequency of PSMF updates
-
Adaption of the EU PSMF for use outside the EU
-
Feedback received from NRAs in other territories
-
Benefits and use of the PSMF
-
Challenges of PSMF maintenance
-
Proposals for improvement and globalisation
Open-ended questions were designed to elicit detailed information and provide insights into company experiences. The intention of the survey was not to generate statistical results, but rather to obtain structured information on experiences from day-to-day work, inspections and/or feedback from NRAs.
Companies affiliated to EU Federation of Pharmaceutical Industries and Associations (EFPIA) and industry stakeholders with experience of PSMFs were contacted, ensuring a broad representation including small, medium and large pharmaceutical companies, contract organisations/consultants and research-driven and generic organisations. Invitations to participate in the survey were routed to EU QPPVs and/or PSMF Managers or their delegates, and respondents were requested to complete the survey in a Word document by return email. Respondents were informed that their responses would support this paper.
All responses for the survey were anonymised, and aggregated by the PSMF NG, as communicated upfront to the survey respondents.
4 Results
Thirty companies responded; of these, 29 provided information relating to their PSMF practices. Figure 1 shows the breakdown of respondents by company type and size. Twenty-four companies were identified as pharma, the remainder being contract organisations/consultants producing PSMFs on behalf of clients. Using the European Commission definition [11], seven respondents were small to medium sized (SMEs) and 22 were large enterprises (> 250 staff headcount).
4.1 Management of PSMF Globally
The survey asked about companies’ approaches to PSMF maintenance (i.e., in-house or outsourced) and update frequency (Fig. 2). The results reflect variability in the approaches but demonstrate that all responding pharma companies manage these documents in-house and perform updates on a regular basis, often quarterly. Update of the EU main body text mostly occurs in parallel with the EU annexes, although some companies noted that they maintain a variable approach to annex updates, for example, depending on size, type and complexity of the annex. Contract Research Organisations’ (CROs’) approaches appear to depend on client requirements. The majority of the companies also update their PSMFs in non-EU territories on a quarterly basis, although the approach appears even more variable than that used for EU updates.
Figure 3 provides an overview of how companies utilise the EU PSMF for countries outside the EU. The information provided by 21 respondents demonstrates heterogenous approaches such as the format they choose and the details they include, however the majority modify their EU PSMF.
Two companies noted that they produce a single, global PSMF with information for all countries with applicable local information placed into additional annexes. One of these companies noted that the main body text is provided as a ‘standalone’ document. Three companies stated that local information was ‘appended’ to the EU PSMF as sub-files or annexes, submitted together. This information could include, for example, details on the country QPPV, local organisations, standard operating procedures (SOPs), product lists and metrics.
Over half of the respondents modify the EU PSMF, be it the main body text, the annexes or both. Table 3 demonstrates the types of modifications, as provided by nine companies (more than one may have been indicated by a single responder).
While the modifications vary, there is a trend to use the main body text and/or annexes of the EU PSMF describing the global PV system, and supplement this with local information in accordance with the company’s understanding of local requirements. In the examples given, companies provide the main body text from EU PSMF Sects. 2–7 along with some EU PSMF annexes. Affiliates may then complete their PSMF by adding local information, for example on the country QPPV, metrics and local SOPs.
Only five participants indicated that they had submitted a PSMF (EU or localised PSMF) to the NRAs in other territories. Without providing details, one indicated that they had received feedback on the PSMF from a NRA.
4.2 Globalisation of the PSMF—Industry Challenges
Survey participants were asked to comment on the three biggest challenges of operationalising and implementing PSMF requirements globally. The answers have been grouped into categories for ease of review. Additional detail from the answers has also been included, where possible, for some categories. The results for both EU and other territories are shown in Figs. 4, 5.
4.3 Industry-Perceived Value of the PSMF
The survey asked respondents to share thoughts on the value of the PSMF. Respondents provided the following examples:
-
for the EU QPPV (e.g., enhancing cross-functional links, tool for training on the PV system);
-
for the company (e.g., centralises, drives quality of compliance data and database outputs);
-
other (e.g., inspection readiness as PSMF components can be used to fulfil pre-inspection requests).
Most of the respondents indicated that the EU PSMF and that of other territories are valuable in providing oversight of the PV system, supporting PV audit planning and the preparation and conduct of PV inspections, as intended in the legislation. Additional value includes training for new colleagues, a document/process to drive quality and compliance, and enhancing cross-functional activities within the organisation.
One respondent noted that, while the EU PSMF provides a good oversight of the PV system and its performance, there remains the “danger that too much [information] is included and oversight is lost”. Three other respondents noted that the benefit of other territories’ PSMFs may be limited and/or outweighed due to the burden of their maintenance.
4.4 PSMF Globalisation
The final question asked for recommendations for a truly globalised PSMF. Table 4 shows the diverse responses, with over two thirds relating to the PSMF structure, in particular regarding the main body text and some annexes, with suggestions to include local data such as local/regional QPPV information and local listings in annexes.
A few comments related to consistency of regulatory guidance and expectations, such as suggesting a common template as well as alignment of requirements. There were also comments suggesting improvements industry could make, such as automating information gathering.
5 Discussion
Overall, there is a clear commitment from the survey participants to operationalise and provide the PSMF in compliance with the respective regulatory requirements and NRA expectations. The results highlight the different approaches taken for PSMF maintenance and the challenges with the globalisation of the PSMF. While there is variability in the approaches due to issues like local requirements, company structure and portfolios, or company assessment of legislation, there is commonality in thinking. This is evidenced in the 29 responses demonstrating that most companies are utilising and/or adapting the EU PSMF process and documents to meet requirements in other territories. Suggestions for standardisation also appear to be similar across the responses received.
It has been over 10 years since the introduction of PSMF requirements in EU GVP Module II. The challenges faced by industry in the preparation and maintenance of the EU PSMF, as discussed in various industry fora (e.g., DIA workshops in November 2019 and November 2020), are well reflected in the responses to the survey. The results in Table 5 indicate that obtaining data in a timely and efficient manner from relevant stakeholders for the EU PSMF is a challenge across companies. The understanding and engagement of contributors, in particular outside the safety function, can be key to the provision of accurate data. The quality of data included in the source systems is highlighted as an additional challenge: company systems/databases may not be set up to provide data specifically for the PSMF, they may not contain the required data or data may need to be pulled from multiple systems with a potential lack of harmonisation across systems.
The survey demonstrates that the introduction of PSMF legislation in other territories brings additional challenges and the operationalisation of the PSMF maintenance process becomes more complex. Some countries have incorporated elements of EU GVP Module II into their legislation without implementing the revisions. The complete PSMF may be requested in other territories outside the EU as part of regulatory dossiers and licence renewals; a purpose not foreseen in EU GVP Module II. The approaches taken across companies are heterogenous: some territories outside the EU accept the EU PSMF, some companies provide the EU PSMF main body text only, some the summary of the applicant’s PV system. This lack of harmonisation, difficulties in interpretation and tracking of legislation in other territories and NRA expectations is burdensome, leading to intra- and inter-company inconsistency.
Producing PSMFs to meet the varying legislation across the globe raises challenges for industry, in particular, how to ensure consistency and scale up production. Large pharma companies may have developed systems or programmes to enable standardised and quick retrieval of data, addressing any country-specific need. However, respondents indicate the obvious resource burden.
The results from the survey nevertheless clearly demonstrate the value placed by industry on the PSMF. Several respondents identified the PSMF as a “critical”, “great” or “useful” tool and indicated that the utilisation of a PSMF can extend beyond the original purpose. The description of the PV system contained in the main body text provides a useful central resource of information for functions outside of safety as well as all new starters to demonstrate the role of the company’s PV system. Many respondents (see Table 4) noted that the PSMF helps to drive quality and compliance through identification and resolution of process gaps, streamlining of PV activities across functions, and the regular review of systems and processes. Furthermore, the PSMF has helped to raise the profile of PV in global functions and affiliates, due to the cross-functional collaboration.
6 Conclusions
The PSMF is recognised as an essential, central point for access to key PV data, enabling oversight of PV system organisation and performance for relevant stakeholders within the MAH (PV Auditors, QPPVs/locally responsible PV persons) and externally, namely NRAs (including their inspectorates). A good PSMF helps to drive PV inspection focus, may reduce inspection requests and time on site, and provides PV inspectors and key internal stakeholders consistent information. Subsequently, EU GVP Module II has been adopted in numerous other countries/regions, although there is variability in the application of the guidance and the implementation status.
Companies are apparently using their EU PSMF as a repository for global PV system information and to comply with local/regional requirements. This has led to heterogeneous approaches—all with an administrative burden of maintaining multiple PSMFs, in multiple jurisdictions, essentially containing the same information, with local variations. Respondents have provided suggestions on how globalisation of the PSMF could be achieved. The overall themes appear similar, highlighting a desire for alignment of requirements across NRAs and a true global approach.
Given the variability in PSMF requirements globally, the PSMF NG wanted to explore and offer potential solutions to address the complex and resource-intensive maintenance of the PSMF based on the survey feedback and their own experience, without detracting from the PSMF objectives. For example, would there be the opportunity to have a harmonised document reflecting the global PV system, such as already seen for the ICH Common Technical Document (ICH M4 guideline) [12]? However, an ICH document on the PSMF has its own complexities, not least that certain regions currently do not have PSMF requirements. Regardless of mechanism, a harmonised approach allowing a global PSMF text to be supplemented by specific country/regional data would reduce the burden on MAHs, streamline the PSMF maintenance efforts and lead to a high-quality output. Such a mutually recognised approach would afford NRAs oversight via a streamlined and efficient review of global PV system information, with a focus on applicable local data.
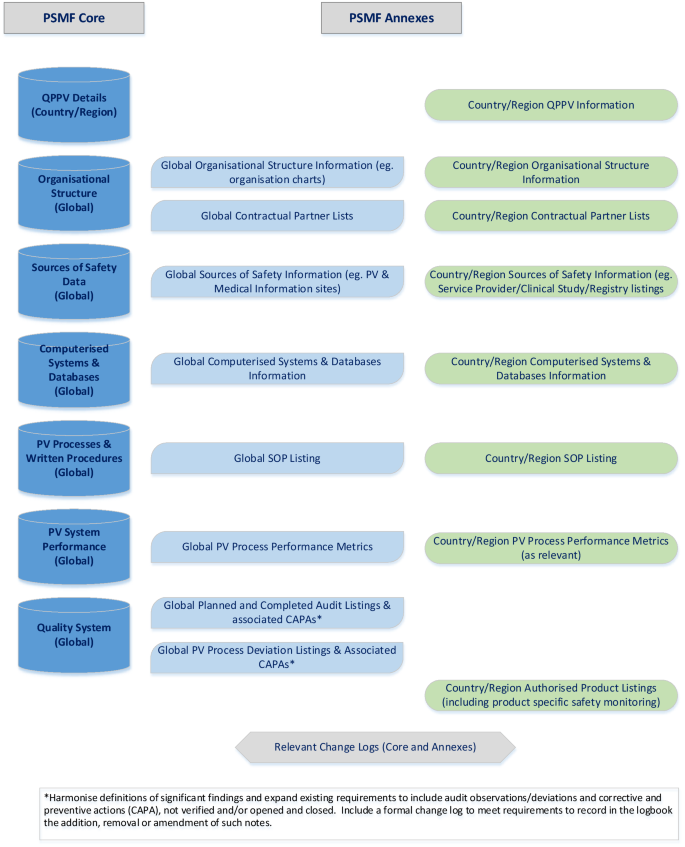
In a systematic review of the PV system, and the implementation of the Arab GVP, Alshammari et al. (2018) [9] pointed to the opportunity regarding harmonisation of PV requirements. In this spirit, the vision of the PSMF NG, as already elaborated in an earlier publication [13], is a pragmatic approach to ensure that the content of a company’s PSMF presents information on the PV system at both global and regional/local levels, in a format that allows easy navigation, in local language if required, which would encompass a modular approach whilst still complying with regulatory requirements such as the Commission Implementing Regulation No. 520/2012. This is summarised below and detailed in Fig. 6.
-
A global ‘main body’ document that provides:
-
o
details of the regional/local QPPV, as applicable;
-
o
a single description of a company’s global PV system, which can be utilised in any territory and is available upon request for inspection and oversight purposes.
-
o
-
Global annexes that provide the operational and compliance details of the PV system.
-
Relevant local/regional annexes in line with applicable legislation.
In the authors’ opinion this proposal would drive simplification within the MAH process; allowing greater flexibility through the use of interchangeable local/regional contents to supplement the global main body/annex content where required for an individual country/region, as opposed to creating multiple versions of largely similar documents. For MAHs, this approach would allow central management of the global main body and annex content and means that affiliate colleagues, who are the experts on regulatory and PV requirements locally, can supplement the global document with the applicable local content, as required. For example, PSMF main body content relating to QPPV details and the PV system performance could be cross-referenced to country/regional-specific annexes that are interchangeable depending on the country/region concerned.
Our survey results, and discussions within the PSMF NG, have highlighted the value of the PSMF as seen by industry and the strong commitment to comply with global requirements for this document. Ongoing dialogue between industry and NRAs (for example through inspections, as well as conference platforms such as DIA) is extremely valuable and the Group would welcome continuation of this dialogue with respect to globalisation of PSMF requirements, including, as proposed in this paper, their simplification and harmonisation.
Notes
The PSMF NG is a subgroup of the European Federation of Pharmaceutical Industries and Association (EFPIA) focusing on PSMF-related matters. The group was formed in 2017 and evolved to become an EFPIA working group in 2019.
References
Regulation (EU) No 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. 2022. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0001:0016:EN:PDF. Accessed 24 Jan 2022.
Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. 2022. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDF. Accessed 24 Jan 2022.
Commission Implementing Regulation (EU) No 520/2012 of 19 June 2012 on the performance of pharmacovigilance activities provided for in Regulation (EC) No 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council. 2022. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:159:0005:0025:EN:PDF. Accessed 24 Jan 2022.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module II – Pharmacovigilance system master file (Rev 2). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-ii-pharmacovigilance-system-master-file-rev-2_en.pdf. Accessed 24 Jan 2022.
Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32004L0027. Accessed 24 Jan 2022.
League of Arab States. Guideline on good pharmacovigilance practices (GVP) for Arab countries, version 2, 2014. 2022. http://www.jfda.jo/EchoBusV3.0/SystemAssets/PDF/AR/PharmVigilance/TheGoodPharmacovigilancePracticev2.pdf. Accessed 24 Jan 2022.
Eurasian Economic Commission. Rules of Good Pharmacovigilance Practice (GVP) of Eurasian Economic Union. 2022. https://docs.eaeunion.org/docs/en-us/01227803/cncd_21112016. Accessed 24 Jan 2022.
Indian Pharmacopoeia Commission. Pharmacovigilance Guidance Document for Marketing Authorisation Holders of Pharmaceutical Products. 2018. http://www.ipc.gov.in/PvPI/pub/Guidance%20Document%20for%20Marketing%20Authorization%20Holders.pdf. Accessed 24 Jan 2022.
Alshammari T, Mendi N, Alsowaida Y. Pharmacovigilance system in Arabic countries: a systematic review of 22 Arab countries. Drug Saf. 2018;41(11):1261.
Exceptions and modifications to the EU guidance on good pharmacovigilance practices that apply to UK marketing authorisation holders and the licensing authority. 2022. https://www.gov.uk/government/publications/exceptions-and-modifications-to-the-eu-guidance-on-good-pharmacovigilance-practices-that-will-apply-to-uk-mahs-and-the-mhra. Accessed 15 Jun 2022.
European Commission Internal Market, Industry, Entrepreneurship and SMEs information page. 2022. https://ec.europa.eu/growth/smes/business-friendly-environment/sme-definition_en. Accessed 24 Jan 2022.
ICH M4 Guideline M4 (R4) on Common Technical Document (CTD) for the Registration of Pharmaceuticals for Human Use—Organisation of CTD. 2022. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m4-r4-common-technical-document-ctd-registration-pharmaceuticals-human-use_en.pdf. Accessed 24 Jan 2022.
Lavery C, Emmott J, Jeck-Thole S, Rouben P, Usher D, van der Spuij W, et al.. Globalizing the pharmacovigilance system master file: challenges and opportunities. DIA Global Forum. 2021. https://globalforum.diaglobal.org/issue/october-2021/globalizing-the-pharmacovigilance-system-master-file-challenges-and-opportunities/. Accessed 27 Jan 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this paper.
Conflicts of interest
CL, JE, SJT, PR, DU, WVDS, and LW are employed by their respective companies and may be share/stock holders but have not received additional funding for this project.
Ethics approval
No human subject data were used in this research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Author contributions
CL, JE, SJT, PR, DU, WVDS, and LW were involved in the concept of the article, the design of the survey, interpretation and analysis of results, and all contributed to the writing of and critical appraisal of the article. All authors have read and approve the final submitted version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lavery, C., Emmott, J., Jeck-Thole, S. et al. An Industry Survey on Managing the Pharmacovigilance System Master File in a Global Environment: The Need for a Pragmatic Approach. Pharm Med 36, 233–245 (2022). https://doi.org/10.1007/s40290-022-00422-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-022-00422-2