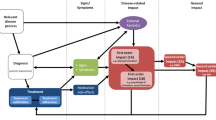
Abstract
The context and setting in which patient-reported outcome (PRO) measures applied in drug development programmes are developed and applied are undergoing rapid changes. The emergence of social media, coupled with advancement of patient-centred methodologies, has led to more connected and empowered patients than before. Drug regulatory agencies are beginning to embrace a new role as collaborators in the drug development process, and new models for defining core outcomes in various disease areas are emerging. Technological advances and social changes have led to the wide use of personal gadgets with greater internet connectivity and computing power than most computers had a generation ago. To explore how progress in the measurement of PROs might be achieved amidst such changes, lessons were drawn from the PRO labelling claims reviewed by the US FDA following the publication of the PRO guidance in 2009. In addition, semi-structured interviews were carried out with seven experts working with drug regulatory agencies and in the pharmaceutical industry to identify major issues and possible solutions. The following recommendations are proposed: (1) that comprehensive hypotheses about the PRO concept be developed; (2) that PRO concepts/measures maintain substantive and clinical validity; (3) that the use of advanced psychometric methods be considered as the new gold standard in the development of PRO measures; (4) that psychometric properties of measures established through “validation by application” be enhanced; (5) that the generalizability of PRO measures be enhanced; (6) that the assessment of ability to detect change, and the interpretability of scores, be improved; and (7) that PRO guidelines by major regulatory authorities be aligned. These entail a greater transparency on the decisions taken during the PRO measure development process and greater understanding of the integration between qualitative aspects of the PRO measure and the score/measurement attributes.
Similar content being viewed by others
Notes
The EXACT is a daily diary for evaluating acute exacerbations of COPD and chronic bronchitis (AECB) [19]. No labelling claim has been granted based on the EXACT to date following its qualification by the FDA.
References
US Food and Drug Administration. US Food and Drug Administration guidance for industry-patient reported outcome measures: use in medical product development to support labelling claims; 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 15 Jan 2012.
Ahmed S, Berzon RA, Revicki DA, Lenderking WR, Moinpour CM, Basch E, et al. The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health care policy. Med Care. 2012;50(12):1060–70.
Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21(8):1305–14.
European Medicines Agency. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: European Medicines Agency, Committee for Medicinal Products for Human Use; 2005.
Marquis P, Caron M, Emery M-P, Scott JA, Arnould B, Acquadro C. The role of health-related quality of life data in the drug approval processes in the US and Europe. Pharm Med. 2011;25(3):147–60.
Baldwin MM, Spong A, Doward L, Gnanasakthy A. Patient-reported outcomes, patient-reported information. Patient. 2011;4(1):11–7.
Frost J, Okun S, Vaughan T, Heywood J, Wicks P. Patient-reported outcomes as a source of evidence in off-label prescribing: analysis of data from PatientsLikeMe. J Med Internet Res. 2011;13(1):e6.
Bender JL, Jimenez-Marroquin M-C, Jadad AR. Seeking support on facebook: a content analysis of breast cancer groups. J Med Internet Res. 2011;13(1):e16.
Hamm MP, Chisholm A, Shulhan J, Milne A, Scott SD, Given LM, et al. Social media use among patients and caregivers: a scoping review. BMJ Open. 2013;3(5). doi:10.1136/bmjopen-2013-002819.
Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc. 2011;86(9):845–50.
DiBenedetti DB, Coles TM, Sharma T. Recruiting patients with a rare blood disorder and their caregivers through social media. Value Health. 2013;16(3):A51.
Gustafson DL, Woodworth CF. Methodological and ethical issues in research using social media: a metamethod of human papillomavirus vaccine studies. BMC Med Res Methodol. 2014;14(1):127.
Rothman M, Gnanaskathy A, Wicks P, Papadopoulos EJ. Can we use social media to support content validity of patient-reported outcome instruments in medical product development? Value Health. 2015;18(1):1–4.
Kamudoni P, Mueller B, Salek M. The development and validation of a disease-specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©). Qual Life Res. 2015;24(4):1017–27.
European Medicines Agency. European Medicines Agency Guidance for applicants seeking scientific advice and protocol assistance. London: European Medicines Agency; 2014.
European Medicines Agency. Best practice guidance for Pilot EMA HTA Parallel Scientific Advice procedures; 2014. European Medicines Agency.
European Medicines Agency. EMA/CHMP/SAWP/178465/2015. Draft qualification opinion of qualification of exacerbations of chronic pulmonary disease tool (EXACT), and EXACT- respiratory symptoms measure (E-RS) for evaluating treatment outcomes in clinical trials in COPD. London: European Medicines Agency; 2015.
US Food and Drug Administration. Draft guidance for industry on qualification of exacerbations of chronic pulmonary disease tool for measurement of symptoms of acute bacterial exacerbation of chronic bronchitis in patients with chronic obstructive pulmonary disease. Federal Register. 2014;79(7).
Leidy NK, Murray LT. Patient-reported outcome (PRO) measures for clinical trials of COPD: the EXACT and E-RS. COPD J Chron Obstr Pulmon Dis. 2013;10(3):393–8.
Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67(7):745–53. doi:10.1016/j.jclinepi.2013.11.013.
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. doi:10.1136/ard.2009.108233.
Coons S, Kothari S, Monz B, Burke L. The patient-reported outcome (PRO) consortium: filling measurement gaps for PRO end points to support labeling claims. Clin Pharmacol Ther. 2011;90(5):743–8.
Leidy NK, Murray LT. Patient-reported outcome (PRO) measures for clinical trials of COPD: the EXACT and E-RS. COPD. 2013;10(3):393–8. doi:10.3109/15412555.2013.795423.
Doward L, Spoorenberg A, Cook S, Whalley D, Helliwell P, Kay L, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. 2003;62(1):20–6.
Reeve BB, Mâsse LC. Item response theory modeling for questionnaire evaluation. In: Presser S, Rothgeb JM, Couper MP, Lessler JT, Martin E, Martin J, et al., editors. Methods for testing and evaluating survey questionnaires. Hoboken: Wiley; 2004. p. 247–73.
Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3.
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94.
Rose M, Fischer F, Nolte S. Who is the norm? Hurdles on the way to an international standardisation of PRO assessments. Qual Life Res. 2014;23(Suppl. 1):12.
Symonds T. Sharing the progress of the PROMIS industry interest committee. Qual Life Res. 2014;23(Supplement 1):12.
Eremenco S, Coons SJ, Paty J, Coyne K, Bennett AV, McEntegart D. PRO data collection in clinical trials using mixed modes: report of the ISPOR PRO mixed modes good research practices task force. Value Health. 2014;17(5):501–16. doi:10.1016/j.jval.2014.06.005.
Coons S, Eremenco S, Lundy JJ, O’Donohoe P, O’Gorman H, Malizia W. Capturing patient-reported outcome (PRO) data electronically: the past, present, and promise of ePRO measurement in clinical trials. Patient. 2014;8:301–9. doi:10.1007/s40271-014-0090-z.
Yeomans A. The future of ePRO platforms. Applied Clinical Trials; 2014.
Gnanasakthy A, Lewis S, Clark M, Mordin M, DeMuro C. Potential of patient-reported outcomes as nonprimary endpoints in clinical trials. Health Qual Life Outcomes. 2013;11:83. doi:10.1186/1477-7525-11-83.
Gnanasakthy A, DeMuro C, Clark M, Mordin M, Thomas S. Role of patient-reported outcome measures in the assessment of central nervous system agents. Ther Innov Regul Sci. 2013;47(5):613–8.
Gnanasakthy A, Mordin M, Clark M, DeMuro C, Fehnel S, Copley-Merriman C. A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health. 2012;15(3):437–42.
Hao Y. Patient-reported outcomes in support of oncology product labeling claims: regulatory context and challenges. Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):407–20.
DeMuro C, Clark M, Mordin M, Fehnel S, Copley-Merriman C, Gnanasakthy A. Reasons for rejection of patient-reported outcome label claims: a compilation based on a review of patient-reported outcome use among new molecular entities and biologic license applications, 2006–2010. Value Health. 2012;15(3):443–8. doi:10.1016/j.jval.2012.01.010.
Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10:S125–37.
Rothman ML, Beltran P, Cappelleri JC, Lipscomb J, Teschendorf B, Mayo/FDA Patient-Reported Outcomes Consensus Meeting Group. Patient-reported outcomes: conceptual issues. Value Health . 2007;10:S66–75. doi:10.1111/j.1524-4733.2007.00269.x.
Leidy NK, Vernon M. Perspectives on patient-reported outcomes : content validity and qualitative research in a changing clinical trial environment. Pharmacoeconomics. 2008;26(5):363–70.
Streiner DL, Norman GR. Health Measurement Scales: a practical guide to their development and use. Oxford: Oxford University Press; 2008.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 2—assessing respondent understanding. Value Health. 2011;14(8):978–88.
Sjodahl Hammarlund C, Nilsson MH, Idvall M, Rosas SR, Hagell P. Conceptualizing and prioritizing clinical trial outcomes from the perspectives of people with Parkinson’s disease versus health care professionals: a concept mapping study. Qual Life Res. 2014;23(6):1687–700. doi:10.1007/s11136-013-0614-3.
Haywood K, Brett J, Salek S, Marlett N, Penman C, Shklarov S, et al. Patient and public engagement in health-related quality of life and patient-reported outcomes research: what is important and why should we care? Findings from the first ISOQOL patient engagement symposium. Qual Life Res. 2014;24:1069–76. doi:10.1007/s11136-014-0796-3.
Forsythe LP, Szydlowski V, Murad MH, Ip S, Wang Z, Elraiyah TA, et al. A systematic review of approaches for engaging patients for research on rare diseases. J Gen Intern Med. 2014;29(Suppl 3):788–800. doi:10.1007/s11606-014-2895-9.
Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, et al. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2011;21:739–46.
Thomas ML. The value of item response theory in clinical assessment: a review. Assessment. 2011;18(3):291–307.
Thompson JR, Cappelleri JC, Getter C, Pleil A, Reichel M, Wolf S. Enhanced interpretation of instrument scales using the Rasch model. Drug Inf J. 2007;41(4):541–50.
Izem R, Kammerman LA, Komo S. Statistical challenges in drug approval trials that use patient-reported outcomes. Stat Methods Med Res. 2013;. doi:10.1177/0962280213476376.
Byrne B. Structural equation modeling with Mplus: basic concepts, applications, and programming. New York: Routledge; 2011.
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR good research practices for evaluating and documenting content validity for the use of existing instruments and their modification PRO task force report. Value Health. 2009;12(8):1075–83.
Reise SP, Ainsworth AT, Haviland MG. Item response theory fundamentals, applications, and promise in psychological research. Curr Dir Psychol Sci. 2005;14(2):95–101.
Terwee C, Dekker F, Wiersinga W, Prummel M, Bossuyt P. On assessing responsiveness of health-related quality of life instruments: guidelines for instrument evaluation. Qual Life Res. 2003;12(4):349–62.
de Vet HC, Bouter LM, Bezemer PD, Beurskens AJ. Reproducibility and responsiveness of evaluative outcome measures. Int J Technol Assess Health Care. 2001;17(04):479–87.
Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–9.
Gerlinger C, Schmelter T. Determining the non-inferiority margin for patient reported outcomes. Pharm Stat. 2011;10(5):410–3. doi:10.1002/pst.507.
McLeod DS, Warner JV, Henman M, Cowley D, Gibbons K, McIntyre HD. Associations of serum vitamin D concentrations with obstetric glucose metabolism in a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study cohort. Diabet Med. 2012;29(8):e199–204. doi:10.1111/j.1464-5491.2011.03551.x.
de Vet HC, Ostelo RW, Terwee CB, van der Roer N, Knol DL, Beckerman H, et al. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131–42.
Lohr KN. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193–205. doi:10.1023/a:1015291021312.
Leidy NK, Revicki DA, Genesté B. Recommendations for evaluating the validity of quality of life claims for labeling and promotion. Value Health. 1999;2(2):113–27.
Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD, Mayo/FDA Patient-Reported Outcomes Consensus Meeting Group. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health. 2007;10(Suppl. 2):S94–105. doi:10.1111/j.1524-4733.2007.00272.x.
Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14(2):285–95.
Salek M, Kamudoni P. Quality of life measurement in dermatology consultation: impact on patient reported outcomes. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2013;148(3):263–75.
Acknowledgments
We gratefully acknowledge the support of the seven experts working with drug regulatory agencies and in the pharmaceutical industry who agreed to be interviewed as part of the research for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation or publication of this article.
Conflict of interest
Professor Salek and Dr. Kamudoni have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salek, S., Kamudoni, P. Streamlining the Validation of Patient Reported Outcome (PRO) Measures in Drug Regulatory Processes. Pharm Med 29, 255–268 (2015). https://doi.org/10.1007/s40290-015-0110-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-015-0110-x