Abstract
Low energy availability, particularly when problematic (i.e., prolonged and/or severe), has numerous negative consequences for health and sports performance as characterized in relative energy deficiency in sport. These consequences may be driven by disturbances in endocrine function, although scientific evidence clearly linking endocrine dysfunction to decreased sports performance and blunted or diminished training adaptations is limited. We describe how low energy availability-induced changes in sex hormones manifest as menstrual dysfunction and accompanying hormonal dysfunction in other endocrine axes that lead to adverse health outcomes, including negative bone health, impaired metabolic activity, undesired outcomes for body composition, altered immune response, problematic cardiovascular outcomes, iron deficiency, as well as impaired endurance performance and force production, all of which ultimately may influence athlete health and performance. Where identifiable menstrual dysfunction indicates hypothalamic-pituitary-ovarian axis dysfunction, concomitant disturbances in other hormonal axes and their impact on the athlete’s health and sports performance must be recognized as well. Given that the margin between podium positions and “losing” in competitive sports can be very small, several important questions regarding low energy availability, endocrinology, and the mechanisms behind impaired training adaptations and sports performance have yet to be explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is insufficient scientific evidence in the sports science literature to directly link endocrine dysfunction (e.g., menstrual dysfunction) to decreased performance and blunted or decreased training adaptations. We can, however, derive the possible mechanistic links between low energy availability-induced hormonal dysfunction and negative health and sports performance outcomes in female athletes from established physiology. |
Monitoring/tracking menstrual bleeding, ovulation (luteinizing hormone surge), and/or peak progesterone during the luteal phase may help to identify menstrual dysfunction associated with low energy availability (e.g., anovulation, luteal phase defect) before more severe menstrual dysfunction (amenorrhea) or marked health or performance decrements occur. |
The endocrine consequences of low energy availability may negatively impact optimal training, recovery, and performance before or after menstrual dysfunction is evident. Concomitant disturbances in other hormonal axes and their impact on an athlete’s health and sports performance must be recognized. |
1 Introduction
Low energy availability (LEA) is a relatively common challenge for physically active and athletic populations [1]. Low energy availability can be problematic and can lead to numerous health and sports performance consequences described in relative energy deficiency in sport (REDs) [2,3,4]. Low energy availability refers to a mismatch between dietary energy intake to cover the energy cost of exercise, resulting in suboptimal energy for other physiological functions in the body, including the maintenance of optimal health and supporting adaptations to training [5]. Low energy availability can be adaptable (i.e., short term and accompanied by benign or even beneficial effects on health and performance), or problematic (i.e., prolonged and/or severe and accompanied by negative consequences for health and performance) [4]. An energy availability (EA) threshold of ~ 30 kcal kg−1 fat-free mass (FFM) day−1, below which disruptions to several hormonal secretory patterns were noted in as few as 4–5 days [6] has been identified in untrained adult women. Presently, a threshold of ~ 45 kcal kg−1 FFM day−1 is suggested for athletes to maintain body mass and support bodily function [7]. Although it is understood that an absolute universal threshold for EA does not exist [7], thresholds can be used to inform both research and practice.
The most studied aspect of REDs to date has been the female athlete triad (Triad) or the interrelationship between problematic LEA, menstrual dysfunction, and poor bone health (low bone mineral density [BMD] and increased risk of bone stress injuries) [8,9,10,11,12,13]. While early research suggested that the hypothalamic-pituitary-ovarian (HPO) axis was primarily responsible for bone decrements, it has become clear that the whole endocrine system, with its numerous feedback loops and various points of physiological interplay, influences athlete health, and ultimately, athlete performance, including the outcomes outlined in REDs [2,3,4]. Although the influences of short-term, medium-term, and long-term LEA on performance have been described in male and female individuals [14], and menstrual dysfunction as a surrogate marker of problematic LEA in female individuals has been linked to performance decrements in REDs [3, 14] (see Table 1), there are only a limited number of studies that actually assess sports performance, or performance changes related to hormonal profiles associated with menstrual dysfunction as summarized in Table 1. Three of these studies are longitudinal [15,16,17], two are cross-sectional [18, 19], and two are case studies [20, 21]. Three of these studies relied on self-reported menstrual status alone [17, 20, 21] while four studies used urinary or blood samples (or their combination) to assess endocrine (menstrual) function [15, 16, 18, 19]. Oligomenorrhea and amenorrhea were most commonly compared to natural/eumenorrheic menstrual cycles while other types of menstrual dysfunction were excluded [19] or not considered/reported. Five studies assessed endurance performance using season best or laboratory testing [15,16,17,18,19], while three studies assessed measures related to strength or power [20, 21], and one study used a published points system [17]. Current research in Table 1 indicates that menstrual dysfunction (e.g., ovarian suppression such as amenorrhea) generally decreases or blunts athletic performance and development whereas natural/eumenorrheic menstrual cycles tend to support performance and athletic development. Regrettably, the relatively limited scope (performance measures) and depth (assessment of mechanisms) of this research hinders our ability to extrapolate results to larger populations and to draw robust conclusions regarding the links between hormone profiles and performance. As such, a gap exists in our understanding regarding the effects of the spectrum and progression of the hormonal profiles characteristic of menstrual dysfunction on sports performance. While most LEA and REDs literature focuses on the components of the Triad, athlete health and performance comprise several other factors, including cardiovascular and ventilatory responses, substrate metabolism, neuromuscular function, nervous system activity, thermoregulation, and psychological factors, all of which are highly pertinent in a sports setting.
The aim of this narrative review is to describe the link between LEA-induced hormonal dysfunction and the various health and sports performance outcomes in female athletes. We focus on describing key evidence-based hormonal pathways responsible for the normal physiological function necessary for sports performance. The review is divided into two parts. Part A: Beyond Menstrual Dysfunction (Sect. 2) illustrates how menstrual dysfunctions (particularly functional hypothalamic amenorrhea [FHA]), per se, are not in themselves the problem for sports performance, but rather that the altered endogenous hormone profiles, characterized by sex hormone deficiencies, contribute to dysfunction in mechanisms that affect both health and ultimately also sports performance. Part B: Beyond Menstrual Dysfunction and Sex Hormones (Sect. 3) describes how the altered endogenous sex hormone profiles associated with menstrual dysfunction are not the only hormonal challenge that arises from problematic LEA and how concurrent dysfunction in other hormonal axes contributes to impairment in mechanisms that affect athlete health and sports performance. Our description of the endocrine consequences of LEA in female athletes is relatively brief, as there are already several excellent reviews on this topic [22,23,24,25,26].
2 Part A: Beyond Menstrual Dysfunction
The HPO axis controls female reproduction via the menstrual cycle [29]. Ideally, gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary, stimulating follicular growth and ovulation, in addition to activating the ovaries to produce estradiol (E2) and inhibin. After ovulation, the follicle remnant becomes the corpus luteum, which is responsible for the production of progesterone (P4). While LH and FSH are important for production of the E2, LH also stimulates thecal cells in the ovaries to secrete testosterone and FSH stimulates granulosa cells in the ovarian follicles to produce aromatase, which then converts thecal cell-produced testosterone into E2. In healthy pre-menopausal women, E2 is the major circulating estrogen, playing a fundamental role in reproduction via the menstrual cycle, as well as in the physiology of the cardiovascular, skeletal, metabolic, and central nervous systems [30,31,32]. Similarly, P4 has several non-reproductive functions related to the cardiovascular system, central nervous system, and bone [33]. For example, P4 influences thermoregulation, ventilation, and metabolism while also having antiestrogenic and androgenic functions [34].
Several other hormones/systems contribute to the regulation of the HPO axis. For example, kisspeptins act via the kisspeptin receptor to stimulate the pulsatile release of GnRH [29]. The kisspeptin system appears to respond to both metabolic status and EA [35]. Kisspeptin activity is decreased by LEA, which, in turn, increases orexigenic factors (ghrelin) and decreases anorexigenic factors (leptin) [36]. This decrease in kisspeptin leads to a downregulation of GnRH thus influencing downstream cascades that affect appetite and feeding behavior [37]. The activin-follistatin-inhibin axis also contributes to regulation of the HPO axis, with activin increasing the synthesis/secretion of FSH and inhibin downregulating it. Inhibin secretion is reduced by GnRH and increased by insulin-like growth factor-1 (IGF-1). Similarly, glucocorticoids, such as cortisol, suppress pituitary gonadotroph responsiveness to hypothalamic input, which may also result in disruptions to the HPO axis [38].
2.1 LEA and Menstrual Dysfunction
The HPO axis requires sufficient energy and nutrients to maintain normal menstrual function [39] or eumenorrhea (i.e., “normal” ovulatory cycles of approximately 21–35 days). Both LEA and stress (emotional and/or physical) may lead to the downregulation of the HPO axis [22, 40] both in the short term [6] and particularly when LEA is problematic or severe (< 10 kcal kg−1 FFM day−1) [41]. Downregulation of the HPO axis is indicated by changes in hormonal profiles, characteristic of menstrual dysfunction, that are recognized as a hallmark of problematic LEA and range in severity from subtle luteal phase defects to anovulation, oligomenorrhea, and secondary amenorrhea (i.e., FHA) [42,43,44,45] (definitions and representative hormonal profiles of menstrual function and dysfunction in Fig. 1). A prolonged follicular phase and luteal phase deficiency characteristic of oligomenorrhea may affect fertility [46], while the occurrence of anovulatory cycles (which can be assessed using a urinary ovulation test [47, 48]) and FHA profoundly impact fertility [49]. Yet menstrual dysfunction is potentially reversible [50] if the root cause is addressed [50]. Regrettably, without regular monitoring/tracking hormones, these changes in hormonal profiles may go unnoticed until attempting pregnancy.

Modified from Allaway et al. [45] with permission
Terminology of menstrual function and dysfunction including representative hormonal profiles. Of note, hormonal profiles of hormonal contraceptive (HC) users (including combined HCs and progestin only) may be different. The solid line represents estradiol (E2), the dashed line represents progesterone (P4), the dotted line represents luteinizing hormone (LH), and the drop symbol represents menstrual bleeding.
A dose–response relationship has been reported between the magnitude (energy deficit of − 470 to − 810 kcal day−1) of LEA and the incidence of menstrual dysfunction in exercising women. However, the severity of menstrual dysfunction appears unrelated to LEA magnitude [51] and there is limited evidence for a specific EA threshold below which menstrual dysfunction is induced [52]. The prevalence of the more severe menstrual dysfunction, such as FHA, is relatively high in elite runners (self-reported = 23/36 of athletes surveyed) [50] and in other endurance athletes (clinically verified = 24/40 of athletes examined) [53]. As FHA is considered a heterogeneous group of disorders that can manifest similarly [54], diagnosis should only be confirmed after other etiologies are excluded [55]. In practice, estimation of ovulation via the LH surge and confirmation of the mid-luteal peak in progesterone indicates normal hormonal function, whereas regular menstrual bleeding alone is not an indicator of eumenorrhea [28].
2.2 Sex Hormones and Health
While the spectrum of menstrual dysfunction (oligo/amenorrhea), as a manifestation of HPO axis dysfunction, is a commonly identified outcome of LEA in women not using hormonal contraceptives (HCs), the non-reproductive actions of suppressed hormones such as E2 and P4 also have the potential to affect health, training responses and adaptations, and ultimately sports performance. Endogenous E2 affects metabolism [32], cardiovascular function [56], bone [57], and muscle [58, 59]. Likewise, endogenous P4 influences thermoregulation, ventilation, and metabolism while having antiestrogenic and androgenic functions [34]. The wide encompassing effects of E2 and P4 are beyond the scope of this review, and we will therefore focus on the effects of E2 and P4 that are most pertinent to sports performance.
2.2.1 Bone
Energy availability and E2 independently and synergistically affect volumetric BMD, bone geometry, and estimates of bone strength [57]. Overall poor bone health is also associated with other LEA-induced hormonal disruptions including decreases in androgens, insulin, IGF-1, triiodothyronine (T3), and leptin in addition to increases in fasting peptide YY (PYY), ghrelin, and cortisol [22, 60, 61]. Athletes and non-athletic women with LEA, as well as athletes with FHA, have lower BMD, impairments of bone microarchitecture, and altered markers of bone remodeling compared with those with adequate EA and eumenorrhea [62,63,64]. Athletes with menstrual dysfunction (oligo/amenorrhea) also have decreased bone strength estimates and higher lifetime fracture rates compared with both eumenorrheic athletes and controls [65, 66]. Women participating in leanness sports have higher rates of menstrual dysfunction, low BMD, and fracture than other sports [67, 68]. Indeed, the prevalence of bone stress injuries is higher in amenorrheic athletes than naturally menstruating athletes [68, 69], whereas even short-term manipulation of EA (15 vs 45 kcal kg−1 FFM day−1) in naturally menstruating women performing daily endurance exercise decreased bone formation and increased bone resorption marker concentrations [70, 71]. In practice, detrimental structural changes in bone resulting from low E2 and accompanying hormonal dysfunction induced by LEA may be undetected for years, but the consequences of low BMD and recurrent bone stress injuries have significant repercussions on both health and ultimately performance (via modified and missed training days). It should be highlighted that the risk for bone stress injuries related to the Triad is found to be higher in teenage athletes than for athletes in their twenties [72]. Furthermore, the accrual of lost BMD when EA is corrected (depending on the timing and duration of LEA) may be difficult, if not impossible [73, 74]. As such, avoidance of LEA and menstrual dysfunction is essential for long-term bone health.
2.2.2 Body Composition
Estrogens are important for the regulation of body weight and body composition. Estrogens influence fat distribution and are associated with lower visceral fat [75]. Endogenous E2 is an anabolic hormone associated with muscle mass and strength in female athletes [59]. Estradiol plays a role in facilitating muscle tissue sensitivity to anabolic stimuli, regulating myofibrillar protein synthesis [59] and skeletal muscle hypertrophy [58, 76]. Endogenous E2 upregulates intracellular signaling pathways that stimulate muscle protein synthesis [77] and may play a role in muscle repair and regeneration [58]. Low energy availability-induced low E2 may affect muscle quality, as E2 is known to protect muscles from damage by acting as an antioxidant or membrane stabilizer or by affecting gene regulation [58] while having antiapoptotic effects [78]. Indeed, estrogen receptors are found in several tissues and organs of the body and are known to modulate cell proliferation, differentiation, and survival. Estrogens also exhibit neuroprotective capabilities by promoting DNA repair, stimulating growth factor expression, and modulating blood flow, whereas E2-dependent signaling pathways are involved in neurogenic processes [79]. Ultimately, ineffective tissue repair and regeneration may impair training adaptations and athletes with low E2 may be more susceptible to muscle damage (i.e., extended recovery times). Generally, lean body composition and low body weight are associated with performance in endurance sports. Lower body fat is associated with better endurance performance while gains in muscle mass are generally associated with increases in performance across sports [80]. A decrease in body mass due to LEA may increase maximal aerobic capacity relative to body mass (maximum oxygen uptake in mL kg−1 min−1), even in the absence of changes in absolute aerobic capacity (maximum oxygen uptake in mL min−1); however, the benefits are likely to be transient when prolonged LEA and menstrual dysfunction are present. Indeed, lower body weight and fat mass in elite amenorrheic endurance athletes do not appear to result in improved aerobic capacity compared with eumenorrheic athletes [19].
2.2.3 Cardiovascular System
Systemic vascular circulation is an important component of health and performance. In a healthy blood vessel, E2 is a potent vasodilator via nitric oxide production; it also mediates inflammation and oxidative stress [81]. Short-term perturbations in E2 might influence blood flow via disturbed endothelial function and low E2 associated with menstrual dysfunction has been linked to lower blood pressure and heart rate response [56]. Perturbations in circulation may impair the transport of oxygen and energy substrates, including glucose and fatty acids, to skeletal muscle, while clearance of metabolic waste may also be affected. Physically active women with low E2 demonstrate lower heart rate and blood pressure response to an orthostatic challenge in which plasma renin, angiotensin II, and aldosterone fail to increase, resulting in a sympathetic vasoconstrictor response to compensate for blood pressure changes [56].
Importantly, LEA may cause endothelial dysfunction independently of low E2 [82, 83] and extreme LEA can lead to cardiac arrythmias [84]. The effects of P4 on the cardiovascular system have received less attention, although there is evidence that P4 lowers blood pressure, inhibits coronary hyperactivity, and has powerful vasodilatory and natriuretic effects [85]. Vascular dysfunction caused, in part, by reduced E2 may be accompanied by impaired/blunted nitric oxide production; early signs of cardiovascular dysfunction have been identified in young amenorrheic athletes including an unfavorable lipid profile: higher total cholesterol and low-density lipoprotein cholesterol [86]. Likewise, reduced endothelium-dependent vasodilation [83], increased vascular tone, lower shear rate, as well as impaired endothelial and/or vascular smooth muscle cell responsiveness to nitric oxide have been reported in female athletes with LEA-induced amenorrhea [82, 86,87,88,89]. Taken together, low E2-induced and P4-induced changes in the circulation and cardiovascular function may, in theory, influence training responses and quality, as well as subsequent adaptations and/or performance.
Despite the unfavorable lipid profile that may present in athletes with LEA, it is important to remember that cholesterol is essential for the metabolism of steroid hormones. Cholesterol is, for example, metabolized to pregnenolone, which is then further metabolized into sex steroids E2 and P4 [90]. As such, it is possible that the observed high cholesterol associated with menstrual dysfunction is a compensatory mechanism for decreased E2 and P4 in LEA or that the metabolism of cholesterol into steroid hormones is disturbed by LEA.
2.3 Summary Part A
Menstrual dysfunction is not in itself a problem for sports performance, but the altered endogenous hormone profiles, characterized by sex hormone deficiencies, contribute to dysfunction in mechanisms that affect health, training quality, and sports performance.
3 Part B: Beyond Menstrual Dysfunction and Sex Hormones
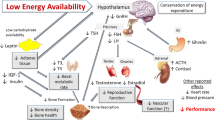
Beyond the HPO axis, several other hormonal axes are affected by LEA. Together, the hypothalamus and pituitary gland control downstream processes related to an athlete’s health and sports performance, including autonomic, endocrine, and somatic responses and adaptations. For example, the hypothalamic–pituitary–adrenal (HPA) axis regulates responses to stress and plays a critical role in energy metabolism, particularly in relation to food intake, energy storage, and energy mobilization [22]. As a catabolic and glucoregulatory hormone, downstream cortisol is secreted in response to physical stress and other challenges to body homeostasis [91]. In turn, the hypothalamic–pituitary–thyroid axis controls metabolic hormones that play a key role in regulating musculoskeletal health and function [92], while several other hormones, including leptin, ghrelin, insulin, and PYY regulate EI via appetite regulation and/or behavioral food intake. Some of these hormones have additional functions, for instance regulating gastric motility, water and electrolyte absorption, and immunological responses [93,94,95].
Hormones such as growth hormone (GH), IGF-1 and its binding proteins, insulin, and testosterone are important for anabolic processes and are major determinants of body composition [96]. Insulin-like growth factor-1 plays a direct role in whole-body glucose homeostasis, influences muscle hypertrophy [97], and is positively associated with muscular endurance and aerobic fitness [98]. Growth hormone modulates insulin sensitivity, glucose homeostasis, and metabolic response to calorie restriction. Importantly, the GH-IGF axis also influences immunity and inflammation [99]. Insulin acts as an anabolic/anticatabolic hormone, mitigating muscle protein breakdown [100, 101] with similar actions by GH, which primarily acts via its actions on IGF-1 [100]. More specifically, IGF-1 is involved in managing muscle protein synthesis, hypertrophy, and inhibition of muscle protein breakdown [102]. Testosterone is produced in female individuals by the ovary, adrenal glands, and peripheral tissues via conversion of androstenedione and dehydroepiandrosterone (pre-androgens synthesized by the ovaries and adrenal glands) to testosterone. Testosterone has both direct and indirect (via aromatization to E2) functions related to vasomotor tone, endothelial function, peripheral vascular resistance, cognition, and musculoskeletal health [103].
3.1 LEA and Endocrine Dysfunction
Short-term LEA has been shown to elevate blood cortisol in a non-linear pattern in naturally menstruating women. A decrease in EA from 45 to 30 or 20 kcal kg−1 FFM day−1 was associated with a small increase in blood cortisol, whereas a more notable increase (~ 150%) was observed at an EA of 10 kcal kg−1 FFM day−1 [6]. However, significant changes in blood cortisol levels were not observed in bodybuilding fitness athletes after a 4-month fat-loss diet combined with a high training volume [104]. In elite female endurance athletes with varying levels of EA, cortisol levels were highest in women reporting menstrual dysfunction compared with their regularly menstruating counterparts [53], which is consistent with previous research [105, 106].
Laboratory-based interventions and cross-sectional investigations have reported decreases in T3, leptin, insulin, and IGF-1, as well as increased growth hormone (GH) and adiponectin due to LEA [22, 23]. Short-term investigations in healthy sedentary women have shown decreased 24-h mean levels of insulin and leptin with decreasing EA (from an adequate EA of 45 kcal kg−1 FFM day−1). In fact, when EA decreased from 45 to 30 kcal kg−1 FFM day−1, there was a 35% decrease in leptin with a further decrease (~ 70%) at an EA of 10 kcal kg−1 FFM day−1. Decreases in fasting levels of IGF-1 and T3 occurred at a threshold of ~ 20–25 kcal kg−1 FFM day−1 [6]. More recently, short-term LEA (15 kcal kg−1 FFM day−1) decreased fasting levels of insulin and leptin in eumenorrheic female individuals when compared with adequate EA [71]. Similarly, a short-term diet or exercise-induced LEA reduced fasting levels of IGF-1, leptin, and T3 [107]. Cross-sectional investigations comparing metabolic hormone profiles between amenorrheic and eumenorrheic female individuals confirm these findings, showing both lower levels of T3 [16, 19, 69] and leptin [108, 109] in athletes with menstrual dysfunction. Similarly, ghrelin levels were higher after 12 weeks of reduced EA that resulted in a minimum of 1.5 kg of weight loss, whereas no change in the anorexigenic PYY was observed [110]. Nevertheless, ghrelin and PYY have been found to be higher in amenorrheic versus eumenorrheic athletes [61, 111].
Research on the relationship between LEA/amenorrhea on androgens in female individuals has so far yielded equivocal results with reports of both decreased [105, 112] and increased [113, 114] levels of androgens. For example, lower levels of testosterone have been reported in amenorrhea and oligomenorrhea resulting from LEA and chronic energy deficit states [105]. Similarly, oligomenorrheic and amenorrheic athletes, in comparison to eumenorrheic athletes, had lower testosterone and dehydroepiandrosterone (DHEA) sulfate levels, as well as higher sex hormone binding globulin (SHBG) levels [112]. Higher levels of testosterone in dancers with menstrual dysfunction (and low daily energy and carbohydrate intake) have been reported (in those without characteristics of hyperandrogenism/polycystic ovary syndrome) [113]. Likewise, endurance athletes with oligomenorrhea or amenorrhea were reported to have higher serum levels of both free and total testosterone as well as androstenedione, which was accompanied by lower SHBG levels when compared with eumenorrheic endurance athletes and non-athletes [114]. Levels of SHBG may help explain differences in androgen availability as SHBG has a high affinity and specificity for binding sex hormones where serum levels are regulated by androgens, estrogens, thyroid hormones, as well as other metabolic factors including EA and physical activity [115]. Sex hormone binding globulin binds to E2, dihydrotestosterone, and testosterone, rendering these hormones biologically inactive. However, higher levels of testosterone in dancers [113] and endurance athletes with menstrual dysfunction [114] could be explained by HPO axis suppression of FSH release, which inhibits aromatase production, potentially resulting in low E2 and high testosterone. Higher testosterone could function as a compensatory mechanism, as testosterone is aromatized to E2, but could also be the result of elevated adrenal activity [116] or due to a decrease in adipose tissue [117]. While the precise mechanisms behind these observations are unclear, other causes of hyperandrogenism (e.g., adrenal hyperplasia, polycystic ovary syndrome) should be considered, as the current LEA and sports science literature does not consistently screen for and exclude other causes of hyperandrogenism. While the effects of LEA on androgens and androgen precursors in women are under-studied and results are inconsistent [118], LEA-induced perturbations in androgen levels in female individuals may influence, among other things, musculoskeletal health [103].
3.2 Other Hormones and Health
A spectrum of downstream hormones are affected by LEA, leading to disturbances in normal physiological and physical function that manifest as metabolic and immunological challenges. These are addressed in the following sub-sections.
3.2.1 Metabolism and Management of Body Composition
In conditions of problematic LEA, the resting metabolic rate (RMR) is reported to decrease, thus affecting the management of body composition in athletes. Indeed, several studies suggest links between problematic LEA, suppressed metabolic hormones, and suppression of RMR. The body has several regulatory systems for mitigating weight loss [119, 120]. For example, leptin acts on the RMR indirectly by suppressing T3 and the activity of the sympathetic nervous system. In addition, decreases in the RMR due to energy restriction may be a result of suppressed catecholamine and thyroid hormone levels [121]. Indeed, reduced RMR has been linked to lower T3 and leptin levels [122, 123], while neither body mass nor FFM appears to explain differences in RMR [123, 124]. Although a difference in body mass or relative fat mass is not consistently observed between amenorrheic and eumenorrheic female individuals, the former appear to have lower levels of T3 [69] and lower RMR [53, 125].
In conditions of LEA, exercise energy expenditure (EEE) tends to decrease [124], contributing to a reduction in total daily energy expenditure, which may affect weight management. The endocrine changes resulting from LEA also appear to affect muscle efficiency and EEE. For example, a 10% loss of body mass led to a 20% increase in skeletal muscle work efficiency during a bicycle ergometer test with light workloads (10, 25, and 50 W), accounting for ~ 75% of the decline in EEE [126]. Similarly, Tornberg and colleagues [19] reported a lower RMR, as well as lower EEE during cycling, concurrent with lower levels of T3 levels in amenorrheic versus eumenorrheic female athletes.
Prolonged concomitant reductions in RMR and EEE are likely to translate into an inability, or extreme difficulty, to maintain or lose body mass, thereby challenging the management of body composition in the athlete. Hormones are also major regulators of muscle protein turnover [77, 100], which has additional implications for the management of body composition, as well as the strength and power capabilities of an athlete. Leanness/thinness may be associated with some forms of menstrual dysfunction [19, 53, 86, 109], but athletes in some sports perceive theoretical benefit from a lean body composition with lower levels of adiposity and higher levels of muscle mass [127, 128]. Lower fat percentage may not actually be beneficial, as has been reported in cross-sectional [129] and longitudinal [130] studies. Most reports characterizing body composition in amenorrheic and eumenorrheic female individuals indicate lower body mass and fat mass in the former group [19, 53, 86, 109]. Whether this is an outcome of LEA, but eventually leads to issues including overcompensation (storage of extra energy as adipose tissue) to sudden increases in EA following a prolonged and/or severe period of LEA [119], remains to be elucidated. Importantly, the hormonal changes associated with long-term LEA are not favorable for maintaining healthy body composition.
Anabolic responses to exercise may be blunted in conditions of LEA [131]. This is supported by findings of GH resistance along with higher levels of cortisol in women with anorexia nervosa [132]. Importantly, nutritional status appears to outweigh the effects of cortisol on GH levels [132]. Nevertheless, glucocorticoids directly inhibit IGF-1 induction of the molecular pathways that stimulate muscle protein synthesis while IGF-1 appears to at least partially reverse glucocorticoid-induced muscle protein breakdown [133]. Areta and colleagues reported a 27% reduction in resting muscle protein synthesis after only 5 days of EA of 30 kcal kg−1 FFM day−1 in both female and male individuals [134]. Low glycogen, a likely consequence of LEA, has been demonstrated to negatively affect cellular growth and adaptation in response to resistance exercise independently of hormonal responses [135], while exercise nutrient interactions influence cascades that affect protein regulatory systems during both exercise and recovery where energy is also needed to fuel cellular pathways [136]. Athletes experiencing short-term LEA might be less prone to muscle catabolism seen in long-term or the most severe forms of LEA, but it is expected that optimal rates of muscle protein synthesis will suffer [137], thus blunting responses and adaptations to resistance training that would otherwise result in muscle hypertrophy. Resistance exercise and amino acid ingestion are crucial to stimulate anabolism, but physiological stress, including LEA, attenuates these effects [138]. Concomitant with nutrient (amino acid and carbohydrate) deficiency, muscle protein synthesis, and muscle remodeling may also be affected by increased catabolic cortisol and decreased levels of anabolic hormones (GH, IGF-1, E2) [139]. Ultimately, impaired muscle protein synthesis and subsequent effects on lean mass may have dramatic implications for sports performance.
3.2.2 Immune Function
The consequences of LEA-induced endocrine dysfunction may predispose athletes to illness [140, 141] as well as injury [69, 142], with endocrine dysfunction affecting the time course of return to play. Indeed, illness and inflammation influence an athlete’s potential to train and compete, while also affecting recovery and healing. Sports that combine exercise training with LEA to modify weight and body composition appear to influence immune function [104, 141,142,143,144,145,146]. Importantly, LEA during recovery from illness/injury may further complicate or delay healing/immune processes whereas the nutritional component of healing is often overlooked [147]. Injury/illness alters an athlete’s nutritional requirements [148], where negative energy balance is known to impair wound healing [149] and increase muscle loss [150] due to down-regulation of muscle protein synthesis and associated intracellular signaling proteins, even during a moderate decrease in EA [134]. It is possible that LEA-induced alterations in hormones concurrent with LEA-induced nutritional deficiencies increase athletes’ susceptibility to illness/infection and injury, while predisposing them to injury cycles due to suboptimal healing. Although several mechanisms related to LEA may reduce the activation and efficacy of the immune system, the significance of a single factor, such as EA, remains unknown.
Many aspects of exercise-induced modifications in immune function may be mediated by increased levels of immunoregulatory hormones such as cortisol [151] while actions of immune cells are also known to be modulated by E2 [152]. Antiviral mechanisms may be modified in women with hormonal profiles associated with menstrual dysfunction. The literature indicates that E2 activates monocytes, macrophages, and neutrophils, which induce the production of proinflammatory cytokines [152]. Similarly, E2 and P4 have been shown to blunt the interleukin-10 response [153], which is associated with more infections in athletes [154]. Finally, E2 promotes hydration of mucous membranes, which could influence the local immune response [155]. The protective mechanism of E2 could be mediated by the increase in the production of nasal mucus that contains immunoglobulin A, an immunoglobin negatively associated with the incidence of respiratory infections in athletes [154]. In a related way, irritative urinary symptoms (including urinary tract infections) may be problematic and indicative of such events in female athletes [156].
3.2.3 Gut Health
LEA can lead to gastrointestinal distress in athletes [157]. Gut health in the context of LEA-induced hormonal dysfunction has not been extensively addressed in the literature, although gut health and function are of great importance to athletes. The gut plays an essential role in the digestion and absorption of nutrients, while also providing a barrier between the external environment and circulation (immune function). Digestion can be impaired during LEA, with symptoms such as constipation, diarrhea, and slowed gastric emptying [158]. In extreme LEA, (e.g., in patients with anorexia nervosa) gut microbiota diversity and richness are reduced, which has been suggested to be linked to compromised bone health [159]. There appears to be a bidirectional relationship between the gut microbiota and sex hormones, although research in athletic female populations is sparse [160]. Nevertheless, E2 is known to strengthen and protect the mucosal and epithelial barriers in the gastrointestinal tract while modulating both intestinal inflammation and immune response [161]. The gastrointestinal tract microbiome contributes to immune function, regulates systemic inflammation, and appears to affect higher cognitive functions [162]. In this sense, gut microbiota may regulate to some degree oxidative stress, inflammatory responses, metabolism, and energy expenditure during intense exercise [163]. While additional research is required, cross-sectional studies have reported associations between physical performance and gut microbiota status [164].
3.2.4 Iron
It has been demonstrated that LEA interacts with iron deficiency [165], where prolonged LEA, concomitant with an inadequate iron intake, can have negative effects on iron stores and eventually hemoglobin, both of which can subsequently affect sports performance [166]. Iron deficiency interacts with LEA to perturb thyroid function and reduce metabolic fuel availability [167]. In addition, iron deficiency affects reproductive function and bone metabolism [168] while several iron-dependent enzymes influence metabolic and immune responses [165]. Hepcidin response appears to be inversely related to EA, while an adequate EA might attenuate the inflammatory response to strenuous exercise [169]. Furthermore, decreased endogenous E2 is associated with higher levels of hepcidin [170].
3.3 Summary Part B
Menstrual dysfunction alone is not a problem for sports performance, but the underlying altered endocrine function, characterized by sex hormone deficiencies and overall endocrine dysfunction, contributes to impairments in mechanisms that affect health and sports performance.
4 LEA-Induced Endocrine Dysfunction Leads to Performance Decrements
While it is reasonable to infer that endocrine dysfunction caused by LEA interferes with training adaptations and performance measures, the research clearly linking endocrine dysfunction to blunted training adaptations or performance decrements is limited and relies, in large part, on self-reported menstrual status and a limited number of performance measures (Table 1). Nevertheless, it is imperative to understand that the impact of hormonal perturbations associated with LEA can be both vast and profound, affecting the homeostasis of various body systems that affect health, as well as training responses/adaptations and sports performance. Healthy training days are essential for long-term athlete development and ultimately sports performance. Therefore, it is important to recognize that hormonal dysfunction (concomitant with nutritional deficiencies/LEA) can affect training quality before menstrual dysfunction is identified. Importantly, adequate EA, energy stores, macronutrient availability, and intermediate metabolites are critical for maintaining quality training sessions with appropriate training volume, intensity, and recovery [171]. Thus, athletes may experience a reduction in training quality or recovery even after short-term LEA [172], although more severe consequences are likely to be experienced after long-term LEA.
At present, relatively little is known about the effects of LEA on maximal aerobic capacity and anaerobic thresholds, although performance decrements and impaired development in endurance performance have been observed [15, 16], which may be related to impaired metabolism and cardiovascular function. One possible mechanism to explain impaired exercise metabolism during LEA is decreased E2 [173]. Lower E2 levels are associated with lower levels of glycogen sparing and fat oxidation [174], while higher levels of E2 promote the availability and metabolism of free fatty acids as well as glucose availability and uptake into type I (oxidative) muscle fibers, although this may be attenuated by higher levels of P4. Exercising at higher intensities and producing force may be hindered during LEA because of reduced muscle glycogen [175] while endurance capacity may be impaired by a decreased ability to utilize fat. Sufficient muscle glycogen stores are necessary for exercise performance, and replenishing these stores is critical for recovery and sustained training [176, 177] where insulin facilitates the transport of glucose into muscle cells (at rest), a process that may be inhibited via decreased levels of E2 [178]. On a cellular level, mitochondrial biogenesis and function (metabolism and morphology) are also known to be influenced by E2 [179] while regulation of mitochondrial dynamics allows the cell to respond and adapt to cellular energy demands [180]. Thyroid hormones also stimulate mitochondrial biogenesis, energy metabolism, and energy transfer while influencing recovery. Mitochondria are essential for the generation of ATP via oxidative phosphorylation in response to energy depletion via AMP-activated protein kinase [181]. Exercise/training should enhance muscle metabolism, but reduced levels of E2 [28] and T3 resulting from LEA [182] could, in theory, blunt or block expected mitochondrial responses [183] and adaptations [19]. Mitochondrial oxidative functions and protein translation appear to be suppressed by LEA and appear to recover after refeeding [184].
Another mechanism affecting endurance performance could be impaired hematopoiesis, as evidenced for example, by lower erythrocyte and platelet counts, and increased white blood cell count in dieting fitness athletes [143]. Prolonged energy restriction and intense exercise training can also reduce iron stores, which, as discussed earlier, are important for oxygen delivery and transport, energy metabolism, cognition, and immune function [165, 185]. It is important to understand that the hormonal changes associated with LEA may be responsible for reduced blood flow related to impaired endothelial function, decreased fat oxidation related to mitochondrial dysfunction, and decreased hematopoiesis associated with impaired iron metabolism or decreased iron stores. We suggest that the synergistic effects of low E2, low T3, and low glycogen may impact mitochondrial remodeling processes, impairing aerobic metabolism and adaptations to endurance training in the longer term with impaired ATP production affecting force production in addition to the cellular repair required for recovery. The literature is currently lacking in studies addressing performance directly, but we postulate that the aforementioned hormonal perturbations and resulting health and functional challenges directly affect training quality, recovery, and performance.
LEA and nutritional deficiency appear to be strongly associated with impairments in muscle protein synthesis [131, 134, 186] and impaired neuromuscular function [19], which may result in blunted/decreased development in force production capabilities. Decreased blood glucose levels and hormonal disruptions in amenorrheic athletes have been associated with lower strength and lean mass of the lower extremities compared with eumenorrheic athletes [19]. A decrease in glycogen due to LEA may be problematic for muscle contraction (myosin cross-bridge interaction) owing to an impaired release of calcium from the sarcoplasmic reticulum [187]. Likewise, amenorrhea may affect metabolism during exercise recovery, possibly impairing the ability of amenorrheic athletes to, for example, optimally complete repeated bouts of exercise [183]. We might also hypothesize that LEA-induced low E2 has a negative influence on force production via central mechanisms [188]. Neuromuscular function and fatigability of the knee extensors change across the menstrual cycle, with greater intracortical inhibition and fatigue during the luteal phase and greater voluntary contraction when the E2 level is elevated [189]. Estradiol is known to alter neuronal excitability and may affect force production capacity via neurotransmitter receptors (direct) and ion channel-activated (indirect) mechanisms. An excitatory neuronal effect is associated with E2, whereas an inhibitory effect is associated with P4 [190]. Thus, in theory, a decrease in E2 related to LEA could reduce cortical excitability due to decreased action on sodium channels that results in attenuated recruitment of excitatory interneurons [191], which may also influence motor control and recruitment of motor units, although this has not been investigated in females with menstrual dysfunction versus those with eumenorrhea. Nevertheless, reduced neuromuscular function due to LEA and LEA-induced hormonal changes may impair mechanical efficiency, which could also increase the individual’s perception of loading. Importantly, even short-term and adaptable LEA may not be without consequences for recovery [172] and longer term adaptations. Differentiating between desired decreases (planned overreaching) and LEA-induced decreases in performance and recovery during training blocks may be difficult but important for long-term athlete development.
5 Summary and Limitations
In female individuals, sex hormones are not only responsible for reproduction, but also play important roles in bone, muscle, and cardiovascular health and function. Menstrual dysfunction secondary to LEA is characterized by changes in hormonal profiles with the combined direct and indirect effects of E2 and P4 on an athlete’s ability to train and recover optimally [4, 14, 22]. Although menstrual dysfunction is indicative of suppression of sex hormones, the concomitant disturbances in other hormonal axes and their impact on athlete health and sports performance must be recognized. Indeed, the hormonal consequences of LEA appear to be controlled, in large part, by the hypothalamus, which connects the nervous system to the endocrine system via the pituitary gland. Low energy availability-induced changes in the levels of several pituitary hormones appear to have unfavorable downstream effects on structural characteristics (muscle protein turnover, adiposity, bone density), energetics (resting and exercise metabolism, mitochondrial function), and adaptation (strength, power, and endurance capacity) of the skeletal muscle and adipose tissue, with direct and indirect negative effects on sports performance (Fig. 2).
Summary of Part A: beyond menstrual dysfunction and Part B: beyond menstrual dysfunction and sex hormones. The endocrine system includes various points for physiological crosstalk and hormones often have pleiotropic effects (see reference [192]). Created with www.biorender.com. ACTH adrenocorticotropic hormone, CRH corticotropin-releasing hormone, E2 estradiol, FSH follicle-stimulating hormone, GH growth hormone, GHRH growth hormone-releasing hormone, IGF-1 insulin-like growth factor 1, LH luteinizing hormone, P4 progesterone, PYY peptide YY, T testosterone, T3 triiodothyronine, TRH thyrotropin-releasing hormone, TSH thyroid-stimulating hormone, ↑ increased, ↓ decreased
While it is understood that problematic LEA disrupts menstrual function, the evidence for dysfunction in other endocrine axes appears to be more scattered. In many cases, information regarding problematic LEA is drawn from studies including amenorrheic athletes or patients with anorexia nervosa. The endocrine system, in general, is regulated by several feedback loops that include various points for physiological crosstalk in which hormones often have pleiotropic effects. Given that hormones play a significant role in maintaining normal physiological function and supporting homeostasis of tissues and processes essential for health, it is reasonable to hypothesize, in line with REDs, that hormonal changes resulting from LEA may negatively affect training responses, adaptations, and performance. Because the temporal relationship between hormonal changes and physiological effects is variable, it is important to recognize that hormonal changes may induce physiological effects that are not always immediate, or even in the same time frame as the physiological responses or adaptations.
Generally, research examining the effects of LEA on health has been laboratory based with a “prescribed” EA that may not translate directly to the field or practical “free-living” situations [20, 69]. In addition, the long-term effects of LEA on performance, training adaptations, and recovery have often been investigated using a cross-sectional approach comparing athletes who already have menstrual dysfunction with naturally menstruating or eumenorrheic athletes. Much of this existing research also relies on self-reporting of menstrual status (i.e., “naturally menstruating” female individuals without hormonal level verification [28]). Finally, female individuals using HCs are not immune to the effects of LEA, although possible endocrine and or performance consequences specific to HC users have not yet been elucidated.
Although several ethical issues may prevent researchers from conducting long-term laboratory-based (especially long-term and severe) LEA studies in athletes, and it would be unethical not to intervene in free-living conditions if an athlete exhibits symptoms or behaviors indicating LEA, a schedule of regular hormonal and physical testing for groups of athletes could allow researchers to elucidate the time course of possible hormonal and performance changes occurring in athletes. Athletes should have access to a network of specialists when faced with REDs [193].
6 Key Findings and Practical Applications
It is worth noting that the etiology behind menstrual dysfunction is not always LEA, but that menstrual dysfunction is indicative of marked hormonal changes that should be assessed by a physician. Additionally, menstrual dysfunction does not immediately translate into performance decrements, although the changes in hormonal profiles may ultimately be profound and detrimental to the health and performance of the athlete. Identification of REDs and hormonal dysfunction should be based on a comprehensive medical evaluation of symptoms (involving a multidisciplinary team), hormone testing, and exclusion of other medical problems.
6.1 Key Findings and Practical Applications
The scientific evidence clearly linking endocrine dysfunction to decreased performance and blunted or decreased training adaptations is limited. We have described how LEA-induced changes in sex hormones that often manifest as menstrual dysfunction and concomitant hormonal dysfunction in other axes could result in several undesirable health outcomes including negative bone health, impaired metabolic activity, undesired outcomes for body composition, altered immune response and gut health, problematic cardiovascular outcomes, and iron deficiency that both directly and indirectly affect training and performance. While it is possible that short-term LEA will not markedly affect performance, it is important to investigate LEA-induced outcomes and their mechanisms in order to better understand the performance decrements associated with the Triad/REDs. As such, we suggest that mechanisms described in this article are influenced by altered endocrine function secondary to LEA and that these impair health and sports performance in female athletes. Based on the totality of the evidence, we suggest that researchers and practitioners:
-
Explore the mechanisms by which endocrine dysfunction, including menstrual dysfunction, affects athlete performance, including the time course of performance decrements and changes in hormonal profiles.
-
Recognize that present cross-sectional studies generally use only FHA as an indicator of prolonged LEA, although menstrual dysfunction such as oligomenorrhea or recurrent anovulation may indicate LEA.
-
Consider the depth and breadth of LEA and the subsequent effects on hormonal homeostasis in free-living conditions (and consider the current literature) [194].
-
Acknowledge that the negative effects of LEA are likely to begin before identifiable menstrual dysfunction, such as FHA. Perturbations in E2 and P4 occur even in less severe forms of menstrual dysfunction while other hormonal axes are also affected. This highlights the importance of going beyond monitoring menstrual bleeding alone and including methods to determine the more subtle menstrual dysfunction, such as monitoring ovulation and/or the P4 peak in the luteal phase [28].
-
Understand that gynecological age may influence responses to LEA. Older female individuals and female individuals with greater gynecological age, i.e., years since onset of menarche, may be more adaptable to LEA than younger female individuals or female individuals of younger gynecological age [41].
-
Monitor markers of menstrual function in female individuals not using HCs (including hormonal intrauterine devices). This may include menstrual bleeding along with an LH surge associated with ovulation (using an ovulation test [47, 48]), P4 peak in the luteal phase, and/or other frequent hormonal sampling [28].
-
Consider the effects of LEA on HC users compared to non-users, as exogenous sex steroids may influence HPO axis function independently of other hormonal axes. Avoid including HC users in “mixed groups” with naturally menstruating/eumenorrheic or amenorrheic participants, as this may affect the interpretation of subsequent results.
-
Monitor HC using athletes by assessing nutritional status proactively.
-
Consider assessing surrogate markers of LEA, including but not limited to T3, testosterone, cortisol, IGF-1, and insulin, in addition to sex hormones, as well as lipid profiles, iron, gut health, and immune function in athletes with LEA or at risk of LEA.
7 Conclusions
Suppression of sex hormones secondary to problematic LEA often manifests as menstrual dysfunction; however, concomitant hormonal dysfunction occurs in other endocrine axes. Taken together, this LEA-induced hormonal dysfunction underpins adverse mechanisms and outcomes that ultimately affect athlete health and impair training quality, thus likely negatively affecting performance. The influence of LEA-induced altered endocrine function on mechanisms of athlete health and components of sports performance requires further research.
References
Logue D, Madigan SM, Delahunt E, Heinen M, Mc Donnell SJ, Corish CA. Low energy availability in athletes: a review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 2018. https://doi.org/10.1007/s40279-017-0790-3.
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, et al. The IOC consensus statement: beyond the female athlete triad-relative energy deficiency in sport (RED-S). Br J Sports Med. 2014. https://doi.org/10.1136/bjsports-2014-093502.
Mountjoy M, Sundgot-Borgen J, Burke L, Ackerman KE, Blauwet C, Constantini N, et al. International Olympic Committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1136/bjsports-2018-099193.
Mountjoy M, Ackerman KE, Bailey DM, Burke LM, Constantini N, Hackney AC, et al. International Olympic Committee’s (IOC) consensus statement on relative energy deficiency in sport (REDs). Br J Sports Med. 2023. https://doi.org/10.1136/bjsports-2023-106994.
Loucks AB, Kiens B, Wright HH. Energy availability in athletes. J Sports Sci. 2011. https://doi.org/10.1080/02640414.2011.588958.
Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003. https://doi.org/10.1210/jc.2002-020369.
Burke LM, Lundy B, Fahrenholtz IL, Melin AK. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2018-0142.
Yeager KK, Agostini R, Nattiv A, Drinkwater B. The female athlete triad: disordered eating, amenorrhea, osteoporosis. Med Sci Sports Exerc. 1993. https://doi.org/10.1249/00005768-199307000-00003.
Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. ACSM position stand: the female athlete triad. Med Sci Sports Exerc. 1997. https://doi.org/10.1097/00005768-199705000-00037.
Drinkwater BL, Nilson K, Chesnut CH, Bremner WJ, Shainholtz S, Southworth MB. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984. https://doi.org/10.1056/NEJM198408023110501.
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. The female athlete triad. Med Sci Sports Exerc. 2007. https://doi.org/10.1249/mss.0b013e318149f111.
De Souza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update. 2004. https://doi.org/10.1093/humupd/dmh033.
De Souza MJ, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, et al. 2014 Female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014. https://doi.org/10.1136/bjsports-2013-093218.
Melin AK, Areta JL, Heikura IA, Stellingwerff T, Torstveit MK, Hackney AC. Direct and indirect impact of low energy availability on sports performance. Scand J Med Sci Sports. 2023. https://doi.org/10.1111/sms.14327.
Schaal K, VanLoan MD, Hausswirth C, Casazza GA. Decreased energy availability during training overload is associated with non-functional overreaching and suppressed ovarian function in female runners. Appl Physiol Nutr Metab. 2021. https://doi.org/10.1139/apnm-2020-0880.
Vanheest JL, Rodgers CD, Mahoney CE, De Souza MJ. Ovarian suppression impairs sport performance in junior elite female swimmers. Med Sci Sports Exerc. 2014. https://doi.org/10.1249/MSS.0b013e3182a32b72.
Ihalainen JK, Kettunen O, McGawley K, Solli GS, Hackney AC, Mero AA, et al. Body composition, energy availability, training, and menstrual status in female runners. Int J Sports Physiol Perform. 2021. https://doi.org/10.1123/ijspp.2020-0276.
De Souza MJ, Maguire MS, Rubin KR, Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc. 1990. https://doi.org/10.1249/00005768-199010000-00006.
Tornberg ÅB, Melin A, Koivula FM, Johansson A, Skouby S, Faber J, et al. Reduced neuromuscular performance in amenorrheic elite endurance athletes. Med Sci Sports Exerc. 2017. https://doi.org/10.1249/MSS.0000000000001383.
Areta JL. Case study: resumption of eumenorrhea in parallel with high training load after 4 years of menstrual dysfunction: a 5-year follow-up of an elite female cyclist. Int J Sport Nutr Exerc Metab. 2020. https://doi.org/10.1123/ijsnem.2019-0284.
Tinsley GM, Trexler ET, Smith-Ryan AE, Paoli A, Graybeal AJ, Campbell BI, et al. Changes in body composition and neuromuscular performance through preparation, 2 competitions, and a recovery period in an experienced female physique athlete. J Strength Cond Res. 2019. https://doi.org/10.1519/JSC.0000000000002758.
Elliott-Sale KJ, Tenforde AS, Parziale AL, Holtzman B, Ackerman KE. Endocrine effects of relative energy deficiency in sport. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2018-0127.
Dipla K, Kraemer RR, Constantini NW, Hackney AC. Relative energy deficiency in sports (RED-S): elucidation of endocrine changes affecting the health of males and females. Hormones. 2020. https://doi.org/10.1007/s42000-020-00214-w.
Areta JL, Taylor HL, Koehler K. Low energy availability: history, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur J Appl Physiol. 2020. https://doi.org/10.1007/s00421-020-04516-0.
Ackerman KE, Misra M. Amenorrhoea in adolescent female athletes. Lancet Child Adolesc Health. 2018. https://doi.org/10.1016/S2352-4642(18)30145-7.
McCall LM, Ackerman KE. Endocrine and metabolic repercussions of relative energy deficiency in sport. Curr Opin Endocr Metab Res. 2019. https://doi.org/10.1016/j.coemr.2019.07.005.
McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022. https://doi.org/10.1123/ijspp.2021-045.
Elliott-Sale KJ, Minahan CL, de Jonge XAKJKJ, Ackerman KE, Sipilä S, Constantini NW, et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021. https://doi.org/10.1007/s40279-021-01435-8.
Hu KL, Zhao H, Chang HM, Yu Y, Qiao J. Kisspeptin/kisspeptin receptor system in the ovary. Front Endocrinol. 2018. https://doi.org/10.3389/fendo.2017.00365.
Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010. https://doi.org/10.1016/j.physbeh.2009.06.010.
Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013. https://doi.org/10.1210/er.2012-1055.
Oosthuyse T, Strauss JA, Hackney AC. Understanding the female athlete: molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur J Appl Physiol. 2022. https://doi.org/10.1007/s00421-022-05090-3.
Taraborrelli S. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand. 2015. https://doi.org/10.1111/aogs.12771.
Constantini NW, Dubnov G, Lebrun CM. The menstrual cycle and sport performance. Clin Sports Med. 2005. https://doi.org/10.1016/j.csm.2005.01.003.
Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006. https://doi.org/10.1016/j.mce.2006.04.026.
Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005. https://doi.org/10.1210/en.2005-0337.
Iwasa T, Matsuzaki T, Yano K, Mayila Y, Yanagihara R, Yamamoto Y, et al. Effects of low energy availability on reproductive functions and their underlying neuroendocrine mechanisms. J Clin Med. 2018. https://doi.org/10.3390/jcm7070166.
Sakakura M, Takebe K, Nakagawa S. Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab. 1975. https://doi.org/10.1210/jcem-40-5-774.
Veldhuis JD, Evans WS, Demers LM, Thorner MO, Wakat D, Rogol AD. Altered neuroendocrine regulation of gonadotropin secretion in women distance runners. J Clin Endocrinol Metab. 1985. https://doi.org/10.1210/jcem-61-3-557.
Hakimi O, Cameron L-C. Effect of exercise on ovulation: a systematic review. Sports Med. 2017. https://doi.org/10.1007/s40279-016-0669-8.
Loucks AB. The response of luteinizing hormone pulsatility to 5 days of low energy availability disappears by 14 years of gynecological age. J Clin Endocrinol Metab. 2006. https://doi.org/10.1210/jc.2006-0570.
De Souza MJ, Toombs RJ, Scheid JL, O’Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2010. https://doi.org/10.1093/humrep/dep411.
Dale E, Gerlach DH, Wilhitte AL. Menstrual dysfunction in distance runners. Obstet Gynecol. 1979. https://doi.org/10.1097/00006250-197907000-00013.
Sonntag B, Ludwig M. An integrated view on the luteal phase: diagnosis and treatment in subfertility. Clin Endocrinol. 2012. https://doi.org/10.1111/j.1365-2265.2012.04464.x.
Allaway HCM, Southmayd EA, De SMJ. The physiology of functional hypothalamic amenorrhea associated with energy deficiency in exercising women and in women with anorexia nervosa. Horm Mol Biol Clin Investig. 2016. https://doi.org/10.1515/hmbci-2015-0053.
Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017. https://doi.org/10.1210/jc.2017-00131.
Schaumberg MA, Jenkins DG, Janse de Jonge XAK, Emmerton LM, Skinner TL. Three-step method for menstrual and oral contraceptive cycle verification. J Sci Med Sport. 2017. https://doi.org/10.1016/j.jsams.2016.08.013.
Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstet Gynecol. 1996. https://doi.org/10.1016/0029-7844(95)00352-5.
Juul A, Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Frederiksen H, et al. Endocrine evaluation of reproductive function in girls during infancy, childhood and adolescence. Endocr Dev. 2012. https://doi.org/10.1159/000326625.
Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, et al. Bone-mineral density and other features of the female athlete triad in elite endurance runners: a longitudinal and cross-sectional observational study. Int J Sport Nutr Exerc Metab. 2010. https://doi.org/10.1123/ijsnem.20.5.418.
Williams NI, Leidy HJ, Hill BR, Lieberman JL, Legro RS, De SMJ. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am J Physiol Endocrinol Metab. 2015. https://doi.org/10.1152/ajpendo.00386.2013/.
Lieberman JL, De Souza MJ, Wagstaff DA, Williams NI. Menstrual disruption with exercise is not linked to an energy availability threshold. Med Sci Sports Exerc. 2018. https://doi.org/10.1249/MSS.0000000000001451.
Melin A, Tornberg ÅB, Skouby S, Møller SS, Sundgot-Borgen J, Faber J, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports. 2015. https://doi.org/10.1111/sms.12261.
Rebar R. Evaluation of amenorrhea, anovulation, and abnormal bleeding. Endotext. MDText.com, Inc.; 2018.
Gordon CM. Functional hypothalamic amenorrhea. N Engl J Med. 2010. https://doi.org/10.1056/NEJMcp0912024.
O’Donnell E, Goodman JM, Morris BL, Floras JS, Harvey PJ. Augmented vagal heart rate modulation in active hypoestrogenic pre-menopausal women with functional hypothalamic amenorrhoea. Clin Sci. 2015. https://doi.org/10.1042/CS20150209.
Southmayd EA, Mallinson RJ, Williams NI, Mallinson DJ, De Souza MJ. Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Osteoporos Int. 2017. https://doi.org/10.1007/s00198-016-3887-x.
Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010. https://doi.org/10.2165/11319760-000000000-00000.
Chidi-Ogbolu N, Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2019. https://doi.org/10.3389/fphys.2018.01834.
Ackerman K, Misra M. Bone health and the female athlete triad in adolescent athletes. Phys Sportsmed. 2011. https://doi.org/10.3810/psm.2011.02.1871.
Ackerman KE, Slusarz K, Guereca G, Pierce L, Slattery M, Mendes N, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab. 2012. https://doi.org/10.1152/ajpendo.00598.2011.
Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014. https://doi.org/10.1016/S2213-8587(13)70180-3.
Papageorgiou M, Dolan E, Elliott-Sale KJ, Sale C. Reduced energy availability: implications for bone health in physically active populations. Eur J Nutr. 2018. https://doi.org/10.1007/s00394-017-1498-8.
Ackerman KE, Misra M. Neuroendocrine abnormalities in female athletes: the female athlete triad. Handb Clin Neurol. 2015. https://doi.org/10.1016/B978-0-444-59602-4.00025-3.
Ackerman KE, Sokoloff NC, De Nardo MG, Clarke HM, Lee H, Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015. https://doi.org/10.1249/MSS.0000000000000574.
Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012. https://doi.org/10.1016/j.bone.2012.07.019.
Hutson MJ, O’Donnell E, Brooke-Wavell K, Sale C, Blagrove RC. Effects of low energy availability on bone health in endurance athletes and high-impact exercise as a potential countermeasure: a narrative review. Sports Med. 2020. https://doi.org/10.1007/s40279-020-01396-4.
Tenforde AS, Carlson JL, Chang A, Sainani KL, Shultz R, Kim JH, et al. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med. 2017. https://doi.org/10.1177/0363546516676262.
Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2017-0313.
Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004. https://doi.org/10.1359/JBMR.040410.
Papageorgiou M, Elliott-Sale KJ, Parsons A, Tang JCY, Greeves JP, Fraser WD, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017. https://doi.org/10.1016/j.bone.2017.08.019.
Nose-Ogura S, Yoshino O, Dohi M, Kigawa M, Harada M, Hiraike O, et al. Risk factors of stress fractures due to the female athlete triad: differences in teens and twenties. Scand J Med Sci Sports. 2019. https://doi.org/10.1111/sms.13464.
Barrack MT, Van Loan MD, Rauh MJ, Nichols JF. Body mass, training, menses, and bone in adolescent runners: a 3-yr follow-up. Med Sci Sports Exerc. 2011. https://doi.org/10.1249/MSS.0b013e318201d7bb.
Barrack MT, Van Loan MD, Rauh M, Nichols JF. Disordered eating, development of menstrual irregularity, and reduced bone mass change after a 3-year follow-up in female adolescent endurance runners. Int J Sport Nutr Exerc Metab. 2021. https://doi.org/10.1123/ijsnem.2021-0011.
Puder JJ, Monaco SE, Sen Gupta S, Wang J, Ferin M, Warren MP. Estrogen and exercise may be related to body fat distribution and leptin in young women. Fertil Steril. 2006. https://doi.org/10.1016/j.fertnstert.2006.02.085.
Hansen M. Female hormones: do they influence muscle and tendon protein metabolism? Proc Nutr Soc. 2018. https://doi.org/10.1017/S0029665117001951.
Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17β-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. Am J Physiol Cell Physiol. 2009. https://doi.org/10.1152/ajpcell001882009.
Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17β-Estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids. 2008. https://doi.org/10.1016/j.steroids.2007.12.027.
Bustamante-Barrientos FA, Méndez-Ruette M, Ortloff A, Luz-Crawford P, Rivera FJ, Figueroa CD, et al. The impact of estrogen and estrogen-like molecules in Neurogenesis and neurodegeneration: bneficial or harmful? Front Cell Neurosci. 2021. https://doi.org/10.3389/fncel.2021.636176.
Mathisen TF, Ackland T, Burke LM, Constantini N, Haudum J, Macnaughton LS, et al. Best practice recommendations for body composition considerations in sport to reduce health and performance risks: a critical review, original survey and expert opinion by a subgroup of the IOC consensus on Relative Energy Deficiency in Sport (REDs). Br J Sports Med. 2023. https://doi.org/10.1136/bjsports-2023-106812.
Ramesh SS, Christopher R, Indira Devi B, Bhat DI. The vascular protective role of oestradiol: a focus on postmenopausal oestradiol deficiency and aneurysmal subarachnoid haemorrhage. Biol Rev Camb Philos Soc. 2019. https://doi.org/10.1111/brv.12541.
Hoch AZ, Papanek P, Szabo A, Widlansky ME, Schimke JE, Gutterman DD. Association between the female athlete triad and endothelial dysfunction in dancers. Clin J Sport Med. 2011. https://doi.org/10.1097/JSM.0b013e3182042a9a.
Hoch AZ, Dempsey RL, Carrera GF, Wilson CR, Chen EH, Barnabei VM, et al. Is there an association between athletic amenorrhea and endothelial cell dysfunction? Med Sci Sports Exerc. 2003. https://doi.org/10.1249/01.MSS.0000053661.27992.75.
Spaulding-Barclay MA, Stern J, Mehler PS. Cardiac changes in anorexia nervosa. Cardiol Young. 2016. https://doi.org/10.1017/S104795111500267X.
Thomas P, Pang Y. Protective actions of progesterone in the cardiovascular system: potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids. 2013. https://doi.org/10.1016/j.steroids.2013.01.003.
Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab. 2005. https://doi.org/10.1210/jc.2004-1286.
O’Donnell E, De Souza MJ. The cardiovascular effects of chronic hypoestrogenism in amenorrhoeic athletes: a critical review. Sports Med. 2004. https://doi.org/10.2165/00007256-200434090-00004.
Hoch AZ, Lynch SL, Jurva JW, Schimke JE, Gutterman DD. Folic acid supplementation improves vascular function in amenorrheic runners. Clin J Sport Med. 2010. https://doi.org/10.1097/JSM.0b013e3181df59f4.
O’Donnell E, Goodman JM, Mak S, Harvey PJ. Impaired vascular function in physically active premenopausal women with functional hypothalamic amenorrhea is associated with low shear stress and increased vascular tone. J Clin Endocrinol Metab. 2014. https://doi.org/10.1210/jc.2013-3398.
Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab. 2010. https://doi.org/10.1186/1743-7075-7-47.
Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones. 2005;4(2):73–89.
Loucks AB, Laughlin GA, Mortola JF, Girton L, Nelson JC, Yen SSC. Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab. 1992. https://doi.org/10.1210/jcem.75.2.1639953.
Wisse BE, Campfield LA, Marliss EB, Morais JA, Tenenbaum R, Gougeon R. Effect of prolonged moderate and severe energy restriction and refeeding on plasma leptin concentrations in obese women. Am J Clin Nutr. 1999. https://doi.org/10.1093/ajcn/70.3.321.
Scheid JL, De Souza MJ. Menstrual irregularities and energy deficiency in physically active women: the role of ghrelin, PYY and adipocytokines. Med Sport Sci. 2010. https://doi.org/10.1159/000321974.
Graybeal AJ, Willis JL, Morales-Marroquin E, Tinsley GM, Messiah SE, Shah M. Emerging evidence of the relationship between fat-free mass and ghrelin, glucagon-like peptide-1, and peptide-YY. Nutrition. 2022. https://doi.org/10.1016/j.nut.2022.111815.
Birzniece V. Exercise and the growth hormone-insulin-like growth factor axis. Curr Opin Endocr Metab Res. 2019. https://doi.org/10.1016/j.coemr.2019.04.006.
Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996. https://doi.org/10.1210/edrv-17-5-481.
Nindl BC, Pierce JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc. 2010. https://doi.org/10.1249/MSS.0b013e3181b07c4d.
Heemskerk VH, Daemen MARC, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999. https://doi.org/10.1016/s1359-6101(98)00022-7.
Rooyackers OE, Nair KS. Hormonal regulation of human muscle protein metabolism. Annu Rev Nutr. 1997. https://doi.org/10.1146/annurev.nutr.17.1.457.
Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016. https://doi.org/10.1007/s00125-015-3751-0.
Svensson J, Lönn L, Johannsson G, Bengtsson BÅ. Effects of GH and insulin-like growth factor-I on body composition. J Endocrinol Investig. 2003. https://doi.org/10.1007/BF03345231.
Davis SR, Wahlin-Jacobsen S. Testosterone in women: the clinical significance. Lancet Diabetes Endocrinol. 2015. https://doi.org/10.1016/S2213-8587(15)00284-3.
Hulmi JJ, Isola V, Suonpää M, Järvinen NJ, Kokkonen M, Wennerström A, et al. The effects of intensive weight reduction on body composition and serum hormones in female fitness competitors. Front Physiol. 2017. https://doi.org/10.3389/fphys.2016.00689.
Rickenlund A, Thorén M, Carlström K, von Schoultz B, Hirschberg AL. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J Clin Endocrinol Metab. 2004. https://doi.org/10.1210/jc.2003-030306.
Ackerman KE, Patel KT, Guereca G, Pierce L, Herzog DB, Misra M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin Endocrinol. 2013. https://doi.org/10.1111/j.1365-2265.2012.04458.x.
Papageorgiou M, Martin D, Colgan H, Cooper S, Greeves JP, Tang JCY, et al. Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone. 2018. https://doi.org/10.1016/j.bone.2018.06.016.
Christo K, Cord J, Mendes N, Miller KK, Goldstein MA, Klibanski A, et al. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol. 2008. https://doi.org/10.1111/j.1365-2265.2008.03237.x.
Thong FSLL, McLean C, Graham TE. Plasma leptin in female athletes: relationship with body fat, reproductive, nutritional, and endocrine factors. J Appl Physiol. 2000. https://doi.org/10.1152/jappl.2000.88.6.2037.
Scheid JL, De Souza MJ, Leidy HJ, Williams NI. Ghrelin but not peptide YY is related to change in body weight and energy availability. Med Sci Sports Exerc. 2011. https://doi.org/10.1249/MSS.0b013e31821e52ab.
Russell M, Stark J, Nayak S, Miller KK, Herzog DB, Klibanski A, et al. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone. 2009. https://doi.org/10.1016/j.bone.2009.03.668.
Bermon S, Garnier PY, Hirschberg AL, Robinson N, Giraud S, Nicoli R, et al. Serum androgen levels in elite female athletes. J Clin Endocrinol Metab. 2014. https://doi.org/10.1210/jc.2014-1391.
Łagowska K, Kapczuk K. Testosterone concentrations in female athletes and ballet dancers with menstrual disorders. Eur J Sport Sci. 2016. https://doi.org/10.1080/17461391.2015.1034786.
Rickenlund A, Carlström K, Ekblom BB, Brismar TB, von Schoultz BB, Hirschberg AL, et al. Hyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performance. Fertil Steril. 2003. https://doi.org/10.1016/s0015-0282(02)04850-1.
Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin: from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015. https://doi.org/10.1016/j.beem.2015.06.005.
Pasquali R, Pignatelli D. Eds. Hyperandrogenism in women: beyond polycystic ovary syndrome. Friburg: Frontiers of Hormone Research; 2019
Boutari C, Pappas PD, Mintziori G, Nigdelis MP, Athanasiadis L, Goulis DG, et al. The effect of underweight on female and male reproduction. Metabolism. 2020. https://doi.org/10.1016/j.metabol.2020.154229.
Enea C, Boisseau N, Fargeas-Gluck MA, Diaz V, Dugué B. Circulating androgens in women exercise-induced changes. Sports Med. 2011. https://doi.org/10.2165/11536920-000000000-00000.
Trexler ET, Smith-Ryan AE, Norton LE. Metabolic adaptation to weight loss: Implications for the athlete. J Int Soc Sports Nutr. 2014. https://doi.org/10.1186/1550-2783-11-7.
Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes. 2008. https://doi.org/10.1038/ijo.2008.246.
Jung RT, Shetty PS, James WP. The effect of refeeding after semistarvation on catecholamine and thyroid metabolism. Int J Obes. 1980;4(2):95–100.
Kaufman BA, Warren MP, Dominguez JE, Wang J, Heymsfield SB, Pierson RN. Bone density and amenorrhea in ballet dancers are related to a decreased resting metabolic rate and lower leptin levels. J Clin Endocrinol Metab. 2002. https://doi.org/10.1210/jcem.87.6.8565.
Koehler K, Williams NI, Mallinson RJ, Southmayd EA, Allaway HCM, De Souza MJ. Low resting metabolic rate in exercise-associated amenorrhea is not due to a reduced proportion of highly active metabolic tissue compartments. Am J Physiol Endocrinol Metab. 2016. https://doi.org/10.1152/ajpendo.00110.2016.
Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci. 2010;14(4):146–54.
Myerson M, Gutin B, Warren MP, May MT, Contento I, Lee M, et al. Resting metabolic rate and energy balance in amenorrheic and eumenorrheic runners. Med Sci Sports Exerc. 1991;23(1):15–22.
Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003. https://doi.org/10.1152/ajpregu.00474.2002.
Stellingwerff T. Case study: body composition periodization in an Olympic-level female middle-distance runner over a 9-year career. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2017-0312.
Aikawa Y, Murata M, Omi N. Relationship of height, body mass, muscle mass, fat mass, and the percentage of fat with athletic performance in male Japanese college sprinters, distance athletes, jumpers, throwers, and decathletes. J Phys Fit Sports Med. 2020. https://doi.org/10.7600/jpfsm.9.7.
Knechtle B, Wirth A, Baumann B, Knechtle P, Rosemann T. Personal best time, percent body fat, and training are differently associated with race time for male and female ironman triathletes. Res Q Exerc Sport. 2010. https://doi.org/10.1080/02701367.2010.10599628.
Burke L, Whitfield J, Ross M, Tee M, Sharma AP, King AJ, et al. Short severe energy restriction with refueling reduces body mass without altering training-associated performance improvement. Med Sci Sports Exerc. 2023. https://doi.org/10.1249/MSS.0000000000003169.
Oxfeldt M, Phillips SM, Andersen OE, Johansen FT, Bangshaab M, Risikesan J, et al. Low energy availability reduces myofibrillar and sarcoplasmic muscle protein synthesis in trained females. J Physiol. 2023. https://doi.org/10.1113/JP284967.
Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003. https://doi.org/10.1210/jc.2003-030532.
Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997. https://doi.org/10.1016/s1359-6101(96)00053-6.
Areta JL, Burke LM, Camera DM, West DWD, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014. https://doi.org/10.1152/ajpendo.00590.2013.
Creer A, Gallagher P, Slivka D, Jemiolo B, Fink W, Trappe S. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol. 2005. https://doi.org/10.1152/japplphysiol.00110.2005.
Smiles W, Hawley JA, Camera DM. Effects of skeletal muscle energy availability on protein turnover responses to exercise. J Exp Biol. 2016. https://doi.org/10.1242/jeb.125104.
Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015. https://doi.org/10.1111/acel.12342.
Church DD, Gwin JA, Wolfe RR, Pasiakos SM, Ferrando AA. Mitigation of muscle loss in stressed physiology: military relevance. Nutrients. 2019. https://doi.org/10.3390/nu11081703.
Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab. 2004. https://doi.org/10.1016/j.tem.2004.02.009.
Drew M, Vlahovich N, Hughes D, Appaneal R, Burke LM, Lundy B, et al. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 summer Olympic games. Br J Sports Med. 2018. https://doi.org/10.1136/bjsports-2017-098208.
Drew MK, Vlahovich N, Hughes D, Appaneal R, Peterson K, Burke L, et al. A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J Sci Med Sport. 2017. https://doi.org/10.1016/j.jsams.2017.02.010.
Raysmith BP, Drew MK. Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: a 5-year prospective study. J Sci Med Sport. 2016. https://doi.org/10.1016/j.jsams.2015.12.515.
Sarin HV, Gudelj I, Honkanen J, Ihalainen JK, Vuorela A, Lee JH, et al. Molecular pathways mediating immunosuppression in response to prolonged intensive physical training, low-energy availability, and intensive weight loss. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.00907.
Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004. https://doi.org/10.1080/0264041031000140590.
Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol. 2017. https://doi.org/10.1152/japplphysiol.00622.2016.
Tritto ACC, Amano MT, De Cillo ME, Oliveira VA, Mendes SH, Yoshioka C, et al. Effect of rapid weight loss and glutamine supplementation on immunosuppression of combat athletes: a double-blind, placebo-controlled study. J Exerc Rehabil. 2018. https://doi.org/10.12965/jer.1835154.577.
Kloubec J, Harris C. Whole foods nutrition for enhanced injury prevention and healing. ACSMs Health Fit J. 2016. https://doi.org/10.1249/FIT.0000000000000189.
Tipton KD. Nutritional support for exercise-induced injuries. Sports Med. 2015;45:93.
Frankenfield D. Energy expenditure and protein requirements after traumatic injury. Nutr Clin Pract. 2006. https://doi.org/10.1177/0115426506021005430.
Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010. https://doi.org/10.1249/MSS.0b013e3181b2ef8e.
Walsh NP. Recommendations to maintain immune health in athletes. Eur J Sport Sci. 2018. https://doi.org/10.1080/17461391.2018.1449895.
Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015. https://doi.org/10.1016/j.cellimm.2015.01.018.
Bommer I, Muzzio DO, Zygmunt M, Jensen F. Progesterone and estradiol exert an inhibitory effect on the production of anti-inflammatory cytokine IL-10 by activated MZ B cells. J Reprod Immunol. 2016. https://doi.org/10.1016/j.jri.2016.05.008.
Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad AS. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012. https://doi.org/10.1111/j.1600-0838.2010.01272.x.
Haeggström A, Östberg B, Stjerna P, Graf P, Hallén H. Nasal mucosal swelling and reactivity during a menstrual cycle. J Otorhinolaryngol Relat Spec. 2000. https://doi.org/10.1159/000027713.
Boos K, Hextall A, Cardozo L, Toozs-Hobson P, Anders K, Treasure J. Lower urinary tract symptoms and their impact on women with anorexia nervosa. Br J Obstet Gynaecol. 1999. https://doi.org/10.1111/j.1471-0528.1999.tb08306.x.
Rankin A, O’Donavon C, Madigan SM, O’Sullivan O, Cotter PD. “Microbes in sport”: the potential role of the gut microbiota in athlete health and performance. Br J Sports Med. 2017. https://doi.org/10.1136/bjsports-2016-097227.
Hughes RL, Holscher HD. Fueling gut microbes: a review of the interaction between diet, exercise, and the gut microbiota in athletes. Adv Nutr. 2021. https://doi.org/10.1093/advances/nmab077.
Aurigemma NC, Koltun KJ, VanEvery H, Rogers CJ, de Souza MJ. Linking the gut microbiota to bone health in anorexia nervosa. Curr Osteoporos Rep. 2018. https://doi.org/10.1007/s11914-018-0420-5.
Pugh JN, Lydon KM, O’Donovan CM, O’Sullivan O, Madigan SM. More than a gut feeling: what is the role of the gastrointestinal tract in female athlete health? Eur J Sport Sci. 2022. https://doi.org/10.1080/17461391.2021.
Nie X, Xie R, Tuo B. Effects of estrogen on the gastrointestinal tract. Dig Dis Sci. 2018. https://doi.org/10.1007/s10620-018-4939-1.
Eisenstein M. Microbiome: bacterial broadband. Nature. 2016. https://doi.org/10.1038/533S104a.
Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017. https://doi.org/10.1016/j.jshs.2016.05.001.
Lensu S, Pekkala S. Gut microbiota, microbial metabolites and human physical performance. Metabolites. 2021. https://doi.org/10.3390/metabo11110716.
McKay AKA, Pyne DB, Burke LM, Peeling P. Iron metabolism: interactions with energy and carbohydrate availability. Nutrients. 2020. https://doi.org/10.3390/nu12123692.
Garza D, Shrier I, Kohl HW, Ford P, Brown M, Matheson GO. The clinical value of serum ferritin tests in endurance athletes. Clin J Sport Med. 1997. https://doi.org/10.1097/00042752-199701000-00009.
Soliman AT, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic anemia and thyroid function. Acta Biomed. 2017. https://doi.org/10.23750/abm.v88i1.6048.
Petkus DL, Murray-Kolb LE, De Souza MJ. The unexplored crossroads of the female athlete triad and iron deficiency: a narrative review. Sports Med. 2017. https://doi.org/10.1007/s40279-017-0706-2.
Pasiakos SM, Margolis LM, Murphy NE, McClung HL, Martini S, Gundersen Y, et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol Rep. 2016. https://doi.org/10.14814/phy2.12820.
Lehtihet M, Bonde Y, Beckman L, Berinder K, Hoybye C, Rudling M, et al. Circulating hepcidin-25 is reduced by endogenous estrogen in humans. PLoS One. 2016. https://doi.org/10.1371/journal.pone.0148802.
Stellingwerff T, Maughan RJ, Burke LM. Nutrition for power sports: middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. J Sports Sci. 2011. https://doi.org/10.1080/02640414.2011.589469.
Kettunen O, Ihalainen JK, Ohtonen O, Valtonen M, Mursu J, Linnamo V. Energy availability during training camp is associated with signs of overreaching and changes in performance in young female cross-country skiers. Biomed Hum Kinet. 2021. https://doi.org/10.2478/bhk-2021-0030.
Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010. https://doi.org/10.2165/11317090-000000000-00000.
Hackney AC. Influence of oestrogen on muscle glycogen utilization during exercise. Acta Physiol Scand. 1999. https://doi.org/10.1046/j.1365-201x.1999.00605.x.
Oxfeldt M, Marsi D, Christensen PM, Andersen OE, Johansen FT, Bangshaab M, et al. Low energy availability followed by optimal energy availability does not benefit performance in trained females. Med Sci Sports Exerc. 2023. https://doi.org/10.1249/MSS.0000000000003370.
Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine joint position statement: nutrition and athletic performance. Med Sci Sports Exerc. 2016. https://doi.org/10.1249/MSS.0000000000000852.
Burke LM, van Loon LJC, Hawley JA. Postexercise muscle glycogen resynthesis in humans. J Appl Physiol. 2017. https://doi.org/10.1152/japplphysiol.00860.2016.
Barros RPA, Gustafsson JÅ. Estrogen receptors and the metabolic network. Cell Metab. 2011. https://doi.org/10.1016/j.cmet.2011.08.005.
Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020. https://doi.org/10.1016/j.redox.2020.101435.
Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017. https://doi.org/10.1111/febs.13820.
Wang C, Youle R. Form follows function for mitochondria. Nature. 2016. https://doi.org/10.1038/530288a.
Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol Regul Integr Comp Physiol. 1994. https://doi.org/10.1152/ajpregu.1994.266.3.R817.
Harber VJ, Petersen SR, Chilibeck PD. Thyroid hormone concentrations and muscle metabolism in amenorrheic and eumenorrheic athletes. Can J Appl Physiol. 2011. https://doi.org/10.1139/h98-017.
Sarin HV, Pirinen E, Pietiläinen KH, Isola V, Häkkinen K, Perola M, et al. Mitochondrial bioenergetic pathways in blood leukocyte transcriptome decrease after intensive weight loss but are rescued following weight regain in female physique athletes. FASEB J. 2021. https://doi.org/10.1096/fj.202002029R.
Sim M, Garvican-Lewis LA, Cox GR, Govus A, McKay AKA, Stellingwerff T, et al. Iron considerations for the athlete: a narrative review. Eur J Appl Physiol. 2019. https://doi.org/10.1007/s00421-019-04157-y.
Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC, et al. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr. 2010. https://doi.org/10.3945/jn.109.118372.
Ørtenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013. https://doi.org/10.1113/jphysiol.2013.251629.
Piirainen JM, Nevanperä S, Tenan MS. Sex hormone effects on the nervous system and their impact on muscle strength and motor performance in Women. In: Hackney AC, editor. Sex hormones, exercise and women. 2nd ed. Cham: Springer International Publishing; 2023. p. 135–49.
Ansdell P, Brownstein CG, Skarabot J, Hicks KM, Simoes DCM, Thomas K, et al. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol. 2019. https://doi.org/10.1152/japplphysiol.01041.2018.
Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002. https://doi.org/10.1002/ana.10180.
Inghilleri M, Conte A, Currà A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability: an rTMS study in humans. Clin Neurophysiol. 2004. https://doi.org/10.1016/j.clinph.2003.12.003.
Hackney AC, Lane AR. Exercise and the regulation of endocrine hormones. Prog Mol Biol Transl Sci. 2015. https://doi.org/10.1016/bs.pmbts.2015.07.001.
Ackerman KE, Stellingwerff T, Elliott-Sale KJ, Baltzell A, Cain M, Goucher K, et al. #REDS (relative energy deficiency in sport): time for a revolution in sports culture and systems to improve athlete health and performance. Br J Sports Med. 2020. https://doi.org/10.1136/bjsports-2019-101926.
Heikura IA, Stellingwerff T, Areta JL. Low energy availability in female athletes: from the lab to the field. Eur J Sport Sci. 2021. https://doi.org/10.1080/17461391.2021.1915391.
Acknowledgements
The authors acknowledge the expert contributions of Prof. Kirsty Elliott-Sale in the preparation of this article.
Funding
Open Access funding provided by University of Jyväskylä (JYU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Johanna K. Ihalainen, Ritva S. Mikkonen, Kathryn E. Ackerman, Ida A. Heikura, Katja Mjøsund, Maarit Valtonen, and Anthony C. Hackney have no conflicts of interest that are relevant to the content of this review.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
MV and JKI conceived the idea for this review. All authors contributed to the first draft of the manuscript. JKI and RSM revised the original manuscript and all authors contributed to subsequent revisions and approved the final version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ihalainen, J.K., Mikkonen, R.S., Ackerman, K.E. et al. Beyond Menstrual Dysfunction: Does Altered Endocrine Function Caused by Problematic Low Energy Availability Impair Health and Sports Performance in Female Athletes?. Sports Med (2024). https://doi.org/10.1007/s40279-024-02065-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40279-024-02065-6