Abstract
Background
A child’s health condition affects family members’ health and well-being. However, pediatric cost-utility analysis (CUA) commonly ignores these family spillover effects leading to an incomplete understanding of the cost and benefits of a child’s health intervention. Methodological challenges exist in assessing, valuing, and incorporating family spillover effects.
Objective
This study systematically reviews and compare methods used to include family spillover effects in pediatric CUAs.
Methods
A literature search was conducted in MEDLINE, Embase, EconLit, Cochrane collection, CINAHL, INAHTA, and the Pediatric Economic Database Evaluation (PEDE) database from inception to 2020 to identify pediatric CUAs that included family spillover effects. The search was updated to 2021 using PEDE. The data describing in which family members spillover effects were measured, and how family spillover effects were measured, incorporated, and reported, were extracted. Common approaches were grouped conceptually. Further, this review identified theories or theoretical frameworks used to justify approaches for integrating family spillover effects into CUA.
Results
Of 878 pediatric CUAs identified, 35 included family spillover effects. Most pediatric CUAs considered family spillover effects on one family member. Pediatric CUAs reported eight different approaches to measure the family spillover effects. The most common method was measuring the quality-adjusted life years (QALY) loss of the caregiver(s) or parent(s) due to a child’s illness or disability using an isolated approach whereby family spillover effects were quantified in individual family members separately from other health effects. Studies used four approaches to integrate family spillover effects into CUA. The most common method was to sum children’s and parents/caregivers’ QALYs. Only two studies used a theoretical framework for incorporation of family spillover effects.
Conclusions
Few pediatric CUAs included family spillover effects and the observed variation indicated no consensus among researchers on how family spillover effects should be measured and incorporated. This heterogeneity is mirrored by a lack of practical guidelines by Health Technology Assessment (HTA) agencies or a theoretical foundation for including family spillover effects in pediatric CUA. The results from this review may encourage researchers to develop a theoretical framework and HTA agencies to develop guidelines for including family spillover effects. Such guidance may lead to more rigorous and standardized methods for including family spillover effects and better–quality evidence to inform decision-makers on the cost-effectiveness of pediatric health interventions.
Similar content being viewed by others
Few pediatric CUAs include family spillover effects; failure to include the positive and/or negative effects of a child’s illness on family members in pediatric CUAs results in an incomplete understanding of the cost and consequences of a child’s health intervention. |
The inconsistency in methods used to incorporate family spillover effects makes the results of CUAs less comparable and may result in biased findings and inequitable policy making. |
Future researchers should strive to develop standardized approaches to measure, assess, value, and incorporate family spillover effects in pediatric CUA. |
1 Introduction
In publicly funded and/or private payer healthcare systems, cost-utility analysis (CUA) is increasingly used in the decision-making process affecting funding, reimbursement, and pricing of a new health intervention. Health Technology Assessment (HTA) agencies such as the Canadian Agency for Drugs and Technologies (CADTH) [1] and the National and Care Excellence (NICE) [2] are responsible for providing information on the efficiency of drugs and medical devices to domestic healthcare decision-makers. These agencies recommend that the health effects of an intervention be captured using quality-adjusted life years (QALYs) with a primary focus on outcomes in individual patients. Global HTA agencies hold diverse views on incorporating effects on individuals with other patients in CUAs [1, 3,4,5,6]. Spillover effects are commonly used to refer to those health and well-being effects of a patient’s illness and treatment on family members and caregivers [7,8,9]. For instance, the NICE’s economic evaluation guidelines state that evaluation should consider all health effects for patients and, when relevant, caregivers in the reference case [i.e., healthcare perspective, the National Health Service and personal social services (NHS PSS)] [3]. According to the CADTH guidelines, spillovers (either costs or effects) for caregivers should be included in the reference case (public healthcare perspective) if the intervention’s target population is patients and caregivers; otherwise, it should be addressed in a nonreference case analysis (societal perspective) [1]. The US Second Panel on Cost-Effectiveness in Health and Medicine (the US Second Panel on CEA) recommends the inclusion of the productivity cost of caregivers in the reference case analysis [6].
Pediatric illness includes a range of conditions, including congenital disorders, infectious diseases, and developmental disorders that impacts infants, children, and adolescents. Children with chronic illnesses or disabilities often require comprehensive caregiving for daily activities such as feeding, bathing, dressing, and attending frequent medical checkups or treatments [10,11,12,13,14,15]. Families play an important role in providing essential services for children with illnesses or disabilities. Having and caring for a child with an illness or disability places significant financial, health, and well-being burdens on family members [16,17,18,19,20,21,22,23,24,25,26]. Family members impacted can include parents, unaffected siblings, grandparents, and other familial relatives, regardless of their caregiver role. Table 1 defines terms used in this paper to describe the impacts of a child’s illness or disability on family members.
The financial impacts on family members due to a child’s illness or disability are family spillover costs [7, 27, 28]. For example, these costs include productivity losses associated with unemployment or reduced work hours, both for paid or unpaid labor, as well as out-of-pocket expenses incurred by family members for medical or nonmedical services related to the child’s health or family member’s health as a result of the child’s health condition. Additionally, household expenditures related to the child’s illness or disability are considered as family spillover costs.
Family spillover effects, on the other hand, refer to the health and nonhealth effects experienced by family members as a result of a child’s illness or disability [7, 9, 27]. The definition of family spillover effects is broad, encompassing various aspects such as psychological health, emotional well-being, quality of life, overall well-being (including happiness and life satisfaction), and bereavement. Family members, especially caregiving parents, may experience positive family spillover effects through feeling appreciated by the child being cared for and by other family members [29,30,31].
Family spillover effects arise from two distinct sources: the family effect and the caregiving effect. Family effects (“caring about others”) arise from the direct impact of a patient’s health (a child) on the health and well-being of other family members. These effects result when family members witness the suffering or declining health of their loved one, i.e., the child [7, 9, 27]. An example of family effects is the impact of a child’s serious illness on their parents’ mental health. In addition, caregiving effects (caring for others) occur in caregivers who provide care for a child who is ill or has a disability [7, 27]. Engaging in physically and emotionally demanding caregiving over extended periods can result in psychological distress, depression, anxiety, and other mental health issues for caregivers [10, 32,33,34]. In families with an ill or disabled child, caregivers are often family members such as the father, mother, and older siblings. Thus, parents and older siblings may experience both types of effects [7, 27, 28, 35].
Family effects can manifest independently from caregiving effects, particularly in the context of child illness, with a larger number of family members experiencing family effects. Note that not all family members experience both types of effects. For example, younger siblings may not experience caregiving effects. For the purpose of this review, “family” is defined as persons living in the same dwelling who are related to a child (≤ 18 years) with chronic illness or disability by blood, adoption, or foster care or close emotional bonds. This typically includes parents, siblings, and other relatives who maintain strong relationships and provide mutual support and care.
Although there is growing recognition of the importance of incorporating family spillover effects in pediatric CUAs, only a small proportion of published CUAs have included family spillover effects [8, 36,37,38]. While both family costs and family spillover effects represent the impacts on family welfare, researchers have usually considered only family cost spillovers, such as productivity losses of parents due to a child’s medical condition or disability or parents’ out-of-pocket costs for transportation or lodging for the child to receive medical and/or nonmedical services [8, 36]. A review of pediatric CUAs conducted from a societal perspective published between 2000 and 2015 found that out of 142 pediatric CUAs, 103 (73%) considered family spillover costs and only 15 (11%) included family spillover effects in the form of caregiver(s) health outcomes [8]. A review of published NICE technology appraisals of health interventions for adults and children found that only 16 of 414 technology appraisals (TA) or appraisals of highly specialized technologies (HSTs) included health effects on a caregiver and/or family member [36].
The differential rate of including family cost versus family spillover effects might be explained by the focus of CEA guidelines on the health consequences in individual patients. While they may consider the inclusion of cost spillovers, they do not explicitly recommend including health and nonhealth spillover effects [1, 3, 4, 6].The observed difference in the rate of inclusion of family spillover costs compared with family spillover effects also reflects methodological challenges in measuring, assessing, and incorporating family spillover effects in pediatric CUAs. These include but are not limited to: (1) identifying and deciding which family members to include, (2) measuring and estimating family spillover effects, (3) establishing the causality of the relationships between a child’s health and family members’ health and well-being, (4) integrating family spillover effects in the analysis, and (5) constructing decision analytic models that capture effects on multiple affected individuals. These challenges might differ in scope for CUAs conducted from different payer perspectives, such as societal or healthcare system perspectives.
Considering these challenges and the absence of guidance on measuring, assessing, and incorporating family spillover effects in pediatric CUAs, it is crucial to understand the ways in which existing pediatric CUAs included family spillover effects. This review aims to investigate methods used by researchers to include family spillover effects in pediatric CUAs. First, it evaluates the specific family members included in studies measuring family spillover effects and the methods by which family spillover effects were measured. Secondly, it compares methods used by researchers to integrate family spillover effects into an analysis and reports the results. Common methods and approaches were grouped conceptually. Finally, this review identifies theories or theoretical frameworks that researchers used to justify their methodological approaches to the integration of family spillover effects in pediatric CUAs. Given the inconsistent incorporation of family spillover effects in pediatric CUAs [8, 9], and considering that welfare economic theory is the foundation of economic evaluation [39, 40], it is crucial to demonstrate to what extent authors drew upon theory in justifying their approaches in integrating family spillover effects within pediatric CUAs.
2 Methods
2.1 Data Sources and Search Strategy
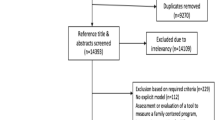
This systematic literature review used the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [41] to identify pediatric CUAs encompassing family spillover effects. Six databases, including MEDLINE, Embase, EconLit, Cochrane collection, Cumulative Index to Nursing and Allied Health Literature, and Internal Network of Agencies for Health Technology Assessment, were searched from inception to 16 November 2020. A comprehensive search strategy was developed using search terms identified from published literature reviews combining search terms to identify cost-effectiveness analysis (CEA) and CUA in neonates, newborns, infants, children, and adolescents [8, 42]. The Pediatric Economic Database Evaluation (PEDE) Registry (pede.ccb.sickkids.ca/pede/index.jsp) was also searched for potentially eligible studies [43]. The PEDE database contains pediatric economic evaluations from a wide array of databases, including all the databases originally searched and over 70 HTA academic and government websites. The database is updated annually. PEDE was used to update the search through December 2021. A single researcher (R.L.) manually searched reference lists of included studies to identify additional eligible studies. Articles generated by all database searches were compiled and duplicates were removed using Endnote X8.2. The search strategy for MEDLINE is provided in Supplementary Fig. 1.
2.2 Study Selection
Initially, a single researcher (R.L.) reviewed titles and abstracts, retaining studies meeting these inclusion criteria: CUA with participants aged 18 years of age or younger (perinate, neonate, infant, child, and adolescent), health outcomes measured in QALYs or DALYs, publication in a peer-reviewed journal, and written in English. Studies or reports published by HTA agencies such as CADTH, NICE, and the Institute for Clinical and Economic Review (ICER) were eligible. Following title and abstract review, a single researcher (R.L.) reviewed full texts of potentially eligible articles and excluded studies that did not meet the aforementioned inclusion criteria and that did not include family spillover effects for one or more family members.
2.3 Data Extraction and Analytic Consideration
The following general data were extracted by a single researcher (R.L.) using a standardized data collection form: (1) bibliographic information including researchers and year of publication, (2) country of the population, (3) disease/condition, (4) participants, (5) aim/objective of the study, (6) perspective and time horizon, and (7) intervention(s) and comparator(s). The health conditions/diseases were categorized by the International Classification of Diseases 10 revision (ICD-code) chapters for description purposes [44].
The following specific information was also extracted: (1) the form in which family spillover effects were measured and expressed, e.g., disutility, QALYs, and QALY loss; (2) family member(s) included; (3) instrument used in family members to measure family spillover effects; (4) instrument used in child to measure utility; (5) the magnitude of family spillover effects reported by researchers; (6) modelling approach; and (7) methods used to integrate family spillover effects with the child’s health-related quality of life (HRQoL) for QALY estimation and the form used to report family spillover effects. Lastly, data were extracted on any theoretical frameworks guiding the integration of family spillover effects in pediatric CUAs.
The methods used to integrate family spillover effects into analyses were tabulated and described. Through the literature synthesis, similar approaches used for capturing family spillover effects were categorized together using appropriate conceptual terms that reflected the observed phenomena.
2.4 Quality Appraisal
Using a quality assessment tool in systematic reviews is essential to ensure the reliability, comparability, and validity of the included studies [45,46,47]. As there are no quality assessment tools or instruments to evaluate the quality of methods used to incorporate spillovers, existing quality appraisal tools such as the Quality of Health Economic Studies (QHES) checklist [48], the Consensus on Health Economic Criteria (CHEC) [49] and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting tool were reviewed for relevance [50]. The QHES checklist has been widely used in assessing economic evaluation methodology [48, 51]. It has been validated, and test–retest reliability has been demonstrated. This tool was therefore selected as the most appropriate to assess the quality of included pediatric CUAs. The QHES scale includes 16 items, each answered as “yes” or “no.” Each item is assigned a fixed weight. The value of the fixed weight depends on the item’s importance in economic evaluation and varies from 1 to 9. Each item is scored as either the full weight or zero, and the sum of the weights (total score) ranges between 0 and 100. Partial points are not allowed; each item should be scored with either full or no points. An economic evaluation with score ≥ 75 is considered high quality. Five pediatric CUAs (NICE TA and HSTs) were not included in the quality appraisal because complete reports were not publicly available but were included in this review.
3 Results
3.1 Search Results
Figure 1 illustrates an overview of the search and retrieval processes. The literature search resulted in 27,070 articles. After duplicates were removed, 24,395 articles were eligible for review. The review of titles and abstracts excluded 23,617 articles. The primary reasons for exclusion were targeting nonpediatric populations and using nonpreference-based health outcome measures or health outcomes that were not QALYs or DALYs. The full texts of 778 remaining studies were screened. A total of 758 studies were excluded because they were not pediatric, included adult populations (n = 26), were not CUAs (n = 12), or did not include family spillover effects (n = 720), resulting in 20 studies that met the inclusion criteria. A search of the PEDE database yielded eight additional studies that considered family spillover effects, including five that were published between November 2020 and December 2021. An additional seven studies that included family spillover effects were identified through manual reference searching of included studies and previous reviews. Of these seven studies, five were NICE appraisals not included in searched databases. Thus, data were extracted from a total of 35 pediatric CUAs.
3.2 Study Characteristics
Table 2 summarizes the 35 included studies. Additional information is available in Supplementary Table 1. Most studies focused on infectious and parasitic diseases (n = 18, 51%), with gastroenteritis being the most common disease studied (n = 11). Vaccines (immunization) were the most common intervention type (n = 20, 57%). Sixteen (46%) studies were conducted from both healthcare system and societal perspectives, followed by healthcare system perspective only, societal perspective only, public sector and societal perspective, and maternal perspective. The United Kingdom was the country where most (n = 11, 31%) of the studies were conducted.
3.3 Quality of Included Studies
Nine of 30 (30%) studies were categorized as poor quality (Supplementary Table 2). The mean (SD) QHES score was 91.04 (4.51) for 21 pediatric CUAs that were deemed high quality (QHES > 75). The mean (SD) QHES score was 70.66 (5.59) for studies that were deemed poor quality. Since the objective was to systematically identify and report the range of approaches employed by researchers to measure and incorporate family spillover effects rather than pooling a quantifiable estimate, studies were not excluded based on the quality appraisal.
3.4 Methods of Inclusion of Family Spillover Effects
There are primarily three steps in incorporating family spillover effects in pediatric CUAs: (1) identifying and deciding which family members should be included, (2) measuring and estimating family spillover effects, and (3) integrating family spillover effects spillovers into the analysis and reporting the results. The following sections describe the findings from this review in this sequence.
3.4.1 Family Members Included
Table 3 summarizes the types and number of family members or caregivers included in the reviewed studies. Additional information is available in Supplementary Table 3. Most studies considered family spillover effects on one parent or caregiver (n = 24, 69%). Five studies incorporated family spillover effects on two caregivers or parents [52,53,54,55,56] and three on family network members [57,58,59]. In an appraisal submitted to NICE, family spillover effects on three caregivers were considered [60]. Finally, one study incorporated family spillover effects on the family/household as a whole but did not mention which family members were included [61].
3.4.2 Measurement and Estimation of Family Spillover Effects
With regard to measuring and estimating family spillover effects, the instruments developed by EuroQol Group (EQ-5D, EQ-5D-5L, EQ-5D-3L) were the most frequently used tools to measure family member utilities (Supplementary Table 3). Other instruments such as HUI-2, AQOL-8D, and other elicitation methods such as TTO, VAS, and SG were used much less frequently.
The forms in which family spillover effects were measured and expressed in the included studies were heterogeneous (Table 3). Of these studies, 11 reported measuring the QALY loss of the caregiver(s) due to a child’s illness or disability [52,53,54,55, 62,63,64,65,66,67,68], 6 studies reported measuring the disutility of a child’s illness on caregivers or parents [60, 69,70,71,72,73], 3 studies reported measuring the QALY loss of the caregiver–child dyad (n = 3) [74,75,76], 3 studies reported measuring the QALY loss of family network members due to a child’s illness or disability (n = 3) [57,58,59], and 1 study reported measuring the QALY loss of the family/household as a whole due to premature death of the child [61]. For example, in a CEA of rotavirus vaccination, Newall et al. included QALY losses for the primary caregiver in the base–case model when a child became infected and required hospitalization [55]. As an example of disutility measurement, in their appraisal submitted to the NICE for ataluren in Duchenne muscular dystrophy treatment, the manufacturer included caregiver disutility for the nonambulatory patient health states. A disutility of 0.11 was subtracted from the patient’s utility [60]. This disutility was obtained from a study by Landfeldt et al., who reported the mean loss of caregiver quality of life compared with the general population. The caregiver quality of life data were collected using the EuroQol EQ-5D instrument [77].
All 11 studies that reported family spillover effects as a QALY loss in caregiver(s) due to a child’s illness or disability were model-based CUAs with inputs obtained from published literature. Most of these studies [52,53,54,55, 64, 65, 68] used estimates of QALY losses due to caregiving from the study by Senecal et al. [78]. This study recruited children younger than 3 years of age presenting with gastroenteritis symptoms and their caregivers from 59 family medicine and pediatric clinics across Canada. Caregivers were asked to evaluate their children’s HRQoL using the HUI2 and their own HRQoL using the EQ-5D over three visits made over a 2-week period. The estimated mean QALY loss for the caregiver per episode of gastroenteritis in the child was 0.002 QALYs.
With regard to reporting of joint family spillover effects, Prosser et al. asked parents to consider their own time spent caring for their child, as well as the time that the child would spend suffering in an undesirable health state, and trade it off with any amount of their own life [76]. The amount of time the parent was willing to trade from their remaining life expectancy was used to estimate the parent–child dyad QALY loss. For example, if a parent was willing to trade 7 days to prevent the recurrence of complex otitis media in their children, this represented a one-time loss of 0.02 parent–child dyad QALYs. The parent–child dyad QALY loss represents the pain and suffering of both the child and parent from the disease in a single estimate, rather than valuing them separately and then adding QALYs together. Two other studies used the estimates from this study to measure the parents’ pain and suffering related to children’s acute otitis media and the impacts of children’s influenza-related disease on parents [74, 75].
Three studies [57,58,59] estimated the QALY loss of family network members due to the impact of meningitis sequelae as 48% of the QALY loss experienced by the meningitis survivor, referencing an unpublished study by Al-Janabi et al. [79]. Al-Janabi et al. conducted a UK-wide prospective cross-sectional study of 1600 family members of meningitis survivors. Aggregating quality of life losses in the family network members, the researchers estimated that QALY losses of the family network members were 48% of QALY losses to the meningitis survivor, in families in which the person with disease had sequelae. These studies considered the additional QALY losses experienced by the family network members due to bereavement to be equivalent to 9% of the QALYs lost by the death of a person with meningococcal disease. This estimate was based on a study by Song et al. [80] who compared the long-term effects of child death on bereaved parents’ HRQoL with the HRQoL of couples with living children.
In the studies described above, family spillover effects were measured as an isolated quantity in individual family members or in the family as a whole (household) independent of other health effects experienced, such as those resulting from family members’ own health conditions or the health conditions of other family members. The term isolated approach was derived to reflect this observation. An isolated approach may include measuring the disutility of a child’s illness or disability on individual family members through elicitation, and/or calculating a QALY loss of individual family members due to a child’s illness or disability, and/or calculating a QALY loss in a parent–child dyad due to a child’s illness or disability, and/or calculating a QALY loss of the family (household) as a whole. For instance, a way to estimate utility decrements through an isolated approach involves estimating the difference between the mean utility of parents who have a child with a specified illness or disability and the mean utility of parents with healthy children, while controlling for or keeping other variables constant, including the parent’s own health conditions. This approach can also be used to determine the incremental family spillover effects due to treatment of a child’s condition.
In contrast to the studies described above, nine studies measured family spillover effects in terms of the health utility of caregiver(s) [56, 81,82,83,84,85,86,87,88] and one study measured the health utility of the caregiver–child dyad (n = 1) [89]. Melliez and colleagues attributed a dyad health utility (equal to 0.884 for mild rotavirus diarrhea and 0.816 for severe diarrhea) to a child and one caregiver based on Senecal et al. [78]. Subsequently, QALYs for caregivers and/or children were calculated based on the inherently derived health utilities. Finally, one study used the General Health Questionnaire-12 (GHQ-12) to assess mental health in parents [90]. The researchers used an algorithm developed by Serrano-Aguilar et al. [91] to convert the GHQ-12 scores into EQ-5D health states utilities and subsequently calculated QALYs.
These nine studies captured family spillover effects using an inherent approach. This term was derived to describe an approach whereby family spillover effects are estimated indirectly, without isolating the effects of a child’s illness. This approach involves measuring the health state utility of each family member or dyad (i.e., the parent–child or caregiver–child dyad joint health state). The utility of the health state reflects the family member’s current HRQoL and consists of family spillover effects of the child’s illness or disability, plus any other health effects they might experience, including those related to their own health conditions as well as health conditions of other family members. For instance, elderly parents who are caregivers are likely to have chronic health conditions simultaneously with their caregiving responsibilities; therefore, their health utility will reflect a combination of family spillover effects and health effects stemming from their own health conditions. This may be expressed as the family member health state utility and/or family member QALYs and/or parent–child dyad QALYs. When this approach is used, the incremental family member QALYs due to treatment of a child’s condition determined for a CUAs will reflect the change in the family spillover effect.
Differentiating between the ways in which family spillover effects are expressed through an isolated approach or an inherent approach is essential to guide how to combine the family spillover effects with a child’s utility. For instance, when family spillover effects are measured as utility decrements using an isolated approach, the typical method for integration involves subtracting the absolute value of the decrement from a child’s health utility to estimate QALYs.
3.4.3 Characteristics of Model Design to Integrate Family Spillover Effects
Table 3 summarizes the various modelling approaches used to capture family spillover effects. Additional information is available in Supplementary Table 3. Seventeen studies used statistical models to examine the costs and benefits of pediatric intervention [53, 54, 56,57,58,59, 62,63,64, 66,67,68, 83, 84, 86, 87, 90]. These included deterministic compartmental static models, dynamic transmission models, cohort models, and static equilibrium models. Fifteen studies were performed using decision-analytic models [52, 55, 60, 61, 65, 69,70,71,72, 75, 81, 82, 85, 88, 89] with a Markov model being the most common (n = 6) [52, 55, 65, 69, 85, 89]. The remaining two model-based CUAs were conducted using microsimulation [73, 74].
Of the 15 decision-analytic studies, 14 were conducted using a single decision-analytic model that consisted of health states and clinical events relevant to children. Partridge et al. constructed two decision-analytic models with both models including health states for children and mothers [88].
3.4.4 Analytic Approaches for Integrating Family Spillover Effects
Table 4 summarizes the various approaches used to integrate family spillover effects with child outcomes. Additional information is available in Supplementary Table 3. Overall, 22 studies incorporated family spillover effects in the reference case analyses, and the remaining 13 studies incorporated them into sensitivity or scenario analyses. These approaches ranged from estimating utility decrements on a parent or caregiver’s utility due to a child’s illness or disability and then applying the decrements to the child’s utility, to measuring health utilities and estimating QALYs for children and caregivers/parents separately and then summing QALYs of children and parents/caregivers within each group.
Of the 22 studies that incorporated family spillover effects in reference case analyses, 13 calculated QALYs separately for children and parents or caregivers [52,53,54,55, 63, 68, 82,83,84, 86,87,88, 90]. In 9 of these 13 studies [52,53,54,55, 63, 68, 86, 88, 90], QALYs of children and parents or caregivers were summed in each comparator group, and then combined QALYs of children and parents or caregivers were estimated at the family level. For instance, Tubeuf et al. measured the utility of adolescents (aged 11–17 years) using the EQ-5D-3L and the utility of parents using HUI2 at baseline, 6 months, and 12 months [86]. The authors calculated QALYs separately for adolescents and parents. Adolescent and parent QALYs were then summed to estimate QALYs at the family level. The authors also used three other quantification methods to capture family spillover effects. Three studies did not combine QALYs of children and parents or caregivers [83, 84, 87] and reported separate QALYs and incremental QALYs between study groups (Δ QALYs) for children and parents or caregivers. The remaining study [82] combined QALYs of children and parents or caregivers, but the method of integration was not specified.
In two studies, caregiver utility decrements (disutilities) were applied to the child’s utility [60, 70]. The caregiver utility decrements represented the caregiver burden. Zanganeh et al. [56] used the multiplier approach proposed by Al Janabi 2016 [92] to incorporate family spillover effects. Beck et al. [57] multiplied the QALY losses of the survivor of acute care with long-term sequelae by a factor of 1.48 to incorporate the impact on the family network members. In a CUA of methylphenidate for children and adolescents with attention deficit hyperactivity disorder, Schawo et al. [85] included 48% of caregiver utility in the model, but no further details were provided. Finally, as described in Section 3.4.2, three studies [74,75,76] measured the QALY losses of a child–caregiver dyad, and one study [89] measured the utility of a child–caregiver dyad. Therefore, these analyses did not require integration of spillover effects on parents or caregivers with child health state utility.
Table 4 summarizes the methods used by researchers to report family spillover effects. Of the 22 studies that incorporated family spillover effects in reference case analyses, 10 reported combined QALYs and between-group Δ QALYs for children and parents or caregivers at the family level [52,53,54, 57, 63, 74,75,76, 85, 89]. Seven studies reported combined QALYs and Δ QALYs for children and parents or caregivers at the family level in addition to separate QALYs and Δ QALYs for children and parents or caregivers [55, 56, 68, 82, 86, 88, 90]. Three studies reported QALYs and Δ QALYs separately for children and caregivers. The remaining two studies reported QALYs and Δ QALYs for children only [60, 70].
Finally, of the 13 studies that incorporated family spillover effects in sensitivity or scenario analyses [61, 62, 64,65,66,67], 6 studies included QALY losses for caregivers due to a child’s illness or disability, but researchers were not explicit regarding how they combined the QALY losses of caregivers and children. In five studies [69, 71,72,73, 81], utility decrements for caregivers or parents due to a child’s illness were applied to the child’s utility within health states to represent the caregiver burden. Finally, two CUAs [58, 59] included QALY losses of family network members due to a child’s illness or disability in sensitivity analysis, but the authors did not state or were unclear regarding how QALY losses of family network members were combined with the QALYs of children.
3.5 Use of Theories or Theoretical Framework to Justify the Methodological Approach to Integrate Family Spillover Effects
Out of 35 included studies, only 2 used theories or theoretical frameworks to justify their methods of integrating family spillover effects [56, 86]. Tubeuf and colleagues [86] proposed quantifying family spillover effects using a household welfare function and an equivalence scale (ES) [93]. The cited household welfare theory states that individual welfare entails a family welfare function. The welfare of each family member can be aggregated to measure family welfare as a whole to enable comparisons between households [93]. The ES allows adjusting all health gains for the rest of the household as an additional individual equivalent QALY or utility gain where all the household members are accounted for [86, 93]. Zanganeh et al. [56] used the framework developed by Al-Janabi and colleagues [92] to incorporate family spillover effects on parents. This theoretical framework is based on an extra-welfarist approach which focuses on maximizing the health benefits of the population from a fixed healthcare budget [94].
4 Discussion
Despite well-established evidence that a child’s health condition significantly affects family members’ health and well-being [20, 95,96,97,98,99], this review found that only a small number of pediatric CUAs (35) included family spillover effects. If a health care intervention improves the HRQoL of a child and therefore improves the HRQoL of family members, ignoring the improvement in family member HRQoL due to the child’s intervention underestimates the value of the intervention. It is recognized that the health of a child and their treatment can impact the health and overall well-being of family members in multiple ways [100] . Researchers were inconsistent in deciding which family member to include, and they employed various approaches to measure, integrate, and report the family spillover effects in pediatric CUA.
4.1 Identification of Family Members
Most studies focused on the family spillover effects of a single primary caregiver, often a mother, while neglecting nonprimary caregivers such as fathers and siblings. However, research shows a child’s illness or disability also affects the health and well-being of noncaregiving family members, i.e., a family effect [20, 22, 96, 98, 99, 101]. Excluding family effects underestimates the true burden of illness and the potential value of pediatric interventions. Identifying all relevant family members is therefore a necessary first step in incorporating family spillover effects into CUA. Researchers should carefully identify the most impacted family members, especially parents (both father and mother, if applicable) with significant caregiving roles. The family spillover effect is likely to be connected to the strength of the relationship between a child with an illness or disability and a family member; therefore, one may expect family effects to be particularly strong in parents and siblings [28, 95, 102,103,104]. Other family members, such as grandparents, may experience caregiving and family effects if they are involved in caregiving or share strong ties with a grandchild [105,106,107]. Determining whom to include is still an active area of research [27, 104, 108, 109].
4.2 Family Effects and Caregiving Effects
Most studies incorporating family spillover effects did not mention whether spillovers effects were restricted to measuring family effects or also included caregiving effects, even though both effects are likely to present in families with children with health conditions [27, 28]. HTA agencies such as NICE [3] support considering what they refer to as “health effects on caregivers” without specifying whether these are caregiving effects or family effects. NICE guidelines state: “Evaluations should consider all health effects for patients, and, when relevant, carers. When presenting health effects for carers, evidence should be provided to show that the condition is associated with a substantial effect on carer’s health-related quality of life and how the technology affects carers” [3]. Previous research has shown that the family effect may be a stronger determinant of HRQoL of the caregiver than the caregiving effect [8, 27, 28, 110]. However, these two effects are often intertwined as family members may engage in varying degrees of caregiving while also demonstrating a higher level of emotional concern [10, 27, 28, 32] and it can be challenging to separate them.
4.3 Approaches for Measuring Family Spillover Effects
The ways in which family spillover effects were measured varied widely across studies. The observed variation can be partially attributed to the limited availability of published data to inform family spillover effects. In model-based CUAs, researchers depend on published estimates. Hence, data availability may determine whether disutilities, QALY losses, or health utilities are used to quantify family spillover effects. For instance, among the 11 studies focused on gastroenteritis, 9 reported measuring spillover effects in caregiver(s) as a QALY loss due to caregiving. The estimates were derived primarily from one previously published study [78]. In only six studies were the family spillover effects reported as disutility, possibly due to a lack of published disutility estimates for specific pediatric conditions [9, 111].
Studies that elicited health utilities in family members (inherent approach) to capture family spillover effects used indirect preference-based HRQoL instruments, such as EQ-5D, EQ-5D-5L, HUI-2 and HUI-3. Providing care can have various positive and negative effects on all aspects of a caregiver’s life, including, but not limited to, their general health [110, 112]. The utilities derived from these instruments will capture many aspects of HRQoL and can be used to calculate QALYs, integrating them into pediatric CUA. However, these instruments may not capture caregiver-relevant dimensions such as fulfillment, financial problems, or relational problems [102, 113]. For example, the EQ-5D has only one question related to mental health (anxiety/depression) and lacks other dimensions such as psychological or emotional distress, which may result from caring for and caring about a child with illness or disability [114]. They therefore may not adequately measure family spillover effects [8, 9, 111]. While the Care-related Quality of Life (CarerQol) instrument [115] captures caregiver-relevant dimensions of burden (fulfillment, support, mental health, physical health, financial problems, relational problems, and problems with daily activities), it cannot be used to estimate QALYs and methods have not been developed to combine it with child health utilities or QALYs [8, 9].
To address these challenges, using the CarerQol alongside indirect preference-based HRQoL instruments such as EQ-5D or HUI-3 may be useful. This may enable capturing the full impact of the pediatric intervention and facilitate separate measurements of family effects and caregiving effects in family members. However, this may overestimate the impact of providing care on caregivers, as some of the current indirect preference-based HRQoL instruments may also capture certain aspects of the caregiving burden [8, 35]. Fully capturing family spillover effects can be enhanced with measures of well-being and capability. Capability-based instruments are designed to capture the impacts of health problems and caring through broader well-being attributes [116,117,118]. Similar to CarerQol, these tools cannot be used to estimate QALYs or as yet be combined with child health utilities or QALYs.
4.4 Analytical Approaches for Integrating and Reporting Family Spillover Effects
The most common method to integrate family spillover effects was the summation of QALYs of index patients and parents or caregivers. QALY summation methods might not be appropriate for integration if utilities used to derive QALYs for the child and family members were measured using preference-based HRQoL instruments that differ with regards to the underlying construct of HRQoL. Aggregating family members and patient QALYs can also lead to equity concerns and distributional implications [7, 117, 119, 120]. If an intervention is effective and reduces negative family spillover effects, the incremental cost-effectiveness ratio (ICER) will be lower for studies where family spillover effects are included compared with studies that excludes family spillover effects [8, 117]. In some cases, the cumulative increase in QALYs that caregivers or family members experience due to an intervention could be greater than QALY gains experience by individual patients. Also, some patients have greater resources, including a wider network of caregivers and/or family members to benefit from reduced spillover effects compared with others [121]. In these cases, summing of QALYs will result in a greater net gain compared with those with limited access or availability of caregivers and family members [117]. Some researchers suggest including equity in estimating caregivers’ QALYs to address some of these concerns [92, 119, 122]. Moreover, the question of whether family and caregiver QALYs should be assigned different weights compared with patient QALYs in CUAs is a subject of ongoing debate [117, 123]. While some researchers suggest prioritizing patients by assigning low or zero weight to caregivers’ HRQoL [86], others suggest weighting caregiver QALYs based on caregivers’ characteristics [27].
The most common method of reporting family spillover effects was as combined QALYs and between-group Δ QALYs for children and parents or caregivers at the family level. However, reporting separate and combined QALYs and Δ QALYs for children and caregivers/family members can allow for precise identification of where the cost and benefits due to an intervention accrue. Also, presenting aggregated and disaggregated results for children and caregivers and/or family members may be useful for funding decision-makers.
4.5 Use of Theory or Theoretical Frameworks to Guide Family Spillover Effects Inclusion
One of the reasons for heterogeneity in approaches to measuring and incorporating family spillover effects is a lack of a theoretical framework to guide their inclusion in pediatric CUA. A few theoretical frameworks for measuring and incorporating spillover effects have been introduced in the context of adult economic evaluations [92, 124, 125]. These frameworks aim to capture the broader impacts of health conditions on individuals and their family networks, providing a more comprehensive evaluation of interventions. Basu and Meltzer use a welfarist approach to propose a theoretical framework for including spillover effects in CUA from a societal perspective, focusing on maximizing the family’s utility [125]. Al-Janabi et al., on the other hand, developed a conceptual framework for including family spillover effects based on the extra-welfarist approach, which focuses on the health benefits to the population from a fixed healthcare budget [92].
Theories guiding inclusion of family spillover effects must recognize the significant differences between child and adult health in terms of development, interdependency, and patterns of resource use [126,127,128]. First, the child’s health and development are intertwined with their parents’ health and the well-being of other family members. This bidirectional relationship has been demonstrated by empirical studies that have observed that children and parents influence each other’s health and well-being [21, 126, 129]. Second, children depend on their parents and other family members to access care [130]. Parents are the decision-makers for their child’s healthcare needs from the start of life until independence is achieved [131]. Finally, parents’ decisions regarding healthcare resource consumption for their child depend on family socioeconomic status, the need to spend on the health and well-being of other family members, including parents and siblings, a family’s cultural values and beliefs, available resources, and parental altruism toward children [132,133,134]. Each of these dimensions affects the health and well-being of children and family members and need to be acknowledged when considering spillover effects in CUA of pediatric interventions. Further research on the theoretical basis for measuring and incorporating spillover effects in the economic evaluation of pediatric interventions is warranted.
4.6 Methodological Considerations for Future Family Spillover Effects Measurement
Based on a close examination of the included studies, this review proposes five considerations to improve methods for measuring and incorporating family spillover effects (see Table 5). First, few studies considered changes in family members’ HRQoL over time after a patient dies [57, 59, 61]. This omission could be due to the current guidelines on CEA limiting analyses to the duration of the patient’s life expectancy. Studies have reported that bereaved parents are at greater risk of poor mental [135,136,137,138] and physical health outcomes [138,139,140], as well as post-traumatic stress disorders [141]. Bereaved parents have also reported suicidal ideation [142] and exhibit higher rates of suicide compared with nonbereaved parents and the general population [143, 144]. Future studies should extend the time horizon beyond the patient’s life expectancy to include the period of bereavement on family members. Second, no studies considered shifts in family spillover effects over time. Caregivers, often parents, may have shorter life expectancies than children with illness or disability. As children with disabilities or chronic illnesses transition into adolescence and adulthood, family spillover effects shift to other individuals, including the spouses, partners, siblings, or others [145,146,147,148]. Adding a spouse or older sibling as a caregiver after a certain age of a patient would be most appropriate for studies that adopt a lifetime horizon. Third, most studies relied on cross-sectional designs for collecting family spillover effects data, making it difficult to establish the temporality of the relationship. Using a matched-comparison group (such as family members of healthy children or general population norms) [149,150,151], collecting HRQoL data on caregivers or family members alongside randomized controlled trials [83, 86, 87] or considering family-level or parent–child dyad health utilities [74, 76, 152] would allow for estimating unbiased family spillover effects. Fourth, the child’s disease or disability is not likely to impact all family members (mothers, fathers, siblings) and caregivers (primary, secondary) equally [153, 154]. Four studies in this review included the family spillover effects on two caregivers [52,53,54,55]. However, these studies incorporated the same magnitude of family spillovers for both caregivers. This could be due to a lack of data on the magnitude of family spillover effects on different family members. Future studies should measure the impacts of a child’s illness or disability on the HRQoL of various family members. Finally, it may take longer to observe the effects of a child’s health treatment on the health outcome of family members compared with the child. Empirical evidence is necessary to support this hypothesis. Family members, such as parents, may first need to experience the improvement in their child’s health due to the treatment [127]. Future studies could extend the time horizon to capture these effects.
4.7 Limitations
A limitation of this review was the potential for relevant articles to be missed. Although the search strategies were comprehensive, the requirements for studies to be published in English and in peer-reviewed journals may have resulted in some eligible studies being excluded. Second, only one reviewer conducted abstract and full-text screening, data extraction, and quality assessment. Third, the quality of five included studies (NICE appraisals) could not be assessed because full texts were inaccessible. Additionally, nine studies scored poorly on the QHES. However, these results do not specifically reflect the quality of methods used to consider family spillover effects. As guidelines for the conduct and reporting of CEA evolve, critical appraisal tools or a component to evaluate the quality of methods used to incorporate spillover costs and effects in pediatric CUAs should be developed. Finally, this review was limited by the information authors provided. Some authors did not explain how combined QALYs or Δ QALYs were estimated.
4.8 Implications for Health Technology Assessment Agencies and Researchers
Considerable progress has been made in understanding, measuring and valuing family spillover effects since the original US Panel and NICE guidelines [9, 155, 156]. Yet, no clear, practical guidelines exist on including family spillover effects in CUAs [5]. While the 2016 Second Panel on CEA recommended that analysts include caregivers’ time costs in a reference case analysis from a societal perspective [6], they did not mention the inclusion of family spillover effects. The most recent NICE guideline states that the NHS PSS perspective on outcomes should include “all direct health effects” for the reference case, whether for patients or other people (carers), but does not offer guidance on how and when to incorporate family spillover effects [3]. The inconsistency observed in methods used to measure and incorporate family spillover effects could be due to a lack of consensus or differing views among HTA agencies regarding the inclusion of caregiver’ HRQoL [5]. Researchers have also advocated for fuller inclusion of family spillover in pediatric CUA, encompassing both family spillover costs and spillover effects, along with establishing standard guidelines for incorporating family spillover effects in CUA [7, 111, 126, 156,157,158,159]. In future guideline updates, HTA agencies may consider adding an explicit category for spillover effects under a societal perspective. A first step may be the US Second Panel on CEA impact inventory, a checklist of health and nonhealth outcomes and costs to be considered in CEA, including those affecting caregivers [6]. Researchers are encouraged to identify criteria for inclusion of family spillover effects, such as the type of pediatric conditions or diseases that significantly impact family members’ health and well-being and/or economic well-being.
5 Conclusion
Few pediatric CUAs have considered family spillover effects, and the observed variation indicated a lack of consensus regarding how family spillover effects should be measured, incorporated, and reported. Future CUAs of child health interventions should incorporate or justify the exclusion of spillover effects on parents and other family members. Development and validation of standard methods for measurement of spillover effects and their inclusion in pediatric CUAs are warranted. The results from this review may encourage HTA agencies to develop guidelines for the inclusion of family spillover effects in pediatric CUAs.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Canadian Agency for Drugs and Technology in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa: CADTH; 2017.
National Institute of Health and Sciences (NICE). Guide to the methods of technology appraisal. 2004 [cited 2021 February 24 ]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/191504/NICE_guide_to_the_methods_of_technology_appraisal.pdf.
National Institute of Health and Sciences (NICE). Guide to the methods of technology appraisal 2013. 2013 [cited 2019 December 1] . https://www.nice.org.uk/process/pmg9/chapter/foreword.
Nederland Zorginstituut. Guideline for economic evaluations in healthcare. Diemen: ZIN 2016 [cited 2022 February 24]. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare.
Pennington B, Eaton J, Hatswell AJ, Taylor H. Carers’ health-related quality of life in global health technology assessment: guidance, case studies and recommendations. Pharmacoeconomics. 2022;40(9):837–50. https://doi.org/10.1007/s40273-022-01164-4.
Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. 2nd ed. New York: Oxford University Press; 2016.
Brouwer WB. The inclusion of spillover effects in economic evaluations: Not an optional extra. Pharmacoeconomics. 2019;37(4):451–6. https://doi.org/10.1007/s40273-018-0730-6.
Lavelle TA, D’Cruz BN, Mohit B, Ungar WJ, Prosser LA, Tsiplova K, et al. Family spillover effects in pediatric cost-utility analyses. Appl Health Econ Health Policy. 2019;17(2):163–74. https://doi.org/10.1007/s40258-018-0436-0.
Wittenberg E, James LP, Prosser LA. Spillover effects on caregivers’ and family members’ utility: a systematic review of the literature. Pharmacoeconomics. 2019;37(4):475–99. https://doi.org/10.1007/s40273-019-00768-7.
Belza C, Ungar WJ, Avitzur Y, Stremler R, Fehlings D, Wales PW. Carrying the burden: informal care requirements by caregivers of children with intestinal failure receiving home parenteral nutrition. J Pediatr. 2022;250:75-82e3. https://doi.org/10.1016/j.jpeds.2022.05.049.
Ireland MJ, Pakenham KI. The nature of youth care tasks in families experiencing chronic illness/disability: Development of the Youth Activities of Caregiving Scale (YACS). Psychol Health. 2010;25(6):713–31. https://doi.org/10.1080/08870440902893724.
Pakenham KI. The nature of caregiving in multiple sclerosis: development of the caregiving tasks in multiple sclerosis scale. Mult Scler. 2007;13(7):929–38. https://doi.org/10.1177/1352458507076973.
Rogers B. Feeding method and health outcomes of children with cerebral palsy. J Pediatr. 2004;145(2 Suppl):S28-32. https://doi.org/10.1016/j.jpeds.2004.05.019.
Bourke-Taylor H, Cotter C, Stephan R. Young children with cerebral palsy: Families self-reported equipment needs and out-of-pocket expenditure. Child Care Health Dev. 2014;40(5):654–62. https://doi.org/10.1111/cch.12098.
Schieve LA, Gonzalez V, Boulet SL, Visser SN, Rice CE, Braun KVN, Boyle CA. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Res Dev Disabil. 2012;33(2):467–76.
Martin CA, Papadopoulos N, Chellew T, Rinehart NJ, Sciberras E. Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: Systematic review. Res Dev Disabil. 2019;93: 103463.
Hulst RY, Gorter JW, Voorman JM, Kolk E, Van Der Vossen S, Visser-Meily JMA, et al. Sleep problems in children with cerebral palsy and their parents. Dev Med Child Neurol. 2021;63(11):1344–50.
McAuliffe T, Cordier R, Chen YW, Vaz S, Thomas Y, Falkmer T. In-the-moment experiences of mothers of children with autism spectrum disorder: a comparison by household status and region of residence. Disabil Rehabil. 2022;44(4):558–72. https://doi.org/10.1080/09638288.2020.1772890.
Pinquart M. Parenting stress in caregivers of children with chronic physical condition—a meta-analysis. Stress Health. 2018;34(2):197–207. https://doi.org/10.1002/smi.2780.
Sharpe D, Rossiter L. Siblings of children with a chronic illness: a meta-analysis. J Pediatr Psychol. 2002;27(8):699–710. https://doi.org/10.1093/jpepsy/27.8.699.
Scherer N, Verhey I, Kuper H. Depression and anxiety in parents of children with intellectual and developmental disabilities: a systematic review and meta-analysis. PLoS ONE. 2019;14(7): e0219888. https://doi.org/10.1371/journal.pone.0219888.
Dey M, Paz Castro R, Haug S, Schaub MP. Quality of life of parents of mentally-ill children: a systematic review and meta-analysis. Epidemiol Psychiatr Sci. 2019;28(5):563–77. https://doi.org/10.1017/S2045796018000409.
Stabile M, Allin S. The economic costs of childhood disability. Future Child. 2012;22(1):65–96. https://doi.org/10.1353/foc.2012.0008.
Cakir J, Frye RE, Walker SJ. The lifetime social cost of autism: 1990–2029. Res Autism Spectr Disord. 2020;72: 101502.
Santacroce SJ, Tan KR, Killela MK. A systematic scoping review of the recent literature about the costs of illness to parents of children diagnosed with cancer. Eur J Oncol Nurs. 2018;35:22–32.
Tsimicalis A, Stevens B, Ungar WJ, McKeever P, Greenberg M. The cost of childhood cancer from the family’s perspective: a critical review. Pediatr Blood Cancer. 2011;56(5):707–17. https://doi.org/10.1002/pbc.22685.
Bobinac A, van Exel NJA, Rutten FF, Brouwer WB. Caring for and caring about: disentangling the caregiver effect and the family effect. J Health Econ. 2010;29(4):549–56.
Bobinac A, van Exel NJA, Rutten FF, Brouwer WB. Health effects in significant others: separating family and care-giving effects. Med Decis Making. 2011;31(2):292–8. https://doi.org/10.1177/0272989X10374212.
Blacher J, Baker BL. Positive impact of intellectual disability on families. Am J Ment Retard. 2007;112(5):330–48. https://doi.org/10.1352/0895-8017(2007)112[0330:PIOIDO]2.0.CO;2.
Brouwer WB, van Exel NJA, van den Berg B, van den Bos GA, Koopmanschap MA. Process utility from providing informal care: the benefit of caring. Health Policy. 2005;74(1):85–99. https://doi.org/10.1016/j.healthpol.2004.12.008.
Murphy NA, Christian B, Caplin DA, Young PC. The health of caregivers for children with disabilities: caregiver perspectives. Child Care Health Dev. 2007;33(2):180–7. https://doi.org/10.1111/j.1365-2214.2006.00644.x.
Belza C, Avitzur Y, Ungar WJ, Stremler R, Fehlings D, Wales PW. Stress, anxiety, depression, and health-related quality of life in caregivers of children with intestinal failure receiving parenteral nutrition: A cross-sectional survey study. JPEN J Parenter Enteral Nutr. 2023;47(3):342–53. https://doi.org/10.1002/jpen.2461.
Page BF, Hinton L, Harrop E, Vincent C. The challenges of caring for children who require complex medical care at home: ‘The go between for everyone is the parent and as the parent that’s an awful lot of responsibility.’ Health Expect. 2020;23(5):1144–54.
McCann D, Bull R, Winzenberg T. The daily patterns of time use for parents of children with complex needs: a systematic review. J Child Health Care. 2012;16(1):26–52. https://doi.org/10.1177/1367493511420186.
Brouwer WB, van Exel NJA, Tilford JM. Incorporating caregiver and family effects in economic evaluations of child health. In: Ungar W, editor. Economic evaluation in child health. New York: Oxford University Press; 2010. p. 55–76.
Pennington BM. Inclusion of carer health-related quality of life in national institute for health and care excellence appraisals. Value Health. 2020;23(10):1349–57. https://doi.org/10.1016/j.jval.2020.05.017.
Goodrich K, Kaambwa B, Al-Janabi H. The inclusion of informal care in applied economic evaluation: a review. Value Health. 2012;15(6):975–81. https://doi.org/10.1016/j.jval.2012.05.009.
Scope A, Bhadhuri A, Pennington B. Systematic review of cost-utility analyses that have included carer and family member health-related quality of life. Value Health. 2022;25(9):1644–53. https://doi.org/10.1016/j.jval.2022.02.008.
Brouwer WB, Koopmanschap MA. On the economic foundations of CEA. Ladies and gentlemen, take your positions! J Health Econ. 2000;19(4):439–59. https://doi.org/10.1016/s0167-6296(99)00038-7.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. New York: Oxford University Press; 2015.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535. https://doi.org/10.1136/bmj.b2535.
Ungar WJ, Santos MT. Trends in paediatric health economic evaluation: 1980 to 1999. Arch Dis Child. 2004;89(1):26–9.
Ungar WJ, Santos MT. The Pediatric Economic Database Evaluation (PEDE) Project: Establishing a database to study trends in pediatric economic evaluation. Med Care. 2003;41(10):1142–52. https://doi.org/10.1097/01.MLR.0000088451.56688.65.
World Health Organization. International statistical classification of diseases and related health problems 10th revision. 2019 [cited 2021 15 August ]. https://icd.who.int/browse10/2019/en. Accessed 14 May 2021
Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12–8. https://doi.org/10.1111/j.1365-2753.2010.01516.x.
Walker DG, Wilson RF, Sharma R, Bridges J, Niessen L, Bass EB, Frick K. Best practices for conducting economic evaluations in health care: A systematic review of quality assessment tools. Rockville: Agency for Healthcare Research and Quality (US); 2012.
Wijnen B, Van Mastrigt G, Redekop W, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):723–32.
Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, Hay JW. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. https://doi.org/10.18553/jmcp.2003.9.1.53.
Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–5.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Pharmacoeconomics. 2022;40(6):601–9. https://doi.org/10.1007/s40273-021-01112-8.
Watts RD, Li IW. Use of checklists in reviews of health economic evaluations, 2010 to 2018. Value Health. 2019;22(3):377–82. https://doi.org/10.1016/j.jval.2018.10.006.
Chodick G, Waisbourd-Zinman O, Shalev V, Kokia E, Rabinovich M, Ashkenazi S. Potential impact and cost-effectiveness analysis of rotavirus vaccination of children in Israel. Eur J Public Health. 2009;19(3):254–9. https://doi.org/10.1093/eurpub/ckp005.
Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine. 2007;25(20):3971–9. https://doi.org/10.1016/j.vaccine.2007.02.070.
Milne RJ, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value Health. 2009;12(6):888–98. https://doi.org/10.1111/j.1524-4733.2009.00534.x.
Newall AT, Beutels P, Macartney K, Wood J, MacIntyre CR. The cost-effectiveness of rotavirus vaccination in Australia. Vaccine. 2007;25(52):8851–60. https://doi.org/10.1016/j.vaccine.2007.10.009.
Zanganeh M, Adab P, Li B, Pallan M, Liu WJ, Hemming K, et al. Cost-effectiveness of a school-and family-based childhood obesity prevention programme in China: The “CHIRPY DRAGON” cluster-randomised controlled trial. Int J Public Health. 2021;66:1–9.
Beck E, Klint J, Neine M, Garcia S, Meszaros K. Cost-Effectiveness of 4CMenB infant vaccination in England: a comprehensive valuation considering the broad impact of serogroup B invasive meningococcal disease. Value Health. 2021;24(1):91–104. https://doi.org/10.1016/j.jval.2020.09.004.
Christensen H, Trotter CL. Modelling the cost-effectiveness of catch-up ‘MenB’ (Bexsero) vaccination in England. Vaccine. 2017;35(2):208–11. https://doi.org/10.1016/j.vaccine.2016.11.076.
Christensen H, Trotter CL, Hickman M, Edmunds WJ. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014;349: g5725. https://doi.org/10.1136/bmj.g5725.
National Institute of Health and Sciences (NICE). Final evaluation determination: ataluren for treating duchenne muscular dystrophy with a nonsense mutation in the dystrophin gene. 2016. https://www.nice.org.uk/guidance/hst3/documents/final-evaluation-determination-document. Accessed 22 July 2021
National Institute of Health and Sciences (NICE). Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency. 2018. https://www.nice.org.uk/guidance/hst7/documents/final-evaluation-determination-document. Accessed 24 August 2021
Hansen Edwards C, de Blasio BF, Salamanca BV, Flem E. Re-evaluation of the cost-effectiveness and effects of childhood rotavirus vaccination in Norway. PLoS ONE. 2017;12(8): e0183306. https://doi.org/10.1371/journal.pone.0183306.
Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine. 2009;27(30):4025–30. https://doi.org/10.1016/j.vaccine.2009.04.030.
Tilson L, Jit M, Schmitz S, Walsh C, Garvey P, McKeown P, Barry M. Cost-effectiveness of universal rotavirus vaccination in reducing rotavirus gastroenteritis in Ireland. Vaccine. 2011;29(43):7463–73. https://doi.org/10.1016/j.vaccine.2011.07.056.
Tu HA, Rozenbaum MH, Coyte PC, Li SC, Woerdenbag HJ, Postma MJ. Health economics of rotavirus immunization in Vietnam: potentials for favorable cost-effectiveness in developing countries. Vaccine. 2012;30(8):1521–8. https://doi.org/10.1016/j.vaccine.2011.11.052.
Christensen H, Irving T, Koch J, Trotter CL, Ultsch B, Weidemann F, et al. Epidemiological impact and cost-effectiveness of universal vaccination with Bexsero((R)) to reduce meningococcal group B disease in Germany. Vaccine. 2016;34(29):3412–9. https://doi.org/10.1016/j.vaccine.2016.04.004.
Bilcke J, van Hoek AJ, Beutels P. Childhood varicella-zoster virus vaccination in Belgium: cost-effective only in the long run or without exogenous boosting? Hum Vaccin Immunother. 2013;9(4):812–22. https://doi.org/10.4161/hv.23334.
Bilcke J, Van Damme P, Beutels P. Cost-effectiveness of rotavirus vaccination: exploring caregiver(s) and “no medical care” disease impact in Belgium. Med Decis Making. 2009;29(1):33–50. https://doi.org/10.1177/0272989X08324955.
Tu HA, Deeks SL, Morris SK, Strifler L, Crowcroft N, Jamieson FB, et al. Economic evaluation of meningococcal serogroup B childhood vaccination in Ontario, Canada. Vaccine. 2014;32(42):5436–46. https://doi.org/10.1016/j.vaccine.2014.07.096.
National Institute of Health and Sciences (NICE). Final evaluation determination: elosulfase alfa for treating mucopolysaccharidosis type IVa. 2015. https://www.nice.org.uk/guidance/hst2/documents/final-evaluation-determination-document. Accessed 22 July 2021.
National Institute of Health and Sciences (NICE). Final evaluation document: burosumab for treating X-linked hypophosphataemia in children and young people. 2018. https://www.nice.org.uk/guidance/hst8/documents/final-evaluation-determination-document. Accessed 22 July 2021
National Institute of Health and Sciences (NICE). Final appraisal determination: abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis. 2015. https://www.nice.org.uk/guidance/ta373/documents/final-appraisal-determination-document. Accessed 24 Aug 2021.
Richardson JS, Kemper AR, Grosse SD, Lam WKK, Rose AM, Ahmad A, et al. Health and economic outcomes of newborn screening for infantile-onset Pompe disease. Genet Med. 2021;23(4):758–66. https://doi.org/10.1038/s41436-020-01038-0.
O’Brien MA, Prosser LA, Paradise JL, Ray GT, Kulldorff M, Kurs-Lasky M, et al. New vaccines against otitis media: Projected benefits and cost-effectiveness. Pediatrics. 2009;123(6):1452–63. https://doi.org/10.1542/peds.2008-1482.
Prosser LA, Meltzer MI, Fiore A, Epperson S, Bridges CB, Hinrichsen V, Lieu TA. Effects of adverse events on the projected population benefits and cost-effectiveness of using live attenuated influenza vaccine in children aged 6 months to 4 years. Arch Pediatr Adolesc Med. 2011;165(2):112–8. https://doi.org/10.1001/archpediatrics.2010.182.
Prosser LA, Ray GT, O’Brien M, Kleinman K, Santoli J, Lieu TA. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics. 2004;113(2):283–90. https://doi.org/10.1542/peds.113.2.283.
Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83(6):529–36. https://doi.org/10.1212/wnl.0000000000000669.
Senecal M, Brisson M, Lebed M. Burden of rotavirus associated gastroenteritis in Canadian families: a prospective community based study. In: Seventh Canadian Immunization Conference; 2006.
Al-Janabi H, van Exel NJA, Brouwer WB, Trotter C, Glennie L, Hannigan L. New studies of QALY loss in patients and carers; family impact of meningitis and septicaemia. In: Meningitis Research Foundation Biennial Conference. Meningitis and Septicaemia in children and adult London, UK, 2013.
Song J, Floyd FJ, Seltzer MM, Greenberg JS, Hong J. Long-term effects of child death on parents’ health related quality of life: a dyadic analysis. Fam Relat. 2010;59(3):269–82. https://doi.org/10.1111/j.1741-3729.2010.00601.x.
Coyle D, Coyle K, Bettinger JA, Halperin SA, Vaudry W, Scheifele DW, Le Saux N. Cost effectiveness of infant vaccination for rotavirus in Canada. Can J Infect Dis Med Microbiol. 2012;23(2):71–7. https://doi.org/10.1155/2012/327054.
Tew M, De Abreu LR, Gordon JR, Thursky KA, Slavin MA, Babl FA, et al. Cost-effectiveness of home-based care of febrile neutropenia in children with cancer. Pediatr Blood Cancer. 2022;69(7): e29469. https://doi.org/10.1002/pbc.29469.
Chatterton ML, Rapee RM, Catchpool M, Lyneham HJ, Wuthrich V, Hudson JL, et al. Economic evaluation of stepped care for the management of childhood anxiety disorders: results from a randomised trial. Aust N Z J Psychiatry. 2019;53(7):673–82. https://doi.org/10.1177/0004867418823272.
Creswell C, Cruddace S, Gerry S, Gitau R, McIntosh E, Mollison J, et al. Treatment of childhood anxiety disorder in the context of maternal anxiety disorder: a randomised controlled trial and economic analysis. Health Technol Assess. 2015;19(38):1–184. https://doi.org/10.3310/hta19380. (vii-viii).
Schawo S, van der Kolk A, Bouwmans C, Annemans L, Postma M, Buitelaar J, et al. Probabilistic Markov model estimating cost effectiveness of methylphenidate osmotic-release oral system versus immediate-release methylphenidate in children and adolescents: Which information is needed? Pharmacoeconomics. 2015;33(5):489–509.
Tubeuf S, Saloniki EC, Cottrell D. Parental health spillover in cost-effectiveness analysis: evidence from self-harming adolescents in England. Pharmacoeconomics. 2019;37(4):513–30. https://doi.org/10.1007/s40273-018-0722-6.
De Kinderen RJ, Lambrechts DA, Wijnen BF, Postulart D, Aldenkamp AP, Majoie MH, Evers SM. An economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy: an interim analysis. Epilepsia. 2016;57(1):41–50.
Partridge JC, Robertson KR, Rogers EE, Landman GO, Allen AJ, Caughey AB. Resuscitation of neonates at 23 weeks’ gestational age: a cost-effectiveness analysis. J Matern Fetal Neonatal Med. 2015;28(2):121–30. https://doi.org/10.3109/14767058.2014.909803.
Melliez H, Levybruhl D, Boelle PY, Dervaux B, Baron S, Yazdanpanah Y. Cost and cost-effectiveness of childhood vaccination against rotavirus in France. Vaccine. 2008;26(5):706–15. https://doi.org/10.1016/j.vaccine.2007.11.064.
Ulfsdotter M, Lindberg L, Mansdotter A. A cost-effectiveness analysis of the Swedish universal parenting program all children in focus. PLoS ONE. 2015;10(12): e0145201. https://doi.org/10.1371/journal.pone.0145201.
Serrano-Aguilar P, Ramallo-Fariña Y, Trujillo-Martín MDM, Muñoz-Navarro SR, Perestelo-Perez L, De Las C-C. The relationship among mental health status (GHQ-12), health related quality of life (EQ-5D) and health-state utilities in a general population. Epidemiol Psychiatr Soc. 2009;18(3):229–39.
Al-Janabi H, van Exel NJA, Brouwer W, Coast J. A framework for including family health spillovers in economic evaluation. Med Decis Making. 2016;36(2):176–86. https://doi.org/10.1177/0272989x15605094.
Buhmann B, Rainwater L, Schmaus G, Smeeding TM. Equivalence scales, well-being, inequality, and poverty: Sensitivity estimates across ten countries using the Luxembourg Income Study (LIS) database. Rev Income Wealth. 1988;34(2):115–42.
Brouwer WB, Culyer AJ, van Exel NJA, Rutten FF. Welfarism vs. extra-welfarism. J Health Econ. 2008;27(2):325–38. https://doi.org/10.1016/j.jhealeco.2007.07.003.
Lamsal R, Ungar WJ. Impact of growing up with a sibling with a neurodevelopmental disorder on the quality of life of an unaffected sibling: a scoping review. Disabil Rehabil. 2021;43(4):586–94. https://doi.org/10.1080/09638288.2019.1615563.
Cohn LN, Pechlivanoglou P, Lee Y, Mahant S, Orkin J, Marson A, Cohen E. Health outcomes of parents of children with chronic illness: a systematic review and meta-analysis. J Pediatr. 2020;218:166-77e2. https://doi.org/10.1016/j.jpeds.2019.10.068.
Pai AL, Greenley RN, Lewandowski A, Drotar D, Youngstrom E, Peterson CC. A meta-analytic review of the influence of pediatric cancer on parent and family functioning. J Fam Psychol. 2007;21(3):407–15. https://doi.org/10.1037/0893-3200.21.3.407.
Martinez B, Pechlivanoglou P, Meng D, Traubici B, Mahood Q, Korczak D, et al. Clinical health outcomes of siblings of children with chronic conditions: a systematic review and meta-analysis. J Pediatr. 2022;250:83-92e8. https://doi.org/10.1016/j.jpeds.2022.07.002.
Zwicker JD, Lamsal R. Evaluating the economic impact of neurodevelopmental disability intervention on the family and community. In: Eisenstat DD, Goldowitz D, Oberlander TF, Yager, JY editors. Neurodevelopmental pediatrics: genetic and environmental influences. Springer; 2023, p. 773-88.
Al-Janabi H, McLoughlin C, Oyebode J, Efstathiou N, Calvert M. Six mechanisms behind carer wellbeing effects: a qualitative study of healthcare delivery. Soc Sci Med. 2019;235: 112382.
Reichman NE, Corman H, Noonan K. Impact of child disability on the family. Matern Child Health J. 2008;12(6):679–83. https://doi.org/10.1007/s10995-007-0307-z.
Lavelle TA, Wittenberg E, Lamarand K, Prosser LA. Variation in the spillover effects of illness on parents, spouses, and children of the chronically ill. Appl Health Econ Health Policy. 2014;12(2):117–24. https://doi.org/10.1007/s40258-014-0079-8.
Al-Janabi H, van Exel NJA, Brouwer W, Trotter C, Glennie L, Hannigan L, Coast J. Measuring health spillovers for economic evaluation: A case study in meningitis. Health Econ. 2016;25(12):1529–44. https://doi.org/10.1002/hec.3259.
Canaway A, Al-Janabi H, Kinghorn P, Bailey C, Coast J. Close-person spillover in end of life care: using hierarchical mapping to identify whose outcomes to include in economic evaluations. Pharmacoeconomics. 2019;37(4):573–83. https://doi.org/10.1007/s40273-019-00786-5.
Chen F, Liu G. The health implications of grandparents caring for grandchildren in China. J Gerontol B Psychol Sci Soc Sci. 2012;67(1):99–112. https://doi.org/10.1093/geronb/gbr132.
Hastings RP. Grandparents of children with disabilities: a review. Int J Disabil Dev Educ. 1997;44(4):329–40.
Hillman JL, Wentzel MC, Anderson CM. Grandparents’ experience of autism spectrum disorder: identifying primary themes and needs. J Autism Dev Disord. 2017;47(10):2957–68. https://doi.org/10.1007/s10803-017-3211-4.
Wittenberg E, Ritter GA, Prosser LA. Evidence of spillover of illness among household members: EQ-5D scores from a US sample. Med Decis Making. 2013;33(2):235–43. https://doi.org/10.1177/0272989X12464434.
Tsimicalis A, Stevens B, Ungar WJ, Greenberg M, McKeever P, Agha M, et al. Determining the costs of families’ support networks following a child’s cancer diagnosis. Cancer Nurs. 2013;36(2):E8–19. https://doi.org/10.1097/NCC.0b013e3182551562.
Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250.
Wittenberg E, Prosser LA. Disutility of illness for caregivers and families: a systematic review of the literature. Pharmacoeconomics. 2013;31(6):489–500. https://doi.org/10.1007/s40273-013-0040-y.
Awad AG, Voruganti LN. The burden of schizophrenia on caregivers: a review. Pharmacoeconomics. 2008;26(2):149–62. https://doi.org/10.2165/00019053-200826020-00005.
Khanna R, Jariwala K, Bentley JP. Psychometric properties of the EuroQol Five Dimensional Questionnaire (EQ-5D-3L) in caregivers of autistic children. Qual Life Res. 2013;22(10):2909–20. https://doi.org/10.1007/s11136-013-0423-8.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Brouwer WB, van Exel NJA, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res. 2006;15(6):1005–21. https://doi.org/10.1007/s11136-005-5994-6.
Karimi M, Brazier J, Basarir H. The capability approach: a critical review of its application in health economics. Value Health. 2016;19(6):795–9. https://doi.org/10.1016/j.jval.2016.05.006.
Al-Janabi H, Flynn TN, Coast J. QALYs and carers. Pharmacoeconomics. 2011;29(12):1015–23. https://doi.org/10.2165/11593940-000000000-00000.
Lorgelly PK, Lawson KD, Fenwick EA, Briggs AH. Outcome measurement in economic evaluations of public health interventions: a role for the capability approach? Int J Environ Res Public Health. 2010;7(5):2274–89. https://doi.org/10.3390/ijerph7052274.
Dixon P, Round J. Caring for carers: Positive and normative challenges for future research on carer spillover effects in economic evaluation. Value Health. 2019;22(5):549–54. https://doi.org/10.1016/j.jval.2018.10.010.
McCabe C. Expanding the scope of costs and benefits for economic evaluations in health: some words of caution. Pharmacoeconomics. 2019;37(4):457–60.
Brock DW, Daniels N, Neumann PJ, Siegel JE. Ethical and distributive considerations. In: Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 2016. p. 319–42.
Dhanji N, Brouwer W, Donaldson C, Wittenberg E, Al-Janabi H. Estimating an exchange-rate between care-related and health-related quality of life outcomes for economic evaluation: an application of the wellbeing valuation method. Health Econ. 2021;30(11):2847–57.
Al-Janabi H, Wittenberg E, Donaldson C, Brouwer W. The relative value of carer and patient quality of life: a person trade-off (PTO) study. Soc Sci Med. 2022;292: 114556. https://doi.org/10.1016/j.socscimed.2021.114556.
Labelle RJ, Hurley JE. Implications of basing health-care resource allocations on cost-utility analysis in the presence of externalities. J Health Econ. 1992;11(3):259–77. https://doi.org/10.1016/0167-6296(92)90003-j.
Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ. 2005;24(4):751–73.
Ungar WJ. Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated? Pharmacoeconomics. 2011;29(8):641–52. https://doi.org/10.2165/11591570-000000000-00000.
Keren R, Pati S, Feudtner C. The generation gap: differences between children and adults pertinent to economic evaluations of health interventions. Pharmacoeconomics. 2004;22:71–81.
Prosser LA. Current challenges and future research in measuring preferences for pediatric health outcomes. J Pediatr. 2009;155(1):7–9. https://doi.org/10.1016/j.jpeds.2009.03.007.
Bakula DM, Sharkey CM, Perez MN, Espeleta HC, Gamwell KL, Baudino M, et al. The relationship between parent and child distress in pediatric cancer: a meta-analysis. J Pediatr Psychol. 2019;44(10):1121–36.
Ungar WJ. Economic evaluation in child health. New York: Oxford University Press; 2010.
Harrison C, Canadian Paediatric Society, Bioethics Committee. Treatment decisions regarding infants, children and adolescents. Paediatr Child Health. 2004;9(2):99–103.
Anderson D, Dumont S, Jacobs P, Azzaria L. The personal costs of caring for a child with a disability: a review of the literature. Public Health Rep. 2007;122(1):3–16. https://doi.org/10.1177/003335490712200102.
Apps P, Rees R. Household production, full consumption and the costs of children. Lab Econ. 2001;8(6):621–48.
Jacobson L. The family as producer of health—an extended Grossman model. J Health Econ. 2000;19(5):611–37.
Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Anxiety and depression in parents 4–9 years after the loss of a child owing to a malignancy: a population-based follow-up. Psychol Med. 2004;34(8):1431–41. https://doi.org/10.1017/s0033291704002740.
October T, Dryden-Palmer K, Copnell B, Meert KL. Caring for parents after the death of a child. Pediatr Crit Care Med. 2018;19(8S Suppl 2):S61–8. https://doi.org/10.1097/PCC.0000000000001466.
Lichtenthal WG, Corner GW, Sweeney CR, Wiener L, Roberts KE, Baser RE, et al. Mental health services for parents who lost a child to cancer: If we build them, will they come? J Clin Oncol. 2015;33(20):2246.
Dias N, Friebert S, Donelan J, Schoemann AM, Morris A, Guard K, Grossoehme DH. Bereaved parents’ health outcomes following the death of their child. Clin Pract Pediatr Psychol. 2021;9(3):272.
Murphy SA, Lohan J, Braun T, Johnson LC, Cain KC, Beaton RD, Baugher R. Parents’ health, health care utilization, and health behaviors following the violent deaths of their 12- to 28-year-old children: a prospective longitudinal analysis. Death Stud. 1999;23(7):589–616. https://doi.org/10.1080/074811899200795.
Schorr L, Burger A, Hochner H, Calderon R, Manor O, Friedlander Y, et al. Mortality, cancer incidence, and survival in parents after bereavement. Ann Epidemiol. 2016;26(2):115–21. https://doi.org/10.1016/j.annepidem.2015.12.008.
Murphy SA, Johnson LC, Chung IJ, Beaton RD. The prevalence of PTSD following the violent death of a child and predictors of change 5 years later. J Trauma Stress. 2003;16(1):17–25. https://doi.org/10.1023/A:1022003126168.
Murphy SA, Tapper VJ, Johnson LC, Lohan J. Suicide ideation among parents bereaved by the violent deaths of their children. Issues Ment Health Nurs. 2003;24(1):5–25.
Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797–802. https://doi.org/10.1001/archpsyc.60.8.797.
Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407–12. https://doi.org/10.1136/jech.2004.024950.
Heller T, Arnold CK. Siblings of adults with developmental disabilities: psychosocial outcomes, relationships, and future planning. J Policy Pract Intellect Disabil. 2010;7(1):16–25.
Eakin L, Minde K, Hechtman L, Ochs E, Krane E, Bouffard R, et al. The marital and family functioning of adults with ADHD and their spouses. J Atten Disord. 2004;8(1):1–10. https://doi.org/10.1177/108705470400800101.
Benderix Y, Sivberg B. Siblings’ experiences of having a brother or sister with autism and mental retardation: a case study of 14 siblings from five families. J Pediatr Nurs. 2007;22(5):410–8. https://doi.org/10.1016/j.pedn.2007.08.013.
Blacher J., Baker B. L. & Braddock D. L. Family response to disability: the evolution of family adjustment and coping. In: Blacher J, Baker BL, & Braddock, DL, editors. Families and Mental Retardation: A Collection of Notable AAMR Journal Articles Across the 20th Century. American Association on Mental Retardation. Washington. 2002. 35–41.
Tilford JM, Grosse SD, Robbins JM, Pyne JM, Cleves MA, Hobbs CA. Health state preference scores of children with spina bifida and their caregivers. Qual Life Res. 2005;14:1087–98.
Acaster S, Lo SH, Nestler-Parr S. A survey exploring caregiver burden and health-related quality of life in hereditary transthyretin amyloidosis. Orphanet J Rare Dis. 2023;18(1):17. https://doi.org/10.1186/s13023-022-02601-5.
Wu Y, Al-Janabi H, Mallett A, Quinlan C, Scheffer IE, Howell KB, et al. Parental health spillover effects of paediatric rare genetic conditions. Qual Life Res. 2020;29(9):2445–54. https://doi.org/10.1007/s11136-020-02497-3.
Rowley PT, Loader S, Kaplan RM. Prenatal screening for cystic fibrosis carriers: an economic evaluation. Am J Hum Genet. 1998;63(4):1160–74. https://doi.org/10.1086/302042.
Feudtner C, Nye RT, Boyden JY, Schwartz KE, Korn ER, Dewitt AG, et al. Association between children with life-threatening conditions and their parents’ and siblings’ mental and physical health. JAMA Netw Open. 2021;4(12):e-2137250-e.
Pisula E, Porebowicz-Dorsmann A. Family functioning, parenting stress and quality of life in mothers and fathers of Polish children with high functioning autism or Asperger syndrome. PLoS ONE. 2017;12(10): e0186536. https://doi.org/10.1371/journal.pone.0186536.
Grosse SD, Pike J, Soelaeman R, Tilford JM. Quantifying family spillover effects in economic evaluations: measurement and valuation of informal care time. Pharmacoeconomics. 2019;37(4):461–73. https://doi.org/10.1007/s40273-019-00782-9.
Prosser LA, Lamarand K, Gebremariam A, Wittenberg E. Measuring family HRQoL spillover effects using direct health utility assessment. Med Decis Making. 2015;35(1):81–93. https://doi.org/10.1177/0272989X14541328.
Tilford JM, Payakachat N. Progress in measuring family spillover effects for economic evaluations. Expert Rev Pharmacoecon Outcomes Res. 2015;15(2):195–8. https://doi.org/10.1586/14737167.2015.997216.
Leech AA, Lin PJ, D’Cruz B, Parsons SK, Lavelle TA. Family spillover effects: are economic evaluations misrepresenting the value of healthcare interventions to society? Appl Health Econ Health Policy. 2023;21(1):5–10. https://doi.org/10.1007/s40258-022-00755-8.
Lamsal R, Zwicker JD. Economic evaluation of interventions for children with neurodevelopmental disorders: opportunities and challenges. Appl Health Econ Health Policy. 2017;15(6):763–72. https://doi.org/10.1007/s40258-017-0343-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
R.L. was supported by the Hospital for Sick Children through a Restracomp Doctoral Scholarship and by the Institute of Health Policy, Management and Evaluation at the University of Toronto. W.J.U. is supported by the Canada Research Chair in Economic Evaluation and Technology Assessment in Child Health.
Conflict of interest
E.A. Yeh has received research funding from NMSS, CMSC, CIHR, NIH, OIRM, SCN, CBMH Chase an Idea, SickKids Foundation, Rare Diseases Foundation, MS Scientific Foundation, McLaughlin Centre, Leong Center, Peterson Foundation. Investigator initiated research funding from Biogen. Scientific advisory: Hoffman-LaRoche. Speaker honoraria: Biogen, Saudi Epilepsy Society, NYU, MS-ATL; ACRS, PRIME, CNPS. R.L., E.P. and W.J.U. declare that they have no conflict of interest.
Author Contributions
All authors contributed to the study’s conception and design. RL designed and executed the search strategy and conducted the analysis and wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final version for publication.
Ethical Approval
Institutional research ethics board approval was not required as no human or animal data were collected or analyzed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lamsal, R., Yeh, E.A., Pullenayegum, E. et al. A Systematic Review of Methods Used by Pediatric Cost-Utility Analyses to Include Family Spillover Effects. PharmacoEconomics 42, 199–217 (2024). https://doi.org/10.1007/s40273-023-01331-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01331-1