Abstract
Background and Objective
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors improve progression-free survival when combined with endocrine therapies in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. However, the comparative cost effectiveness of utilizing three US Food and Drug Administration-approved CDK4/6 inhibitors is unknown. Therefore, we aimed to evaluate the cost effectiveness of individual CDK4/6 inhibitors (palbociclib, ribociclib, abemaciclib) with letrozole versus letrozole monotherapy in the first-line treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in the USA.
Methods
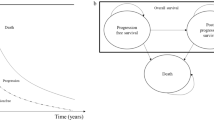
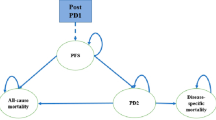
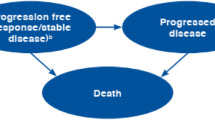
We constructed a Markov-based decision-analytic model to evaluate the cost effectiveness of CDK4/6 inhibitors plus endocrine therapies over a 40-year lifetime from a third-party payer perspective. The model incorporated health states (progression-free disease, progressive disease, and death), major adverse events (neutropenia), and cancer-specific and all-cause mortality. Using clinical efficacy and quality-of-life scores (utility) data from clinical trials, we estimated quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios using Medicare charges reported in US dollars per 2022 valuation and a discount rate of 3% applied to costs and outcomes. We performed deterministic and probabilistic sensitivity analyses to evaluate parametric and decision uncertainty.
Results
Compared to letrozole, the model estimated an increase of 5.72, 5.87, and 6.39 in QALYs and costs of $799,178, $788,168, and $741,102 in combining palbociclib, ribociclib, and abemaciclib plus letrozole, respectively. Palbociclib or ribociclib plus letrozole were dominated by abemaciclib plus letrozole. Compared with letrozole, abemaciclib plus letrozole resulted in an incremental cost-effectiveness ratio of $457,538 per QALY with an incremental cost of $553,621 and an incremental QALY gain of 1.21. The results were sensitive to the cost of abemaciclib, disease progression utility, and patients’ age.
Conclusions
At a willingness to pay of $100,000/QALY gained, our model predicts that combining CDK4/6 inhibitors plus letrozole is not cost effective with a marginal increase in QALYs at a high cost. Lowering the cost of these drugs or identifying patients who can receive maximal benefit from CDK4/6 inhibitors would improve the value of this regimen in patients.


Similar content being viewed by others
References
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020 Dec;31(12):1623–49.
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013 Oct;141(3):507–14.
den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017 Feb;161(3):549–56.
Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017 Jun;26(6):809–15.
Eggersmann TK, Degenhardt T, Gluz O, Wuerstlein R, Harbeck N. CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib. BioDrugs. 2019 Apr;33(2):125–35
Hartkopf AD, Grischke EM, Brucker SY. Endocrine-Resistant Breast Cancer: Mechanisms and Treatment. Breast Care (Basel). 2020 Aug;15(4):347–54.
Nardone A, De Angelis C, Trivedi MV, Osborne CK, Schiff R. The changing role of ER in endocrine resistance. Breast. 2015 Nov;24 Suppl 2:S60–6.
Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020 Mar 7;395(10226):817–27.
Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020 Feb;21(2):250–60.
O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016 Jul;13(7):417–30
Ingham M, Schwartz GK. Cell-Cycle Therapeutics Come of Age. J Clin Oncol. 2017 Sep 1;35(25):2949–59.
Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017 Jan 27;17(2):93–115.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74.
Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016 Apr;6(4):353–67.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015 Feb;14(2):130–46.
Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006 Apr 10;24(11):1770–83.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018 Aug 20;36(24):2465–72.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018 Jul 1;29(7):1541–7
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017 Nov 10;35(32):3638–46.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016 Nov 17;375(20):1925–36.
Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017 Sep 1;35(25):2875–84.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17(4):425–39.
Zhang B, Long EF. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res Treat. 2019 Jun;175(3):775–9.
Wang Y, Rui M, Guan X, Cao Y, Chen P. Cost-Effectiveness Analysis of Abemaciclib Plus Fulvestrant in the Second-Line Treatment of Women With HR+/HER2- Advanced or Metastatic Breast Cancer: A US Payer Perspective. Front Med (Lausanne). 2021;8:658747.
Loke L, Lee SC, Pearce F, Ng K, Aziz MIA. Cost-effectiveness of ribociclib as initial treatment for premenopausal women with advanced breast cancer in Singapore. Cancer Rep (Hoboken). 2021 Feb;4(1):e1308.
Jeong E, Wang C, Wilson L, Zhong L. Cost-Effectiveness of Adding Ribociclib to Endocrine Therapy for Patients With HR-Positive, HER2-Negative Advanced Breast Cancer Among Premenopausal or Perimenopausal Women. Front Oncol. 2021;11:658054.
Giuliani J, Bonetti A. The introduction of a third CDK4/6 inhibitor does not change the cost-effectiveness profile in first and subsequent-lines after progression or relapse during previous endocrine therapy in patients with hormone receptor positive (HR+)/human epidermal receptor-2 negative (HER-2) advanced or metastatic breast cancer. J Oncol Pharm Pract. 2020 Sep;26(6):1486–91
Giuliani J, Bonetti A. Palbociclib or Ribociclib in First-Line Treatment in Patients With Hormone Receptor-Positive/Human Epidermal Receptor 2-Negative Advanced or Metastatic Breast Cancer? A Perspective Based on Pharmacologic Costs. Clin Breast Cancer. 2019 Aug;19(4):e519–e21.
Galve-Calvo E, González-Haba E, Gostkorzewicz J, Martínez I, Pérez-Mitru A. Cost-effectiveness analysis of ribociclib versus palbociclib in the first-line treatment of HR+/HER2- advanced or metastatic breast cancer in Spain. Clinicoecon Outcomes Res. 2018;10:773–90
Matter-Walstra K, Schwenkglenks M, Dedes KJ. Cost-effectiveness of palbociclib plus letrozole versus letrozole alone as a first-line treatment in women with oestrogen receptor-positive, HER2-negative, advanced breast cancer. Revised results for the Swiss health care setting. Breast Cancer Res Treat. 2017 Jun;163(3):635.
Iwata H, Im SA, Masuda N, Im YH, Inoue K, Rai Y, et al. PALOMA-3: Phase III Trial of Fulvestrant With or Without Palbociclib in Premenopausal and Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy-Safety and Efficacy in Asian Patients. J Glob Oncol. 2017 Aug;3(4):289–303.
Finn RS, Rugo HS, Dieras VC, Harbeck N, Im S-A, Gelmon KA, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. Journal of Clinical Oncology. 2022;40(17_suppl):LBA1003-LBA.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006 Sep 18;95(6):683–90.
Mistry R, May JR, Suri G, Young K, Brixner D, Oderda G, et al. Cost-Effectiveness of Ribociclib plus Letrozole Versus Palbociclib plus Letrozole and Letrozole Monotherapy in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: A U.S. Payer Perspective. J Manag Care Spec Pharm. 2018 Jun;24(6):514–23.
Delea TE, Hawkes C, Amonkar MM, Lykopoulos K, Johnston SR. Cost-Effectiveness of Lapatinib plus Letrozole in Post-Menopausal Women with Hormone Receptor-and HER2-Positive Metastatic Breast Cancer. Breast Care (Basel). 2013 Dec;8(6):429–37.
Stokes ME, Muehlenbein CE, Marciniak MD, Faries DE, Motabar S, Gillespie TW, et al. Neutropenia-related costs in patients treated with first-line chemotherapy for advanced non-small cell lung cancer. J Manag Care Pharm. 2009 Oct;15(8):669–82.
Sorensen SV, Goh JW, Pan F, Chen C, Yardley D, Martin M, et al. Incidence-based cost-of-illness model for metastatic breast cancer in the United States. Int J Technol Assess Health Care. 2012 Jan;28(1):12–21.
Hudgens S, Taylor-Strokes G, De Courcy J, Kontoudis I, Tremblay G, Forsythe A, Lloyd A. Real-world evidence on health states utilities in metastatic breast cancer patients: data from a retrospective patient record form study and a cross-sectional patient survey. Value Health. 2016;19(3):A157.
Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006 Jul-Aug;26(4):391–400.
Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015 Jan;16(1):25–35.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016 Nov 3;375(18):1738–48.
Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Hart L, et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. New England Journal of Medicine. 2022;386(10):942–50.
Beauchemin C, Letarte N, Mathurin K, Yelle L, Lachaine J. A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J Med Econ. 2016 Jun;19(6):619–29.
CMS.gov. Centers for Medicare & Medicaid Services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs; Accessed Dec 26, 2022.
US Bureau of Labor Statistics CPIfaucMc. Available at:https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed Dec 26, 2022.
Mamiya H, Tahara RK, Tolaney SM, Choudhry NK, Najafzadeh M. Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol. 2017 Aug 1;28(8):1825–31.
Deshmukh AA, Shirvani SM, Lal L, Swint JM, Cantor SB, Smith BD, et al. Cost-effectiveness Analysis Comparing Conventional, Hypofractionated, and Intraoperative Radiotherapy for Early-Stage Breast Cancer. JNCI: Journal of the National Cancer Institute. 2017;109(11).
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000 May;17(5):479–500.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation: Oup Oxford; 2006.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Medicine. 2022/01/12;20(1):23.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: A Validation-Assessment Tool of Health-Economic Models for Decision Makers and Model Users. Pharmacoeconomics. 2016 Apr;34(4):349–61.
Torres-Guzman R, Calsina B, Hermoso A, Baquero C, Alvarez B, Amat J, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017 Sep 19;8(41):69493–507.
Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol Cancer Ther. 2016 Oct;15(10):2273–81.
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014 Oct;32(5):825–37.
O'Brien N, Conklin D, Beckmann R, Luo T, Chau K, Thomas J, et al. Preclinical Activity of Abemaciclib Alone or in Combination with Antimitotic and Targeted Therapies in Breast Cancer. Mol Cancer Ther. 2018 May;17(5):897–907.
Tate SC, Cai S, Ajamie RT, Burke T, Beckmann RP, Chan EM, et al. Semi-mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin Cancer Res. 2014 Jul 15;20(14):3763–74.
Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019 Oct;20(10):1360–9.
Le V, Zhong L, Narsipur N, Hays E, Tran DK, Rosario K, et al. Cost-effectiveness of ribociclib plus endocrine therapy versus placebo plus endocrine therapy in HR-positive, HER2-negative breast cancer. J Manag Care Spec Pharm. 2021 Mar;27(3):327–38.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016 Sep 13;316(10):1093–103.
Stellato D, Thabane ME, Park J, Chandiwana D, Delea TE. Cost Effectiveness of Ribociclib in Combination with Fulvestrant for the Treatment of Postmenopausal Women with HR+/HER2- Advanced Breast Cancer Who Have Received No or Only One Prior Line of Endocrine Therapy: A Canadian Healthcare Perspective. Pharmacoeconomics. 2021 Sep;39(9):1045–58.
Stellato D, Thabane ME, Chandiwana D, Park J, Delea TE. Cost Effectiveness of Ribociclib Plus a Nonsteroidal Aromatase Inhibitor in Pre-/Perimenopausal, HR+ and HER2- Advanced Breast Cancer: A Canadian Healthcare Perspective. Pharmacoeconomics. 2021 Jul;39(7):853–67.
Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med. 1990 Nov;9(11):1259–76.
Suri G, Chandiwana D, Lee A, Mistry R. Cost-effectiveness analysis of ribociclib plus letrozole versus palbociclib plus letrozole in the United Kingdom. J Health Econ Outcomes Res. 2019;6(2):20–31.
Bonetti A, Giuliani J. Implications of drugs with rebate in Europe. Lancet Reg Health Eur. 2021 Apr;3:100060.
Lieberman SM, Ginsburg PB, Patel KK. Balancing lower U.S. prescription drug prices and innovation: part 1. Health Aff. 2020. https://doi.org/10.1377/forefront.20201123.114048. (Accessed 1 Feb 2023).
Cherla A, Renwick M, Jha A, Mossialos E. Cost-effectiveness of cancer drugs: Comparative analysis of the United States and England. EClinicalMedicine. 2020 Dec;29-30:100625.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship, and/or publication of this article. Meghana Trivedi was supported by National Institutes of Health 1P20CA221729-01A1 and Cancer Prevention and Treatment Institute of Texas RP210148.
Conflicts of Interest/Competing Interests
Prajakta P. Masurkar, Haluk Damgacioglu, Ashish A. Deshmukh, and Meghana Trivedi have no relevant financial or non-financial interests to disclose.
Ethics Approval
Data used for this study were publicly available; therefore, no ethics approval was required.
Consent to Participate
Data used for this study were publicly available; therefore, no informed consent was required.
Consent for Publication
Not applicable.
Availability of Data and Material
All data used for this study are provided in the article or are publicly available. Additional details are available from the corresponding author on request.
Code Availability
Not applicable.
Authors’ Contributions
PPM reviewed the literature, collected data, performed the analysis, and drafted the manuscript. AAD reviewed the study design, analysis, and manuscript. HD reviewed the analysis and manuscript. MVT conceived the study and supervised the literature review, data collection, and manuscript preparation. All authors read and approved the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masurkar, P.P., Damgacioglu, H., Deshmukh, A.A. et al. Cost Effectiveness of CDK4/6 Inhibitors in the First-Line Treatment of HR+/HER2− Metastatic Breast Cancer in Postmenopausal Women in the USA. PharmacoEconomics 41, 709–718 (2023). https://doi.org/10.1007/s40273-023-01245-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01245-y