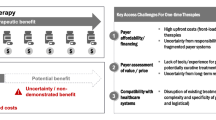
Abstract
Objective
Decision-makers need to resolve constraints on delivering cell and gene therapies to patients as these treatments move into routine care. This study aimed to investigate if, and how, constraints that affect the expected cost and health consequences of cell and gene therapies have been included in published examples of cost-effectiveness analyses (CEAs).
Method
A systematic review identified CEAs of cell and gene therapies. Studies were identified from previous systematic reviews and by searching Medline and Embase until 21 January 2022. Constraints described qualitatively were categorised by theme and summarised by a narrative synthesis. Constraints evaluated in quantitative scenario analyses were appraised by whether they changed the decision to recommend treatment.
Results
Thirty-two CEAs of cell (n = 20) and gene therapies (n = 12) were included. Twenty-one studies described constraints qualitatively (70% cell therapy CEAs; 58% gene therapy CEAs). Qualitative constraints were categorised by four themes: single payment models; long-term affordability; delivery by providers; manufacturing capability. Thirteen studies assessed constraints quantitatively (60% cell therapy CEAs; 8% gene therapy CEAs). Two types of constraint were assessed quantitatively across four jurisdictions (USA, Canada, Singapore, The Netherlands): alternatives to single payment models (n = 9 scenario analyses); improving manufacturing (n = 12 scenario analyses). The impact on decision-making was determined by whether the estimated incremental cost-effectiveness ratios crossed a relevant cost-effectiveness threshold for each jurisdiction (outcome-based payment models: n = 25 threshold comparisons made, 28% decisions changed; improving manufacturing: n = 24 threshold comparisons made, 4% decisions changed).
Conclusion
The net health impact of constraints is vital evidence to help decision-makers scale up the delivery of cell and gene therapies as patient volume increases and more advanced therapy medicinal products are launched. CEAs will be essential to quantify how constraints affect the cost-effectiveness of care, prioritise constraints to be resolved, and establish the value of strategies to implement cell and gene therapies by accounting for their health opportunity cost.

Similar content being viewed by others
References
Ma C, Wang Z, Xu T, He Z, Wei Y. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40(May-June(107502)):1–14.
Wotherspoon L, Buchan R, Morrison E, Amatt G. Evaluation of institutional readiness at sites within the UK NHS using a novel advanced therapy medicinal product assessment tool. Regen Med. 2021;16(3):253–68.
Ogunbayo D. Horizon Scan for Advanced Therapy Medicinal Products. Newcastle upon Tyne: NIHR Innovation Observatory; 2021.
Pillai M, Davies M, Thistlethwaite F. Delivery of adoptive cell therapy in the context of the health-care system in the UK: challenges for clinical sites. Ther Adv Vaccines Immunother. 2020;8(2515135520944355):1–8.
Elverum K, Whitman M. Delivering cellular and gene therapies to patients: solutions for realizing the potential of the next generation of medicine. Gene Ther. 2020;27(12):537–44.
Fenwick E, Claxton K, Sculpher M. The value of implementation and the value of information: combined and uneven development. Med Decis Making. 2008;28(1):21–32.
Sharpe M, Barry J, Kefalas P. Clinical adoption of advanced therapies: challenges and opportunities. J Pharm Sci. 2021;110(5):1877–84.
June C, Riddell S, Schumacher T. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7(280(ps7)):1–8.
High K, Roncarolo M. Gene therapy. N Engl J Med. 2019;381(5):455–64.
Hettle R, Corbett M, Hinde S, Hodgson R, Jones-Diette J, Woolacott N, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204.
Aballéa S, Thokagevistk K, Velikanova R, Simoens S, Annemans L, Antonanzas F, et al. Health economic evaluation of gene replacement therapies: methodological issues and recommendations. J Mark Access Health Policy. 2020;8(1(1822666)):1–16.
ten Ham R, Klungel O, Leufkens H, Frederix G. A review of methodological considerations for economic evaluations of gene therapies and their application in literature. Value Health. 2020;23(9):1268–80.
Drummond M, Neumann P, Sullivan S, Fricke F, Tunis S, Dabbous O, et al. Analytic considerations in applying a general economic evaluation reference case to gene therapy. Value Health. 2019;22(6):661–8.
Hampson G, Towse A, Pearson S, Dreitlein W, Henshall C. Gene therapy: evidence, value and affordability in the US health care system. J Comp Eff Res. 2018;7(1):15–28.
Angelis A, Naci H, Hackshaw A. Recalibrating health technology assessment methods for cell and gene therapies. Pharmacoeconomics. 2020;38(12):1297–308.
Jönsson B, Hampson G, Michaels J, Towse A, von der Schulenburg J, Wong O. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J Health Econ. 2019;20(3):427–38.
Coyle D, Durand-Zaleski I, Farrington J, Garrison L, von der Schulenburg J, Greiner W, et al. HTA methodology and value frameworks for evaluation and policy making for cell and gene therapies. Eur J Health Econ. 2020;21(9):1421–37.
Raymakers A, Regier D, Peacock S. Modelling uncertainty in survival and cost-effectiveness is vital in the era of gene therapies: the case of axicabtagene ciloleucel. Health Policy Technol. 2019;8(2):103–4.
Gavan S, Lu C, Payne K. Assessing the joint value of genomic-based diagnostic tests and gene therapies. J Personal Med. 2019;9(2(28)):1–9.
Kansagra A, Farnia S, Majhail N. Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2020;40(1):e27–34.
Jørgensen J, Mungapen L, Kefalas P. Data collection infrastructure for patient outcomes in the UK—opportunities and challenges for cell and gene therapies launching. J Mark Access Health Policy. 2019;7(1(1573164)):1–13.
Jørgensen J, Kefalas P. The use of innovative payment mechanisms for gene therapies in Europe and the USA. Regen Med. 2021;16(4):405–22.
Ramsay C, Grant A, Wallace S, Garthwaite P, Monk A, Russell I. Statistical assessment of the learning curves of health technologies. Health Technol Assess. 2001;5(12):1–79.
Wright S, Paulden M, Payne K. Implementing interventions with varying marginal cost-effectiveness: an application in precision medicine. Med Decis Making. 2020;40(7):924–38.
Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):1–9.
Wright S, Newman W, Payne K. Accounting for capacity constraints in economic evaluations of precision medicine: a systematic review. Pharmacoeconomics. 2019;37(8):1011–27.
Ho J, Borle K, Dragojlovic N, Dhillon M, Kitchin V, Kopac N, et al. Economic evidence on potentially curative gene therapy products: a systematic literature review. Pharmacoeconomics. 2021;39(9):995–1019.
Lloyd-Williams H, Hughes D. A systematic review of economic evaluations of advanced therapy medicinal products. Br J Clin Pharmacol. 2021;87(6):2428–43.
Centre for Reviews and Dissemination. Search Strategies. 2014. https://www.crd.york.ac.uk/crdweb/searchstrategies.asp. Accessed 20 Jan 2022.
Food and Drug Administration. Approved Cellular and Gene Therapy Products. 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed 21 Jan 2022.
European Medicines Agency. Download medicine data: European public assessment reports (EPAR). 2022. https://www.ema.europa.eu/en/medicines/download-medicine-data#european-public-assessment-reports-(epar)-section. Accessed 21 Jan 2022.
Morgan S, Vogler S, Wagner A. Payers’ experiences with confidential pharmaceutical price discounts: a survey of public and statutory health systems in North America, Europe, and Australasia. Health Policy. 2017;121(4):354–62.
Wang X, Wang Y, Li S, Gkitzia C, Lim S, Koh L, et al. Cost-effectiveness and budget impact analyses of tisagenlecleucel in adult patients with relapsed or refractory diffuse large B-cell lymphoma from Singapore’s private insurance payer’s perspective. J Med Econ. 2021;24(1):637–53.
Qi C, Bollu V, Yang H, Dalal A, Zhang S, Zhang J. Cost-effectiveness analysis of tisagenlecleucel for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma in the United States. Clin Ther. 2021;43(8):1300-19.e8.
Wakase S, Teshima T, Zhang J, Ma Q, Fujita T, Yang H, et al. Cost effectiveness analysis of tisagenlecleucel for the treatment of adult patients with relapsed or refractory diffuse large B cell lymphoma in Japan. Transpl Cell Ther. 2021;27(6):506.e1–.e10.
Cher B, Gan K, Aziz M, Lin L, Hwang W, Poon L, et al. Cost utility analysis of tisagenlecleucel vs salvage chemotherapy in the treatment of relapsed/refractory diffuse large B-cell lymphoma from Singapore’s healthcare system perspective. J Med Econ. 2020;23(11):1321–9.
Liu R, Oluwole O, Diakite I, Botteman M, Snider J, Locke F. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24(1):458–68.
Lin J, Muffly L, Spinner M, Barnes J, Owens D, Goldhaber-Fiebert J. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37(24):2105–19.
Whittington M, McQueen R, Ollendorf D, Kumar V, Chapman R, Tice J, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2(2(e190035)):1–9.
Roth J, Sullivan S, Lin V, Bansal A, Purdum A, Navale L, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238–45.
Wakase S, Teshima T, Zhang J, Ma Q, Watanabe Y, Yang H, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia in Japan. Transpl Cell Ther. 2021;27(3):241.e1–.e11.
Furzer J, Gupta S, Nathan P, Schechter T, Pole J, Krueger J, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020;6(3):393–401.
Santasusana J, Saldaña A, García-Muñoz N, Gostkorzewicz J, Llinàs D, de Heredia C. Cost-effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res. 2020;2020(12):253–64.
Thielen F, van Dongen-Leunis A, Arons A, Ladestein J, Hoogerbrugge P, de Groot C. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-Cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105(2):203–15.
Sarkar R, Gloude N, Schiff D, Murphy J. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–26.
Lin J, Lerman B, Barnes J, Boursiquot B, Tan Y, Robinson A, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–202.
Whittington M, McQueen R, Ollendorf D, Kumar V, Chapman R, Tice J, et al. Long-term survival and value of chimeric antigen receptor T-Cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172(12):1161–8.
Moradi-Lakeh M, Yaghoubi M, Seitz P, Javanbakht M, Brock E. Cost-effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38(6):3427–43.
Simons C, Malone D, Wang M, Maglinte G, Inocencio T, Wade S, et al. Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J Med Econ. 2021;24(1):421–31.
Lindenberg M, Retèl V, Rohaan M, van den Berg J, Haanen J, van Harten W. Evaluating different adoption scenarios for TIL-therapy and the influence on its (early) cost-effectiveness. BMC Cancer. 2020;20(1(712)):1–14.
Retèl V, Steuten L, Foppen M, Mewes J, Lindenberg M, Haanen J, et al. Early cost-effectiveness of tumor infiltrating lymphocytes (TIL) for second line treatment in advanced melanoma: a model-based economic evaluation. BMC Cancer. 2018;18(1(895)).
Gong C, Hay J. Cost-effectiveness analysis of abiraterone and sipuleucel-T in asymptomatic metastatic castration-resistant prostate cancer. J Natl Compr Canc Netw. 2014;12(10):1417–25.
Uhrmann M, Lorenz B, Gisse C. Cost Effectiveness of Voretigene Neparvovec for RPE65-Mediated Inherited Retinal Degeneration in Germany. Transl Vis Sci Technol. 2020;9(9(17)):1–8.
Viriato D, Bennett N, Sidhu R, Hancock E, Lomax H, Trueman D, et al. An economic evaluation of voretigene neparvovec for the treatment of biallelic RPE65-mediated inherited retinal dystrophies in the UK. Adv Ther. 2020;37(3):1233–47.
Johnson S, Buessing M, O’Connell T, Pitluck S, Ciulla T. Cost-effectiveness of voretigene neparvovec-rzyl vs standard care for RPE65-mediated inherited retinal disease. JAMA Ophthalmol. 2019;137(10):1115–23.
Zimmermann M, Lubinga S, Banken R, Rind D, Cramer G, Synnott P, et al. Cost utility of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease. Value Health. 2019;22(2):161–7.
Cook K, Forbes S, Adamski K, Ma J, Chawla A, Garrison L Jr. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia A. J Med Econ. 2020;23(5):501–12.
Machin N, Ragni M, Smith K. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv. 2018;2(14):1792–8.
Shih S, Farrar M, Wiley V, Chambers G. Newborn screening for spinal muscular atrophy with disease-modifying therapies: a cost-effectiveness analysis. J Neurol Neurosurg Psychiatry. 2021;92(12):1296–304.
Broekhoff T, Sweegers C, Krijkamp E, Mantel-Teeuwisse A, Leufkens H, Goettsch W, et al. Early cost-effectiveness of onasemnogene abeparvovec-xioi (zolgensma) and nusinersen (spinraza) treatment for spinal muscular atrophy i in the netherlands with relapse scenarios. Value Health. 2021;24(6):759–69.
Dean R, Jensen I, Cyr P, Miller B, Maru B, Sproule D, et al. An updated cost-utility model for onasemnogene abeparvovec (Zolgensma®) in spinal muscular atrophy type 1 patients and comparison with evaluation by the Institute for Clinical and Effectiveness Review (ICER). J Mark Access Health Policy. 2021;9(1(1889841)):1–12.
Malone D, Dean R, Arjunji R, Jensen I, Cyr P, Miller B, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1(1601484)):1–14.
Kansal A, Reifsnider O, Brand S, Hawkins N, Coughlan A, Li S, et al. Economic evaluation of betibeglogene autotemcel (Beti-cel) gene addition therapy in transfusion-dependent β-thalassemia. J Mark Access Health Policy. 2021;9(1(1922028)):1–13.
Almutairi A, Alkhatib N, Oh M, Curiel-Lewandrowski C, Babiker H, Cranmer L, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155(1):22–8.
Institute for Clinical and Economic Review. 2020–2023 value assessment framework. Boston: Institute for Clinical and Economic Review; 2020.
Binder L, Ghadban M, Sit C, Barnard K. Health technology assessment process for oncology drugs: impact of CADTH changes on public payer reimbursement recommendations. Curr Oncol. 2022;29(3):1514–26.
Zwaap J, Knies S, van der Meijden C, Staal P, van der Heiden L. Cost-effectiveness in practice. Diemen: Zorginstituut Nederland; 2015.
Eisman A, Quanbeck A, Bounthavong M, Panattoni L, Glasgow R. Implementation science issues in understanding, collecting, and using cost estimates: a multi-stakeholder perspective. Implement Sci. 2021;16(1(75)):1–12.
Garrison L Jr, Pauly M, Willke R, Neumann P. An overview of value, perspective, and decision context-a health economics approach: an ISPOR Special Task Force Report [2]. Value Health. 2018;21(2):124–30.
Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(50):1–15.
Sanders G, Neumann P, Basu A, Brock D, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Walker S, Griffin S, Asaria M, Tsuchiya A, Sculpher M. Striving for a societal perspective: a framework for economic evaluations when costs and effects fall on multiple sectors and decision makers. Appl Health Econ Health Policy. 2019;17(5):577–90.
Advanced Therapy Treatment Centres. Working together to accelerate patient access to advanced therapies. 2022. https://www.theattcnetwork.co.uk. Accessed 23 Sep 2022.
Cell and Gene Therapy Catapult. Healthcare system readiness for the adoption of advanced therapies: learnings from the introduction of CAR T cell therapies in the UK. London: Cell and Gene Therapy Catapult; 2021.
Panzar J, Willig R. Economies of scope. Am Econ Rev. 1981;71(2):268–72.
World Health Organization. Everybody’s business: strengthening health systems to improve health outcomes: WHO’s framework for action. Geneva: World Health Organization; 2007.
Hauck K, Morton A, Chalkidou K, Chi Y, Culyer A, Levin C, et al. How can we evaluate the cost-effectiveness of health system strengthening? A typology and illustrations. Soc Sci Med. 2019;220(1):141–9.
Carlson J, Sullivan S, Garrison L, Neumann P, Veenstra D. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy. 2010;96(3):179–90.
Lomas J, Claxton K, Martin S, Soares M. Resolving the “cost-effective but unaffordable” paradox: estimating the health opportunity costs of nonmarginal budget impacts. Value Health. 2018;21(3):266–75.
Salleh S, Thokala P, Brennan A, Hughes R, Dixon S. Discrete event simulation-based resource modelling in health technology assessment. Pharmacoeconomics. 2017;35(10):989–1006.
Paulden M. Calculating and interpreting ICERs and net benefit. Pharmacoeconomics. 2020;38(8):785.
Stinnett A, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(S2):S68–80.
Faria R, Walker S, Whyte S, Dixon S, Palmer S, Sculpher M. How to invest in getting cost-effective technologies into practice? A framework for value of implementation analysis applied to novel oral anticoagulants. Med Decis Making. 2017;37(2):148–61.
Whyte S, Dixon S, Faria R, Walker S, Palmer S, Sculpher M, et al. Estimating the cost-effectiveness of implementation: is sufficient evidence available? Value Health. 2016;19(2):138–44.
Whittington M, McQueen R, Campbell J. Valuing chimeric antigen receptor T-cell therapy: current evidence, uncertainties, and payment implications. J Clin Oncol. 2020;38(4):359–66.
Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Sullivan W, Payne K. The appropriate elicitation of expert opinion in economic models: making expert data fit for purpose. Pharmacoeconomics. 2011;29(6):455–9.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SPG, SJW, and KP. The first draft of the manuscript was written by SPG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sean P. Gavan and Katherine Payne declare that they have no conflict of interest. Stuart J. Wright reports consultancy fees for acting on a steering group for a project investigating the impact of including supply constraints in economic evaluations for PHMR on a project funded by Roche. Fiona Thistlethwaite reports institutional research grant income from Instil Bio; honoraria from GSK, Bristol Myers Squibb, Janssen, and Pfizer; advisory committee membership for Immatics; and speaker fees from Kite/Gilead; is principal investigator for commercial ATMP trials (Adaptimmune, GSK, T-knife Therapeutics, Instil Bio), but does not receive personal payment; and is director of iMATCH (Innovate Manchester Advanced Therapy Centre Hub), supported by Innovate UK.
Funding
This work was supported by Innovate UK (grant number 104234). For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavan, S.P., Wright, S.J., Thistlethwaite, F. et al. Capturing the Impact of Constraints on the Cost-Effectiveness of Cell and Gene Therapies: A Systematic Review. PharmacoEconomics 41, 675–692 (2023). https://doi.org/10.1007/s40273-022-01234-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01234-7