Abstract
Objective
The efficacy and safety of propranolol for retinopathy of prematurity (ROP) remain under debate. This network meta-analysis (NMA) focuses on whether a ranking may be established for different dose levels of propranolol as treatment of ROP in terms of stage progression as the primary outcome, with appearance of plus disease and need for anti-vascular endothelial growth factors (anti-VEGFs) or laser therapy as secondary endpoints.
Methods
Fourteen studies (10 randomised controlled trials, three single-arm trials and one retrospective observational study) of 474 patients treated with oral or ocular propranolol were retrieved from databases up to April 2024. Meta-insight and model-based NMA were undertaken to evaluate the propranolol dose–response relationship. Studies were evaluated for model fit, risk of bias and Confidence of evidence In Network Meta-Analysis (CINeMA). Effect sizes were determined as odds ratio (OR) with 95% credible interval (CrI).
Results
Bayesian analysis showed a trend towards improved effects for propranolol given at late stages (stages 2–3; S23) of ROP progression compared with its administration at earlier stages (stages 0–1; S01). OR values for oral propranolol 1.5 and 2 mg/kg/day given at S23 were 0.13 (95% CrI 0.04–0.37) and 0.16 (95% CrI 0.04–0.61), respectively, while given at S01 were 0.28 (95% CrI 0.02–2.96) and 0.78 (95% CrI 0.14–4.43), respectively. Similarly, OR of eye propranolol 0.2% at S23 was 0.37 (95% CrI 0.09–1.00) versus an S01 OR of 0.64 (95% CrI 0.21–2.04). Surface under the cumulative ranking curve (SUCRA) analyses confirmed best probability values for oral propranolol 1.5–2 mg/kg followed by eye propranolol 0.2%, all at S23. Model-based NMA showed nonlinearity in the dose–response for oral propranolol with a trend to greater maximal effect for its administration at late versus early stages. For secondary endpoints, lower risk values were found with oral propranolol 1.5 mg/kg/day at S23 for progression to plus disease (OR 0.14; 95% CrI 0.02–0.84) and need for anti-VEGFs (OR 0.23; 95% CrI 0.05–0.93) and laser (OR 0.16; 95% CrI 0.02–1.10) therapies also followed by eye propranolol 0.2%, and a similar profile was obtained with SUCRA analysis. Lower doses (0.5–1.0 mg/kg/day) of oral propranolol retained efficacy. Threat of adverse events was estimated as risk difference versus control with no difference for eye propranolol 0.2% and oral propranolol 0.5 mg/kg/day, modest increases of risk for oral propranolol 1.0 and 1.5 mg/kg/day and the highest risk difference for oral propranolol 2.0 mg/kg/day (0.06; 95% CI −0.01 to 0.13).
Conclusion
A diminished risk of disease progression and need for additional treatment was obtained with propranolol in ROP, but safety is a potential concern. Propranolol eye micro-drops (0.2%) can be as efficacious as oral propranolol. Nonetheless, the evidence is limited due to the paucity and quality of the available studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Retinopathy of prematurity (ROP) is a serious neovascular disorder of increasing global burden. Propranolol is effective for ROP but its clinical use remains under debate due to limited evidence. |
Oral propranolol (1.5–2.0 mg/kg/day) has the highest probability of reducing risk of disease progression and need for anti-vascular endothelial growth factor (VEGF) and laser therapies, with a trend towards best values when given at stages 2–3 of ROP according to Bayesian forest and surface under the cumulative ranking curve (SUCRA) analyses and a model-based network meta-analysis. |
Bayesian analysis showed ocular propranolol (0.2%) to be as efficacious as oral propranolol. Oral but not ocular propranolol augmented the risk difference of adverse events. |
1 Introduction
Retinopathy of prematurity (ROP) is a serious neovascular disorder largely observed in preterm infants. It is a multifaceted condition linked to visual impairment which may ultimately lead to childhood blindness [1]. The prevalence and global burden of ROP appear to have been steadily increasing in recent decades, likely due to the rising numbers of extremely immature newborns, showing variable incidence and inequalities between countries [2, 3].
Normal retinal vasculature development is highly sensitive to oxygen dynamics, and premature birth results in untimely exposures of blood vessels to a more oxygen-rich environment, which disrupts their regular progress. ROP is therefore deemed a biphasic disease with initial oxygen-induced suppression of retinal blood vessel growth followed by ischaemic-induced uncontrolled pathological proliferation of retinal vascularisation and fibrous formation driven by activation of the signalling pathways of hypoxia-regulated angiogenic growth factors [4].
Vascular endothelial growth factor (VEGF) is a selective mitogen for endothelial cells that acts as a key element in a chain of events leading to vascular leakage and angiogenesis [4]. The current strategy for treating ROP involves a wait and watch approach until the imminent risk of disease progression mandates the use of procedures such as laser photocoagulation and/or intraocular administration of anti-VEGF agents [5, 6]. Laser is an established primary treatment for ROP and likely works by destroying the hypoxic, VEGF-producing, peripheral retina [7]. Dose-adjusted intravitreal anti-VEGF therapy has demonstrated beneficial effects while avoiding some of the drawbacks of laser [8, 9]. Nonetheless, a growing body of clinical evidence reveals limitations and adverse effects related to laser and intravitreal anti-VEGFs [10], highlighting the need for further research on additional treatments.
Propranolol is a non-selective β-adrenergic receptor blocker with a long-standing clinical use in paediatrics [11]. Following a report on propranolol action in reducing infantile haemangioma [12], the potential effectiveness of this drug on ROP was also proposed, given that both diseases appear pathogenetically related [13]; since then, a number of researchers have contributed with further evidence [11, 14]. Adrenergic receptors modulate VEGF levels with a firmly established role for β2-adrenoceptor in ROP while the importance of the β3-receptors remains under debate. By inhibiting sympathetic excitement, propranolol impairs retinal neoangiogenesis, although some additional activity unrelated to receptor blockade may also be contributing to its therapeutic effect on ROP [14, 15]. Furthermore, unlike established therapies, propranolol can be used not only as a therapeutic option for pre-existing ROP in preterm neonates, but also prophylactically to potentially slow disease progression as an early preventive approach for ROP. Although most clinical research has focused on oral propranolol, other studies have focused on ocular propranolol, which retains clinical efficacy with minimal occurrence of severe adverse events [14].
However, a consensus has not yet been reached on the advantages and risks of using propranolol for ROP therapy, since doubts still remain regarding its precise mechanism of action, assessment of pretreatment hazards and the most suitable dosage, time and route of administration [4, 11, 14, 15]. To address these concerns, we conducted a network meta-analysis (NMA) to compare different doses of oral and ocular propranolol in terms of disease progression, need for additional treatment and adverse events, with the aim of ranking results by different dose levels whenever available. In this work, we retrieved a greater number of studies than a previously published systematic review [11] and four meta-analyses [16,17,18,19] on this topic. These previous reviews were not designed as a network meta-analysis, aggregated the different doses of propranolol for main analysis, and considered only the oral administration route. In this NMA, we examined the results of both oral and ocular delivery of propranolol in the same analysis and addressed the effects of different doses individually, thus avoiding the heterogeneity of treating them together as a pseudo-homogeneous cluster. This enabled broader evidence and effect estimates of interventions to be generated for comparison. Further, we provide new data on probability ranking metrics to establish a hierarchy of different propranolol dose levels from the surface under the cumulative ranking curve (SUCRA) values alongside an approach analysing the dose–effect relationship from a model-based, dose–response NMA within a Bayesian framework. Given the medical relevance of the propranolol dosage and administration route, this analytic approach may serve to inform its clinical use in the arduous decision-making process required to manage this sight-threatening disorder in preterm neonates.
2 Methods
2.1 Literature Search Strategy
This NMA was conducted in accordance with a pre-specified protocol registered with PROSPERO (Prospective Register of Systematic Reviews), with identification number CRD42022353416, which conformed with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) NMA extension statement [20] and recommendations outlined in the Cochrane Handbook (see Electronic Supplementary Material [ESM] 1—PRISMA NMA Checklist).
2.2 Eligibility Criteria and Definition of Outcomes
2.2.1 Study Types
The following criteria were used to add eligible studies: (1) studies including the use of oral or ocular propranolol to protect against ROP in preterm neonates with gestational age under 32 weeks; (2) study designs using randomised controlled trials (RCTs), non-randomised clinical trials and observational studies; and (3) studies that use placebo or a control group of patients. We excluded narrative and systematic reviews, meta-analyses, case reports, letters to the editor, editorials and commentaries. No restrictions were imposed in terms of language, location or journal of publication.
2.2.2 Participant Criteria
Criteria for participants were (1) preterm infants with ROP, (2) diagnosis of ROP after clinical exam and (3) no evidence of other vitreoretinal conditions aside from ROP.
2.2.3 Type of Interventions and Comparators
We divided the interventions into groups according to the different propranolol dose levels and administration routes used for ROP treatment in published studies to find differences in intervention effect estimates and establish the probability that some treatments will perform better than others (a treatment hierarchy). The comparator was either a placebo or a control arm.
2.2.4 Type of Outcome Measures
The primary outcome was stage progression of ROP. Surge of plus disease and need for additional treatment with laser or intravitreal anti-VEGFs were the secondary outcomes. After an initial analysis of the effects of propranolol on stage progression, the remaining analyses separately considered propranolol effects administered at earlier (stages 0 and 1) or later (stages 2 and 3) stages of ROP. Studies were included if they provided data on at least one primary or secondary outcome. Reporting of adverse events was also analysed.
2.3 Study Retrieval and Data Extraction
The databases systematically queried were EMBASE, PubMed, Cochrane Library and Web of Science (WOS). Grey literature databases were also examined. Searches were performed using medical subject headings (MeSH) and free-text terms. The database search was completed on 30 April, 2024. Citation searching was also performed in both included and excluded studies. Two independent reviewers (JLO and AOS) separately screened abstracts then performed full-text assessment and data extraction from eligible studies. Discrepancies were resolved by discussion and consensus with two other authors (EM and PR). The literature was organised using EndNote reference management software (version 21.2). The data extracted from the recruited studies included first author name, year of publication, study design, size of cohorts, patient characteristics (gender, gestational age, birth weight, location and severity of ROP), the intervention and dose, route and duration, outcome, intervention-related adverse events and follow-up. For studies presumably involving the same patient populations, duplication of data was avoided by including only the most complete data set. Search strategies for each database are indicated in ESM 2.
2.4 Data Analysis
Data synthesis and analysis were first performed using the software MetaInsight v5.2.1 [21] as detailed in a previous work [8]. We used the ‘gemtc’ and ‘netmeta’ R packages for the Bayesian and frequentist framework, respectively, and the Markov Chain Monte Carlo method. Effect estimates were synthesised with a Bayesian NMA for disease progression (stage progression and appearance of plus disease) and need for additional treatment (laser or intraocular anti-VEGF agents) outcome measures. A secondary analysis using the frequentist framework was performed, obtaining a league table and forest plot. Given the diversity among studies, all statistical analyses were executed using the random-effects model. The effect size for dichotomous variables was estimated as odds ratio (OR) with 95% credible intervals (CrI). Since OR values cannot be estimated in cases where there are zero events, as is the case for adverse events reporting, absolute risk differences (RD) were calculated instead to accommodate zero counts. Where appropriate, forest plots showing the summary effects of treatments versus comparator, the Litmus Rank-O-Gram alongside a litmus strip of the surface under the cumulative ranking curve (SUCRA) and radial SUCRA plots were also generated. To further examine the dose–effect relationship of oral propranolol, a model-based, network meta-analysis [22, 23] was applied for analysing the effects of the different dose levels of propranolol using the ‘MBNMAdose’ package software in a random effects Bayesian framework [24] performed in R software 4.3.1.
The model fit was evaluated by posterior mean of the residual deviance (Dbar), effective degrees of freedom (pD) and Deviance Information Criterion (DIC) estimates and visualised via the unrelated mean effect model (UME), the stem and leverage plots using the MetaInsight v5.2.1.
2.5 Assessment of Risk of Bias and Confidence
The Cochrane risk-of-bias tool for randomised trials (RoB2) was employed by two independent authors (JLO and AOS) to assess the risk of bias in the eligible studies. The RoB2 tool has five domains of bias assessment: randomisation process, deviation from the intended intervention, missing outcome data, outcome measurement and selective reporting of outcomes. Each study was scored as high risk, low risk or some concerns. For non-randomised studies, we performed the risk-of-bias analysis using the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool. This tool covers six fields: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Between-reviewer discrepancies were resolved through discussion until consensus with a third researcher (PR). For visualising risk-of-bias assessments we used the Risk-Of-Bias VISualization (robvis) package.
The confidence of evidence generated across the trials was estimated using the Confidence in Network Meta-Analysis (CINeMA approach) and performed in the CINeMA app. CINeMA uses six domains to explore confidence in the network result. All the domains (within-study bias, reporting bias, indirectness, imprecision, heterogeneity and incoherence) were rated as ‘no concerns’, ‘some concerns’ or ‘major concerns’ apart from reporting bias, which was reported as ‘suspected’ or ‘undetected’. Within-study bias was estimated by adding the scores of the ROB2 and ROBINS-I judgements. To assess within-study and across-study bias and small-study effects, the online ROB-MEN (Risk Of Bias due to Missing Evidence in Network meta-analysis) tool was used. The final overall judgment on the confidence of evidence for each treatment comparison was rated ‘very low’, ‘low’, ‘moderate’ or ‘high’. For all outcomes, we assessed potential breaches in the transitivity assumption required for NMA by checking the distribution of potential effect modifiers across studies grouped by treatment comparison. To check the consistency assumption, the inconsistency test with the node-splitting model was undertaken using the MetaInsight v5.2.1.
3 Results
3.1 Characteristics of Included Studies
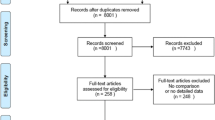
The electronic databases search identified a total of 103 references. Removing duplicated records left 58 articles, whose titles and abstracts were screened for relevance to our research question. This yielded 38 papers, for which full texts were retrieved and assessed for eligibility. Of these, 28 studies failed meet the eligibility criteria and were excluded, while four studies identified from citation searching were added. A total of 14 studies were finally included in the network meta-analysis, of which 10 were RCTs (two double-blind [25, 26], seven randomised but not double-blinded [27,28,29,30,31,32,33] and one randomised pilot study [34]), three were single-arm controlled trials [35,36,37] and one was a retrospective observational study [38] (see Table 1—Characteristics of included studies). One of the RCTs [31] was available only as an abstract. Another RCT [26] supplemented the data of a previous study [30]. The only study available with propranolol 0.1% eye micro-drops [35] was discontinued, so the data of this study were excluded from efficacy analyses. No results from unpublished clinical trials were found in the clinicaltrials.gov, trialsearch.who.int, or clinicaltrialsregister.eu registries. No other records from grey literature databases fulfilled the inclusion criteria. A PRISMA flow diagram summarises the search process and the selection results (Fig. 1—PRISMA flow chart).
This NMA included 474 propranolol-treated preterm infants. Our primary endpoint (stage progression of ROP) was reported in all included studies. The secondary outcomes (appearance of plus disease and need for anti-VEGFs or laser therapy) were reported by 7, 10 and 10 studies, respectively. Demographic data are displayed in Table 1.
3.2 Confidence in Evidence
The quality of the included clinical trials and the observational study is assessed in ESM 3—Risk of bias. Three out of the ten RCTs included had a low risk of bias, six showed some concerns and one was considered high risk. The three non-randomised studies showed moderate risk of bias. The grading of paired comparisons in the network with the CINeMA approach showed low confidence across most estimates (see ESM 4—CINeMA grading), mainly due to the imprecision of most direct and indirect estimates. Assessment of heterogeneity showed a number with “some concerns”, while indirectness showed “no concerns”.
3.3 Network Meta-Analysis of Stage Progression of ROP as the Primary Outcome
3.3.1 Bayesian Analysis
The Bayesian network meta-analysis (Fig. 2) showed that oral propranolol 1.5 and 2.0 mg/kg/day had the lowest OR values, 0.15 (95% CrI 0.05–0.44) and 0.31 (95% CrI 0.09–0.99), respectively, statistically significant versus control, followed by propranolol 1.0 and 0.5 mg/kg/day with OR values approaching yet not reaching significance. The OR value for propranolol 0.2% eye micro-drops (144 μg/eye) was 0.35 (95% CrI 0.07–1.11), close to the OR values of oral propranolol but failing to reach statistical significance.
Effects of propranolol on the stage progression of retinopathy of prematurity (ROP) as the primary outcome. The upper panels (a–d) correspond to effects of propranolol analysed without separation by stages of disease progression, and the lower panels (e–h) indicate results for separate analyses of propranolol given at earlier (stages 0 to 1) vs later stages (stages 2 and 3) of ROP. Panels a and e: Network plot. Number on the lines indicate the number of studies conducted for each comparison. Panels b and f: Forest plot depicting the effect estimates (odds ratio) for stage progression with the corresponding 95% credible intervals (95% CrI) for each treatment compared with placebo/control (Plc). Panels c and g: The Litmus Rank-O-Gram presents a cumulative rank-o-gram alongside a litmus strip of the Surface Under the Cumulative RAnking curve (SUCRA) values. Highest values and cumulative curves correspond to a better position in the ranking and are situated nearer the top. Panels d and h: Radial SUCRA plots. Size of the nodes represent number of patients and thickness of lines indicate number of studies conducted. Abbreviations for propranolol treatments: D_02: 0.2% eye micro-drops; O_05: oral 0.5 mg/kg/day; O_10: oral 1.0 mg/kg/day; O_15: oral 1.5 mg/kg/day; O_20: oral 2.0 mg/kg/day; S01_D_02: eye drops 0.2% given at stages 0–1; S01_O_10: oral 1.0 mg/kg/day given at stages 0–1; S01_O_15: oral 1.5 mg/kg/day given at stages 0–1; S01_O_20: oral 2.0 mg/kg/day given at stages 0–1; S23_D_02: eye drops 0.2% given at stages 2–3; S23_O_05: oral 0.5 mg/kg/day given at stages 2–3; S23_O_10: oral 1.0 mg/kg/day given at stages 2–3; S23_O_15: oral 1.5 mg/kg/day given at stages 2–3; S23_O_20: oral 2.0 mg/kg/day given at stages 2–3
3.3.2 SUCRA Ranking
Consistent with the data on the forest plot, the SUCRA values (Fig. 2) showed that oral propranolol 1.5 mg/kg/day ranks best followed by a cluster composed of oral propranolol 0.5 and 2 mg/kg/day and eye propranolol 0.2%, and in turn these appear somewhat better than oral propranolol 1 mg/kg/day.
3.3.3 Model Fit
The computed measures of fit (Dbar 25.226, pD 22.161, DIC 47.387) indicate a good model performance in the Bayesian analysis. The UME residual deviance plots, stem and leverage plots are included in ESM 5: Model fit assessment and outlier detection. The goodness of fit is indicated by inspecting these plots, showing small residual deviance and good leverage.
3.3.4 Frequentist Analysis
The Bayesian analysis was complemented with frequentist analysis, and the OR values for stage progression were found to be mostly comparable with those of Bayesian analysis with an OR of 0.42 (95% confidence interval [CI] 0.18–0.96) for ocular propranolol 0.2% reaching statistical significance. The forest plot and league table are provided in ESM 6: Frequentist NMA.
3.3.5 Propranolol at Early Versus Late Stages
The preceding analyses of the stage progression after different oral and ocular doses of propranolol did not differentiate between effects of propranolol given at early stages (stages 0 and 1; ‘S01’) of ROP compared with later stages (stages 2 and 3; ‘S23’) of disease progression. The lower row of panels in Fig. 2 shows the network plot and the results for Bayesian analysis of the effects of propranolol on the rate of stage progression with this segregation. There was a trend to obtain greater effect sizes for propranolol given at S23 in comparison to S01. Thus, the OR values for oral propranolol 1.5 and 2 mg/kg/day given at S23 were 0.13 (95% CrI 0.04–0.37) and 0.16 (95% CrI 0.04–0.61), respectively, while given at S01 were 0.28 (95% CrI 0.02–2.96) and 0.78 (95% CrI 0.14–4.43), respectively. A similar tendency was observed for eye propranolol 0.2% with an OR at S23 of 0.37 (95% CrI 0.09–1.00) versus an S01 OR value of 0.64 (95% CrI 0.21–2.04). The same general disposition was obtained with SUCRA plots in which best probability values were obtained for drug administration at S23 versus S01, particularly for oral propranolol 1.5 and 2.0 mg/kg/day, which ranked above the cluster conformed by oral propranolol 1.0 and 0.5 mg/kg/day and eye propranolol 0.2%. Oral propranolol 1.5 mg/kg/day at S01 ranked better than the other interventions at early stages. Computed measures of fit (Dbar 33.382, pD 29.914 and DIC 63.296) as well as the UME residual deviance plots, stem and leverage plots indicated a good model performance (ESM 5: Model fit assessment and outlier detection). Frequentist analysis displayed values comparable to those obtained for the Bayesian approach with eye propranolol 0.2% reaching significance at S23 (OR 0.50; 95% CI 0.28–0.89). The forest plot and league table are provided in ESM 6: Frequentist NMA.
3.4 Network Meta-Analysis of the ROP Progression to Plus Disease and the Need For Anti-VEGF Agents or Laser Therapy as Secondary Outcomes
Given the observed trend of differences in propranolol effects on stage progression when administered at earlier compared with later stages of ROP, the analyses for secondary outcomes were carried out following the same scheme, as displayed in Fig. 3.
Effects of propranolol on the secondary outcomes. Upper (a–c), middle (d–f) and lower (g–i) panels correspond to analysis of plus disease appearance, need for treatment with an anti-VEGF agent, and requirement for laser therapy, respectively. Panels on the left side show the corresponding network plots; middle panels show forest plots with odds ratio and 95% credible intervals (CrI) compared with placebo/control (Plc); and panels on the right side show the Litmus Rank-O-Gram with a litmus strip of the Surface Under the Cumulative RAnking curve (SUCRA) values. Values nearer the top represent a better ranking position. Abbreviations for propranolol treatments: D_02: 0.2% eye micro-drops; O_05: oral 0.5 mg/kg/day; O_10: oral 1.0 mg/kg/day; O_15: oral 1.5 mg/kg/day; O_20: oral 2.0 mg/kg/day; S01_D_02: eye drops 0.2% given at stages 0–1; S01_O_10: oral 1.0 mg/kg/day given at stages 0–1; S01_O_15: oral 1.5 mg/kg/day given at stages 0–1; S01_O_20: oral 2.0 mg/kg/day given at stages 0–1; S23_D_02: eye drops 0.2% given at stages 2–3; S23_O_05: oral 0.5 mg/kg/day given at stages 2–3; S23_O_10: oral 1.0 mg/kg/day given at stages 2–3; S23_O_15: oral 1.5 mg/kg/day given at stages 2–3; S23_O_20: oral 2.0 mg/kg/day given at stages 2–3
3.4.1 Plus Disease
Results for Bayesian analysis showed a significant beneficial effect only for oral propranolol 1.5 mg/kg/day (OR 0.14; 95% CrI 0.02–0.84) at S23. The same profile was obtained in the SUCRA ranking, in which the best probability value was obtained for 1.5 mg/kg/day of oral propranolol at S23. Computed measures of fit (Dbar 14.944; pD 14.521; DIC 29.465), UME residual deviance plots, stem and leverage plots indicated a good model performance (ESM 5: Model fit assessment and outlier detection). Frequentist analysis displayed OR values comparable to those obtained for the Bayesian approach, with significance confirmed for oral propranolol 1.5 mg/kg/day at S23 but also attained by 2.0 mg/kg/day of oral propranolol (OR 0.30; 95% CI 0.10–0.87) as well as by eye propranolol 0.2% (OR 0.43; 95% CI 0.23–0.80) at S23 (forest plot and league table are provided in ESM 6: Frequentist NMA).
3.4.2 Anti-VEGF
A significant decrease in the need for anti-VEGF treatment was achieved for 1.5 mg/kg/day of oral propranolol (OR 0.23; 95% CrI 0.05–0.93) at S23. A cluster of good probability values was obtained in the SUCRA ranking for 1.5 and 2.0 mg/kg/day of oral propranolol at S23 along with oral propranolol 1.0 mg/kg/day at S01 followed by eye propranolol 0.2% at S23. Computed measures of fit (Dbar 15.615, pD 14.383, DIC 29.998), UME residual deviance plots, stem and leverage plots indicated a good model performance (ESM 5: Model fit assessment and outlier detection). Frequentist analysis displayed OR values comparable to those obtained for the Bayesian approach with significance confirmed for oral propranolol 1.5 mg/kg/day at S23, but no other intervention reached statistical significance (forest plot and league table are provided in ESM 6: Frequentist NMA).
3.4.3 Laser
Results for Bayesian analysis showed no statistically significant differences among interventions. A cluster of good probability values was obtained in the SUCRA ranking for 0.5, 1.5 and 2.0 mg/kg/day of oral propranolol followed by eye propranolol 0.2%, all given at S23. Computed measures of fit (Dbar 24.972, pD 21.319, DIC 46.291), UME residual deviance plots, stem and leverage plots indicated a good model performance (ESM 5: Model fit assessment and outlier detection). Frequentist analysis displayed OR values comparable to those obtained for the Bayesian approach with significance attained for oral propranolol 1.5 (OR 0.19; 95% CI 0.05–0.72) and 2.0 (OR 0.25; 95% CI 0.10–0.65) mg/kg/day at S23 as well as for eye propranolol 0.2% at S23 (OR 0.41; 95% CI 0.20–0.86) with no other intervention reaching statistical significance (forest plot and league table are provided in ESM 6: Frequentist NMA).
3.5 Model-Based, Dose–Response, Network Meta-Analysis
Given that the results obtained with oral propranolol in decreasing the risk of stage progression indicated a dose–response association, we further analysed the dose–effect relationship with the MBNMAdose software programme. This package was applied to the data obtained for the effects of the different doses of oral propranolol administered at early (S01) and late (S23) stages of ROP in reducing the rate for stage progression as the primary outcome. This dataset was selected since it is derived from all the studies included in this meta-analysis. This model-based, dose–response NMA was not carried out with data from ocular propranolol because efficacy information was limited to one dose point only.
Following the established procedure in this package, we first viewed a ‘split’ NMA in which each dose is examined as unrelated to each other, not assuming any dose–response association. This analysis showed that no studies were disconnected at the dose level with good model fit (pD 21.9, DIC 127.7). Next, the MBNMAdose was applied to calculate model predictions. Figure 4 shows the predicted dose–response function plotted with the observed data overlaid. The tighter 95% CrI on the predicted MBNNMA line indicates a reasonable modelling not yielding a poorer fit (pD 17.0, DIC 120.1) as required. Thus, the dose–response prediction plots exhibited nonlinearities with an apparent plateauing within the predicted variation in the exposure response. The predicted maximal effect for oral propranolol was greater when given at late stages (S23) compared with early (S01) stages administration with a difference in magnitude nearly reaching statistical significance (OR 0.16; 95% CrI 0.08–0.29 and OR 0.61; 95% CrI 0.26–1.34, respectively).
Dose–response relationship for oral propranolol on the primary outcome (stage progression of retinopathy of prematurity [ROP]) plotted with the model-based, network meta-analysis (MBNMA) software programme. The upper panels represent the observed effects for the different doses of propranolol in reducing the rate of stage progression. The x-axis represents the dose (μg/kg/day) and the y-axis shows the effect size of the observed responses (odds ratio [OR] with 95% credible interval [CrI] as natural logarithm). Lower panels represent the dose–response prediction plots. The solid lines represent the mean predictions as a function of dose of oral propranolol. Shaded areas represent the 95% CrI. The embed points represent the mean observed values with their variation. Oral propranolol was administered at early (stages 0 and 1) and late (stages 2 and 3) stages of progression of ROP as indicated by the respective legends O-S01 and O-S23 at the upper part of each of the panels
3.6 Analysis of Adverse Events
Several of the included studies with oral propranolol recorded adverse events in the intervention and control cohorts [25,26,27,28, 30, 31, 34], while others reported no adverse effects [29, 32, 33, 38]. The adverse events disclosed in the oral propranolol arm comprised death, apnoea, increased ventilator need, bradycardia, hypotension, hypoglycaemia, bronchospasm, viral infection and insufficient weight gain. Some of these adverse events were also reported in the untreated arm. In the three studies with propranolol eye micro-drops [35,36,37], neither local nor severe adverse events related to propranolol were observed. Since OR values are not quantifiable where there are zero adverse events, risk differences (RD) were calculated instead to enable comparative intervention effect estimates for all studies, thus including the largest possible dataset as shown in the network plot (Fig. 5). The forest plot shows that only eye propranolol 0.2% and oral propranolol 0.5 mg/kg/day had no risk difference with respect to control while positive differences were obtained for higher doses of oral propranolol versus control, with the highest risk difference found for 2.0 mg/kg/day (RD 0.06; 95% CI −0.01 to 0.13). Nevertheless, these values failed to reach statistical significance. The corresponding league table is shown in ESM 6.
Risk differences for adverse events after propranolol. Left panel: Network plot of included studies. Right panel: Frequentist forest plot showing pairwise risk differences versus placebo/control (Plc) for total counts of adverse events. Effect estimates are shown with their 95% confidence intervals (95% CI)
4 Discussion
The pathogenic similarities between infantile haemangioma and ROP, alongside promising results from preclinical and clinical studies, have prompted the use of propranolol in the management of ROP. Subsequently, one systematic review [11] and four systematic review and meta-analyses [16,17,18,19] have summarised the oral effects of propranolol, supporting its clinical application as a preventive treatment in preterm newborns with ROP. Nevertheless, there is a lack of consensus on the true clinical relevance of this use of oral propranolol due to limited evidence on the most appropriate dosage and duration, time and route of administration and because of lack of data on long-term outcomes, particularly in relation to visual impairment and other potentially serious harmful effects [4].
This work is the first study to use network meta-analytical methodology to analyse the effects of propranolol on ROP. In the analyses, we included data from direct ocular administration of propranolol not considered in previous meta-analyses. This NMA was fully comprehensive, including ten randomised clinical trials [25,26,27,28,29,30,31,32,33,34], three non-randomised clinical trials [35,36,37] and one observational study [38], with updated published information about propranolol in ROP. Our work also stands out from previous meta-analyses in that we have examined each dose tested in clinical studies as separate interventions. Moreover, we aimed to provide estimates of intervention hierarchies for different propranolol doses and administration routes at different stages of disease progression. One advantage of the NMA technique is that it can quantitatively address both direct and indirect evidence regarding the different interventions, thus strengthening inferences about their relative efficacy. This is pivotal, since all the available evidence on propranolol effects comes from direct comparisons against placebo or control cohorts.
Stage progression of ROP is commonly taken as a primary measure of the relative efficacy of interventions, together with the appearance of signs indicating severe ROP (such as the emergence of plus disease and the need for anti-VEGF or laser therapy) that have been considered as secondary outcomes. In primary outcome analysis, without discriminating between early versus late-stage propranolol administration, the best position on forest and SUCRA plots was at 1.5 mg/kg/day of oral propranolol, followed by a cluster formed by 0.5 and 2.0 mg/kg/day oral propranolol and 0.2% eye propranolol. When a separate analysis was carried out distinguishing propranolol administration at early stages (stages 0 and 1) from later stages (stages 2 and 3) of disease progression, the highest ranked interventions were oral propranolol 1.5–2 mg/kg/day followed by oral propranolol 0.5–1 mg/kg/day and eye propranolol 0.2%, all given at later stages with the exception of oral propranolol 1.5 mg/kg/day given at early stages. An overview of these results indicate that oral and ocular propranolol show a tendency to be more efficacious in reducing risk of stage progression when given at later stages compared with their administration at earlier stages. A subgroup analysis by Kong et al. [18] failed to find significant differences in clinical effects at different oral doses of propranolol, but the present study shows that a probabilistic rank order for oral and ocular doses of propranolol may be established by means of Bayesian forest plot and SUCRA analysis. These results are also consistent with a subgroup analysis by stages made by Kong et al. [18] as well as with those from the separate analyses of primary and secondary prevention with propranolol performed by Kaempfen et al. [16] and Stritzke et al. [17]. Hence, propranolol seems possibly more effective on disease progression, particularly when given at later stages in concert with the notion that this drug primarily affects the VEGF levels overexpressed during the proliferative phase of ROP. Our study further contributed to this concept since the Bayesian forest and SUCRA analyses positioned oral and ocular propranolol doses given at later stages as potentially better treatments. By contrast, oral and ocular propranolol delivered at earlier stages were generally located at the lower probability side of the ranking.
Since these results indicated the existence of an apparent dose–response association, we further explored this relationship with a model-based network meta-analysis under a Bayesian framework. This procedure confirmed a non-linear dose–response functionality, with the effects of oral propranolol rising in parallel with increasing doses until a plateau was reached at 1.5–2.0 mg/kg/day according to the predicted maximal effect. Indeed, 1.5 mg appears to be a dose capable of producing a maximal or near maximal effect. Although oral propranolol doses of up to 3 mg/kg/day are used for medical management of infantile haemangioma [39], this high dose seems unlikely to result in greater clinical impact on ROP progression but rather represents a precautionary overtreatment.
The secondary outcomes analysed in this study are also commonly used indicators in ROP management. The highest probability of decreasing the risk of progression to plus disease was shown in forest and SUCRA plots to be patients with stages 2 or 3 treated with oral propranolol 1.5 mg/kg/day, with the next cluster formed by oral propranolol 2.0 mg/kg/day and eye propranolol 0.2%, both at stages 2–3. Regarding the need for treatment with anti-VEGF agents or laser when ROP worsens, the better positions are also taken by the same interventions mentioned for plus disease. As an overview on the efficacy of propranolol for primary and secondary outcomes, oral doses of 1.5 and 2.0 mg/kg/day of propranolol followed by propranolol 0.2% eye micro-drops given at later stages showed the highest likelihood of being discretely superior to the other doses studied and were consistently found to be a preferable option. Nevertheless, congruent with the subgroup analysis of Kong et al. [18], lower oral doses of propranolol were also efficacious, as observed in the forest and SUCRA plots.
The extent and variety of all harm outcomes reported after propranolol dosage in ROP are relatively low, particularly as regards ocular administration of this drug [11, 14]. Overall, medical use of propranolol for ROP showed reasonable safety and tolerability, although its systemic dosing may not be sufficiently safe, particularly at high doses. Some included studies reported no adverse effects for propranolol [29, 32, 33, 38]. Others recorded a number of adverse events [25,26,27,28, 30, 31, 34], most of which were likely related to propranolol (bradycardia, hypotension, hypoglycaemia, bronchospasm, apnoea, increased ventilator need, augmented serum potassium), although others potentially were not (cataract, retinal haemorrhage, insufficient weight gain, infections and sepsis); nonetheless, many of these events also occurred in the untreated group. Premature infants typically have several comorbidities and a high risk of associated complications, which may enhance the appearance of unwanted effects. Previous meta-analyses found that the relative risk of total adverse events in propranolol-treated patients was about twice that of untreated premature newborns [18, 19]. The incidence of death was not significantly different between treated and control groups, nor were other particular side effects. These analyses have a limited number of comparisons, as quantifying relative risk is not feasible for studies that report no events. Calculating absolute risk difference instead enabled a more comprehensive analysis revealing a trend towards propranolol dose-related total adverse events, although statistical significance was not reached. Most studies in the analysis were not designed to specifically visualised adverse effects associated with propranolol; only one [34] declared safety evaluation as its main objective, a few include both safety and efficacy in equal terms [29, 32, 35, 36] and the others were clearly focused on efficacy as their main endpoint. Understandably, further well-designed controlled clinical trials are advisable to pinpoint the potential undesirable effects of propranolol in this particular population of unstable newborns, its drug interactions and the optimal protocol for its administration in clinical practice.
Our study has a number of limitations. A key weakness arises from the dearth of clinical studies combined with a certain narrowness in the demographic diversity of the study cohorts, skewing results mainly toward Asia and the Middle East. This geographical bias means that extrapolation of findings should be exercised with caution and variability in patient populations and differences in clinical practices between countries should be considered. Furthermore, combining randomised controlled trials, single-arm trials and an observational study resulted in a heterogenous overall population and accompanying risk of confounding factors. Only two of the clinical trials were double-blinded, several were not randomised, in some the therapeutic option was unmasked and some studies used a historical control group. To minimise this heterogeneity, we used a random-effect model. Another limitation is the grouping of different stages for analysis, since conducting a separate analysis for each ROP stage would lead to an insufficiently connected network due to the paucity of studies available, not all of which provided separate data for different stages. In this way, considering stages 0 and 1 together may have resulted in added variability within this grouping. Nevertheless, recent data from tear VEGF levels in progressing ROP show a different VEGF profile for controls and stage 1 compared with stages 2 and 3 [40], although this is an indirect biomarker. As previously mentioned, the risk–benefit safety analysis of propranolol for treating ROP in this vulnerable population was hindered by the lack of detailed reporting of propranolol-related adverse events. Future research should prioritise robust adverse event monitoring with extended follow-up observation periods to detect detrimental delayed-onset events and ensure patient safety.
5 Conclusion
This systematic review and network meta-analysis investigated the efficacy and safety of propranolol in the treatment of ROP in preterm neonates. The risk of disease progression and need for additional treatment with anti-VEGFs and/or laser were significantly lower after either oral or ocular propranolol compared with controls. A tendency to better responses against disease progression was noticed when propranolol was given at later compared with earlier ROP stages according to available data from the included studies. However, this particular finding requires skilled interpretation due to the limitations of the study, highlighting a need to align propranolol dosage with VEGF dynamics. Doses currently reported for oral (0.5–2.0 mg/kg/day) and ocular (0.2%, 144 μg/eye) propranolol appear to produce sound responses with little room for further improvement at higher oral doses according to the predictions of the model-based, dose-response, network meta-analysis. Nevertheless, nuances in the rank order of interventions need to be interpreted with caution. The demonstrated efficacy of propranolol at the strategically appropriate time of administration validates it as an alternative to more invasive options, though attention must be paid to potential safety concerns. Nevertheless, the number of clinical studies with propranolol on ROP is considerably lower than that of other drug treatments, and best practices in its application in this setting have yet to be ascertained. Additional efforts to achieve demographic diversity in further well-designed clinical trials will improve our understanding of the position of propranolol in managing ROP.
References
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–57. https://doi.org/10.1016/S0140-6736(13)60178-6.
Chen J, Zhu Y, Li L, Lv J, Li Z, Chen X, Chen X, Huang S, Xie R, Zhang Y, Ye G, Luo R, Shen X, Fu M, Zhuo Y. Visual impairment burden in retinopathy of prematurity: trends, inequalities, and improvement gaps. Eur J Pediatr. 2024. https://doi.org/10.1007/s00431-024-05450-5.
Scarboro SD, Harper CA 3rd, Karsaliya G, Ghoraba H, Moshfeghi DM, Al-Khersan H, Robles-Holmes HK, Fan KC, Berrocal AM, Hoyek S, Patel NA, Sbrocca R, Capone A Jr, Drenser KA, Wood EH. The incidence and timing of treatment-requiring retinopathy of prematurity in nanopremature and micropremature infants in the United States: a national multicenter retrospective cohort study. Ophthalmol Retina. 2024;8(3):279–87. https://doi.org/10.1016/j.oret.2023.10.003.
Zhang L, Buonfiglio F, Fiess A, Pfeiffer N, Gericke A. Retinopathy of prematurity-targeting hypoxic and redox signaling pathways. Antioxidants (Basel). 2024;13(2):148. https://doi.org/10.3390/antiox13020148.
Bancalari A, Schade R. Update in the treatment of retinopathy of prematurity. Am J Perinatol. 2022;39(1):22–30. https://doi.org/10.1055/s-0040-1713181.
Tsai AS, Chou HD, Ling XC, Al-Khaled T, Valikodath N, Cole E, Yap VL, Chiang MF, Chan RVP, Wu WC. Assessment and management of retinopathy of prematurity in the era of anti-vascular endothelial growth factor (VEGF). Prog Retin Eye Res. 2022;88: 101018. https://doi.org/10.1016/j.preteyeres.2021.101018.
McNamara JA. Laser treatment for retinopathy of prematurity. Curr Opin Ophthalmol. 1993;4(3):76–80. https://doi.org/10.1097/00055735-199306000-00013.
Ortiz-Seller A, Martorell P, Barranco H, Pascual-Camps I, Morcillo E, Ortiz JL. Comparison of different agents and doses of anti-vascular endothelial growth factors (aflibercept, bevacizumab, conbercept, ranibizumab) versus laser for retinopathy of prematurity: a network meta-analysis. Surv Ophthalmol. 2024. https://doi.org/10.1016/j.survophthal.2024.02.005.
Zhang C, Reynolds AL, Beiter A, Lillvis JH, Reynolds JD. Effect of low-dose intravitreal bevacizumab and ranibizumab on regression and late reactivation in retinopathy of prematurity in the treatment-naive eyes. Ophthalmol Retina. 2022;6(4):328–30. https://doi.org/10.1016/j.oret.2021.12.016.
Tsiropoulos GN, Seliniotaki AK, Haidich AB, Ziakas N, Mataftsi A. Comparison of adverse events between intravitreal anti-VEGF and laser photocoagulation for treatment-requiring retinopathy of prematurity: a systematic review. Int Ophthalmol. 2023;43(3):1027–62. https://doi.org/10.1007/s10792-022-02480-6.
Bührer C, Bassler D. Oral propranolol: a new treatment for infants with retinopathy of prematurity? Neonatology. 2015;108(1):49–52. https://doi.org/10.1159/000381659.
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–51. https://doi.org/10.1056/NEJMc0708819.
Praveen V, Vidavalur R, Rosenkrantz TS, Hussain N. Infantile hemangiomas and retinopathy of prematurity: possible association. Pediatrics. 2009;123(3):e484-489. https://doi.org/10.1542/peds.2007-0803.
Pascarella F, Scaramuzzo RT, Pini A, Cammalleri M, Bagnoli P, Ciantelli M, Filippi L. Propranolol: a new pharmacologic approach to counter retinopathy of prematurity progression. Front Pediatr. 2024;12:1322783. https://doi.org/10.3389/fped.2024.1322783.
Filippi L, Cammalleri M, Amato R, Ciantelli M, Pini A, Bagnoli P, Dal Monte M. Decoupling oxygen tension from retinal vascularization as a new perspective for management of retinopathy of prematurity. New opportunities from beta-adrenoceptors. Front Pharmacol. 2022;13: 835771. https://doi.org/10.3389/fphar.2022.835771.
Kaempfen S, Neumann RP, Jost K, Schulzke SM. Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev. 2018;3(3): CD011893. https://doi.org/10.1002/14651858.CD011893.pub2.
Stritzke A, Kabra N, Kaur S, Robertson HL, Lodha A. Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol. 2019;39(12):1584–94. https://doi.org/10.1038/s41372-019-0503-x.
Kong HB, Zheng GY, He BM, Zhang Y, Zhou Q. Clinical efficacy and safety of propranolol in the prevention and treatment of retinopathy of prematurity: a meta-analysis of randomized controlled trials. Front Pediatr. 2021;9: 631673. https://doi.org/10.3389/fped.2021.631673.
Shafique MA, Haseeb A, Uddin MMN, Asghar B, Chaudhry ER, Raqib MA, Ali SMS, Mustafa MS. Effectiveness of propranolol in preventing severe retinopathy of prematurity: a comprehensive systematic review and meta-analysis. Am J Ophthalmol. 2024;259:141–50. https://doi.org/10.1016/j.ajo.2023.11.012.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385.
Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10(4):569–81. https://doi.org/10.1002/jrsm.1373.
Mawdsley D, Bennetts M, Dias S, Boucher M, Welton NJ. Model-based network meta-analysis: a framework for evidence synthesis of clinical trial data. CPT Pharmacometr Syst Pharmacol. 2016;5(8):393–401. https://doi.org/10.1002/psp4.12091.
Gallardo-Gómez D, Del Pozo-Cruz J, Noetel M, Álvarez-Barbosa F, Alfonso-Rosa RM, Del Pozo CB. Optimal dose and type of exercise to improve cognitive function in older adults: a systematic review and bayesian model-based network meta-analysis of RCTs. Ageing Res Rev. 2022;76: 101591. https://doi.org/10.1016/j.arr.2022.101591.
Pedder H. MBNMAdose: dose–response model-based network meta-analysis. The comprehensive R archive network; 2022. https://doi.org/10.5281/zenodo.8221452
Sanghvi KP, Kabra NS, Padhi P, Singh U, Dash SK, Avasthi BS. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial). Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F389–94. https://doi.org/10.1136/archdischild-2016-311548.
Ozturk MA, Korkmaz L. The efficacy of propranolol in very preterm infants at the risk of retinopathy of prematurity: which newborn and when? Int Ophthalmol. 2019;39(9):1921–30. https://doi.org/10.1007/s10792-018-1018-8.
Mirjalili A, Manaviat MR, Noori-Shadkam M. Evaluation of oral propranolol effect on retinopathy of prematurity: randomized clinical trial. World J Peri Neonatol. 2019;2(1):37–43. https://doi.org/10.18502/wjpn.v2i1.2810.
Hosseini M, Gharebaghi MM, Bagheri M, Yeganehdoost S, Milani AE, Niyousha MR. Efficacy of oral propranolol on the treatment of retinopathy of prematurity. Crescent J Med Biol Sci. 2022;9(1):56–62. https://doi.org/10.34172/cjmb.2022.10.
Makhoul IR, Peleg O, Miller B, Bar-Oz B, Kochavi O, Mechoulam H, Mezer E, Ulanovsky I, Smolkin T, Yahalom C, Khoury A, Lorber A, Nir A, Blazer S. Oral propranolol versus placebo for retinopathy of prematurity: a pilot, randomised, double-blind prospective study. Arch Dis Child. 2013;98(7):565–7. https://doi.org/10.1136/archdischild-2013-303951.
Korkmaz L, Baştuğ O, Ozdemir A, Korkut S, Karaca C, Akin MA, Gunes T, Kurtoglu S, Ozturk MA. The Efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Curr Eye Res. 2017;42(1):88–97. https://doi.org/10.3109/02713683.2016.1158272.
Bancalari A. Treatment of retinopathy of prematurity with propranolol: a randomized control trial. Toronto, Canada: Pediatric Academic Sciences; 2018.
Sun H, Li M, Yu Z, Xing S, Yuan M, Cheng P. The efficacy and safety of oral propranolol in the treatment of retinopathy of prematurity. Chin J Neonatol. 2018;6:266–70.
Mohammadi P, Babaei H, Mohsenpour H, Azadi P. Efficacy of oral propranolol in prevention of severe retinopathy of prematurity: a randomized clinical trial study. Iran J Neonatol. 2023;14(1):1–7. https://doi.org/10.22038/ijn.2023.70115.2361.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Donzelli G, Tinelli F, Araimo G, Cristofori G, La Marca G, Della Bona ML, La Torre A, Fortunato P, Furlanetto S, Osnaghi S, Mosca F. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr. 2013;163(6):1570-1577 e1576. https://doi.org/10.1016/j.jpeds.2013.07.049.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Berti E, Padrini L, Donzelli G, Araimo G, Cristofori G, Fumagalli M, la Marca G, Della Bona ML, Pasqualetti R, Fortunato P, Osnaghi S, Tomasini B, Vanni M, Calvani AM, Milani S, Cortinovis I, Pugi A, Agosti M, Mosca F. Propranolol 0.1% eye micro-drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr Res. 2017;81(2):307–14. https://doi.org/10.1038/pr.2016.230.
Filippi L, Cavallaro G, Berti E, Padrini L, Araimo G, Regiroli G, Raffaeli G, Bozzetti V, Tagliabue P, Tomasini B, Mori A, Buonocore G, Agosti M, Bossi A, Chirico G, Aversa S, Fortunato P, Osnaghi S, Cavallotti B, Suzani M, Vanni M, Borsari G, Donati S, Nascimbeni G, Nardo D, Piermarocchi S, la Marca G, Forni G, Milani S, Cortinovis I, Calvani M, Bagnoli P, Dal Monte M, Calvani AM, Pugi A, Villamor E, Donzelli G, Mosca F. Propranolol 0.2% eye micro-drops for retinopathy of prematurity: a prospective phase IIB study. Front Pediatr. 2019;7:180. https://doi.org/10.3389/fped.2019.00180.
Scaramuzzo RT, Bagnoli P, Dal Monte M, Cammalleri M, Pini A, Ballini S, Bendinelli A, Desideri I, Ciantelli M, Filippi L. Treating infants with 0.2% propranolol eye micro-drops drastically reduced the progression of retinopathy of prematurity. Acta Paediatr. 2023;112(9):1905–6. https://doi.org/10.1111/apa.16850.
Bancalari A, Schade R, Munoz T, Lazcano C, Parada R, Pena R. Oral propranolol in early stages of retinopathy of prematurity. J Perinat Med. 2016;44(5):499–503. https://doi.org/10.1515/jpm-2015-0357.
Colmant C, Powell J. Medical management of infantile hemangiomas: an update. Paediatr Drugs. 2022;24(1):29–43. https://doi.org/10.1007/s40272-021-00477-9.
Murugeswari P, Vinekar A, Prakalapakorn SG, Anandula VR, Subramani M, Vaidya TA, Nair AP, Jayadev C, Ghosh A, Kumaramanickavel G, Shetty R, Das D. Correlation between tear levels of vascular endothelial growth factor and vitamin D at retinopathy of prematurity stages in preterm infants. Sci Rep. 2023;13(1):16175. https://doi.org/10.1038/s41598-023-43338-w.
Acknowledgements
The authors would like to acknowledge research assistance from the technical staff of INCLIVA and the University of Valencia.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare that no funds, grants, or other financial support were received during the preparation of this manuscript.
Conflict of Interest
The authors (AO, PM, PR, EM, JLO) declare no financial or non-financial interests that are directly or indirectly related to this work.
Ethics Approval
Not applicable for meta-analyses.
Consent to Participate
Not applicable for meta-analyses.
Consent for Publication
Not applicable for meta-analyses.
Availability of Data and Material
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Methodology, software: JLO, AOS, PM. Material preparation and data acquisition: JLO, AOS. Data analysis and interpretation: JLO, AOS, EM, PR. First draft of manuscript: EM and JLO. All authors commented on the succeeding versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ortiz-Seller, A., Martorell, P., Roselló, P. et al. Comparison of Different Doses of Oral and Ocular Propranolol for Retinopathy of Prematurity: A Network Meta-Analysis. Pediatr Drugs 26, 499–518 (2024). https://doi.org/10.1007/s40272-024-00647-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-024-00647-5