Abstract
Given the heterogenous etiology of pediatric heart failure (pHF), evidence-based studies improving pHF are unlikely. A paradigm shift towards updated medicine-based evidence is therefore necessary. In view of the life expectancy of children, cardiac regeneration strategies are required. Therefore, age- and disease-related differences in myocardial (receptor) physiology require individualized precision medicine. First-line diuretic therapy, adopted from the treatment of adults with HF with no chance for recovery, should be questioned in the treatment of pHF with potential for recovery. Inadequate use of diuretics is a common reason for additional stimulation of the neurohumoral axis. Consecutive intravascular volume depletion led to an inadequate treatment with β-blocker and renin–angiotensin–aldosterone antagonists. Given the age-related catecholamine-driven cardiovascular (patho-) physiology, highly selective β1-blockers (bisoprolol) protect against β1-(noradrenaline)-related myocytic apoptosis and necrosis, but allow β2-receptor-mediated myocardial regeneration. Based on its high safety–efficacy profile with rarely seen adverse effects but easily monitorable efficacy by the surrogate of heart rate (reduction), bisoprolol is our first-line drug in infancy. Reduced heart rate economizes the heart and full body oxygen consumption and extends the diastolic filling and coronary perfusion time. Based on our many years of institutional experience, physicians should be encouraged to use β1-selected blockers in infants with dilated cardiomyopathy and hypoplastic left heart syndrome after stage-1 procedure, but also to treat ventricular septal defects with a significant left-to-right shunt. In summary, individualized pHF therapy is the prerequisite for a causal treatment to improve HF symptoms, but above all for the most functional regeneration possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Improving pediatric heart failure (pHF) therapy requires a paradigm shift from ‘evidence-based medicine’ to ‘medicine-based evidence’. |
The age-dependent catecholamine-controlled cardiovascular system and the activated neurohumoral axis during pHF, in particular the catecholamine cascade, make an age- and disease-related β-blocker therapy overdue. |
Based on the review article by Weisert et al. [1] and critical reading of other recently published articles [2, 3], evaluating all cardiovascular drugs for the treatment of pediatric heart failure (pHF) led me to ask “but, how concretely can pHF therapy be improved?” These reviews nicely summarize cardiovascular drugs that are currently available or will be approved in the near future. However, the works also show why little progress has been made in drug therapy for pHF in recent decades. Drugs with cardioprotective properties such as the differentiated use of β-blockers and ACE inhibitors are questioned for their use in children due to a lack of evidence. However, the same does not apply to first-line therapy with diuretics, despite the fact that evidence has always been lacking. Therefore, in the interests of especially young HF patients, a paradigm shift from the fixation on ‘evidence-based medicine’ (EbM) to updated ‘medicine-based evidence’ (MbE) is urgently needed. There is no time to wait any longer for pediatric EbM data. They will almost never become available and if they do, then ultimately only ‘background’ knowledge that is already known from adult medicine with large case numbers will be allowed. In my view, clinicians need detailed instructions for individualized or personalized treatment based on current pathophysiology and pharmacology knowledge. This is particularly important when treating young patients with heterogeneous pHF diseases but with a chance of recovery [4]. The effectiveness of drugs that counteract the neurohumoral response in HF, in particular the catecholamine cascade by β-blockers (βB), has long been known [5]. A detailed treatment strategy with βBs, which would correspond to sophisticated use as cardioprotective and regeneration promoting agents, has been neglected or even questioned. Therefore, knowledge is already available for more than just a differentiated use of βBs [6, 7].
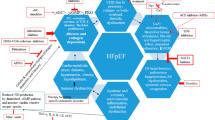
Based on our more than 15 years of institutional experience [7,8,9,10,11], it is time to recommend physicians preferentially use selective β1-blocker therapy in three clinical scenarios of HF in infants with (a) ventricular septal defect (VSD) with a significant left-to-right shunt; (b) hypoplastic left heart syndrome (HLHS) during the interstage after stage 1 procedure (S1P); and (c) dilative cardiomyopathy (DCM), also in connection with the application of a pulmonary arterial banding (PAB) for forcing functional regeneration of left ventricular (LV)-DCM if right ventricular (RV) function is preserved. The extent of heart rate reduction to the lowest effective level is an important indicator of the effect on ventricular function and myocardial and whole-body economization. Thereby, it can be easily monitored. The only contraindications to βB are cardiogenic shock, symptomatic bradycardia and second/third degree heart block. The benefits of comprehensive disease-modifying therapies, particularly for young patients with HF with reduced ejection fraction (EF), are summarized as a practical example (Fig. 1). For decision making in infants with DCM referred for end-stage treatment, or inquiries as to whether PAB is an option for treating the affected young child with DCM, the following basic information is required: a brief summary of the patient’s medical history (age, weight, functional class); cardiac morphology and function (LV-EF, left ventricular end-diastolic diameter [LVEDD] with z score, extent of mitral valve regurgitation, if present, and residual RV function); and some hemodynamic data, especially heart rate under the current overall treatment, not just the heart-specific drug therapy. It remains a mystery why such a simple and well established medical strategy as summarized in Fig. 1 is so problematic to establish. Also, the risk of placing a PAB is low because it is inversely correlated with the patient’s age. There was no procedural mortality at any of the participating centers in the LV-DCM registry [11]. Infants can grow into the PAB, there is also no need to make the PAB too tight. However, if you consider that the regenerative potentials decrease dramatically, every week counts. Waiting for a spontaneous recovery makes no sense if the LVEDD has already exceeded a certain level (z score + 5). Therefore, the protective and regenerative aspects of pHF treatment in infancy should be considered when cardiomyocytes are at their peak in mitosis and cytokinesis [14]. Reducing β-adrenergic signaling is a cornerstone to avoid cytokinesis failure [15]. In addition, the adrenergic receptors of an insufficient heart must be re-differentiated, at least from the point of view of age and disease [16, 17]. The β1 and β2 adrenoceptor distribution, the expression pattern and in particular receptor-specific stimulatory and inhibitory signaling pathways should be taken into account. From a clinical point of view, this is most possible on the basis of MbE. It is therefore also a matter of perspective how ‘end-stage’ is defined in pHF. Patients with ventriculo-ventricular interaction (RV compression) caused by LV-DCM, as shown in Fig. 1, are almost typical for infants and quite different from older children and young adults. However, the chances of recovery are so enormous that it is time to integrate the peculiarities of age and disease [18] into a thoughtful treatment strategy that includes concomitant drug treatment [7,8,9,10,11,12,13]. ‘Heart failure’ is not a syndrome, but a sequence of symptoms. The continued goal of obtaining data analogous to large cohort studies in adults remains unrealistic for pHF, especially if a single drug is to be analyzed with regard to an endpoint such as mortality.
Echocardiographic (ECHO) four-chamber view of a 4-month-old infant with left ventricular dilated cardiomyopathy and preserved right ventricular function is shown. The consecutive pathophysiology can easily be deduced. Briefly, the four-chamber view is from a 4-month-old infant (4.9 kg body weight) who was referred for heart transplantation, but also with the question about being a candidate for pulmonary arterial banding (PAB). The medication for the infant, who was intubated and continuously ventilated at this time, consisted of continuous infusion of epinephrine (0.075 µg/kg/min), milrinone (1 µg/kg/min), nitroprusside-natrium (15 µg/kg/min) and intermittent applications of furosemide (6 × 4 mg), hydrochlorothiazide (2 × 5 mg), spironolactone 2 × 5 mg. The resting heart rate was 170/min. Interpretation of the ECHO image (banana-shaped RV, collapsed inferior caval vein, non-congestive LA) immediately revealed that the young patient was overtreated with intubation, ventilation, sedation, diuretics and catecholamines. Upon recommendation, epinephrine and nitroprusside were discontinued after a volume challenge of at least 50 mL (10 mL/kg) Ringer® solution to avoid hypotension. After extubation, oral treatment was given with bisoprolol (B) in a single dose of 1 × 0.6125 mg (1/4 of 2.5-mg tablet) followed by lisinopril (L) in the same dose of 1 × 0.615 mg when volume deficit was definitively ruled out. Spironolactone (S) was administered once daily at a non-diuretic dosage of 1 × 6.25 mg. Clonidine 3–6 ×/day (1–2 µg/kg per dosage) was continued orally due to weaning from previous continuous analgo-sedation and its additional heart rate lowering effect. Oral feeding of the spontaneously breathing baby became possible. Upon admission at our center, the resting heart rate was < 120/min. Continuous infusion of milrinone and oral application of B-L-S were maintained. After decision to PAB, levosimendan (0.1 µg/kg/min) without a loading dose was additionally infused for 24 h, beginning 12 h prior to PAB placement. This is consistent with our institutional protocol [9], including differential use of cardiovascular drugs in DCM-induced pHF in infancy [7, 12]. The indication for PAB was based on age, normal RV function (TAPSE 12 mm) and the distinct left-right displacement of the interventricular septum shift by the ‘apple-shaped’ dilated left ventricle (LV) with a z score of the LVEDD > 5. In relation to the low chance (< 10%) of spontaneous DCM regeneration, infants in high-income countries are listed for heart transplantation (HTX) with or without the additional use of an assist device [13]. Here, the patient showed a functional regeneration after 6 months of follow-up care. IAS interatrial septum, LA left atrium, LV left ventricle, LV-DCM left ventricular dilated cardiomyopathy, LVEDD left ventricular end-diastolic diameter (z score), rASD restrictive atrial septum defect, RV right ventricle, TAPSE tricuspid annular plane systolic excursion
Either the pHF patients included are too heterogenous in term of diseases, age and functional class or the number of homogeneous and comparably severely diseased patient cohorts is too small. A randomized, double-blind study design alone cannot form the basis for EbM. The inclusion of a clearly defined homogenous patient group is a must for an EbM study. If, despite a random design, the results of an incomplete, non-homogeneous ‘evidence’ design are negative or indifferent, as shown in the carvedilol or enalapril studies [19, 20], this is particularly problematic because the studies are permanently classified as EbM. The results of the two pediatric randomized studies led to an exaggerated ‘discrediting’ of the two drug groups investigated, β-blockers for the treatment of severe pHF and enalapril in failing single ventricle after stage-II or stage-III surgery [21]. Based on these data, it has been postulated that adult data cannot be extrapolated from adults to children [22]. This may apply to some pharmacokinetic aspects, but not to the indication for blocking the neurohumoral overreaction, which is also and especially evident with pHF. In addition, the indication for targeted drug treatment to support cardioprotective, age-related and possibly even cardio-regenerative goals is made. With significant heart failure in children, it is almost impossible that one drug class alone could alter mortality, whether due to the blockade of the adrenergic, the renin-angiotensin or neuropeptide system. However, it becomes problematic when such protective drugs are not used, but drugs are tested in children [23] with unanswered questions about possible long-term side effects, such as the inhibition of neprilysin, an important enzyme that prevents β-amyloid deposits in the brain [24].
Nevertheless, an answer is needed for the current pHF patients who are being treated now. Especially in the field of pediatric cardiology, the switch from EbM to updated MbE is long overdue. The necessary age- and disease-specific physiological and pharmacological knowledge has existed for a long time and is constantly being expanded. An international platform that enables the exchange of knowledge and data as a basis for MbE strategies could serve as control. Experience in pediatric cardiac surgery is a strong example [25]. The history of surgery for congenital heart defects is based on risk-taking innovations. The lineage of survivalism largely goes back to pioneers such as Blalock, Lillihei, Gibbon, Senning, Mustard, Jatene, Rastelli, Fontan, Norwood, Castaneda etc., who made sophisticated surgery a success story. Today it could be summarized as an MbE strategy or personalized medicine. They realized that the risk of doing nothing was unbearable for the suffering patients. Gradual success was based on experience and mastery of a variety of surgical procedures.
After all, what would be the consequence if pediatric EbM studies were to confirm the effectiveness of β-blockers, ACE inhibitors or mineralocorticoid-receptor blockers again, and in principle for children as well as adult studies? Nevertheless, a targeted design of drug therapy with regard to the disease, age and the individualized therapeutic goal that depends on it, as shown in Fig. 1 for an infant with DCM, would still be absolutely necessary. The question also remains open as to whether a palliative measure (organ replacement) or an accompanying strategy for functional regeneration should be the goal of the pHF therapy, and whether a drug should be used for prophylactic purposes or to protect against harmful side effects, as is the case with transient myocardial stimulating treatment with catecholamines in DCM.
In conclusion, given the dominant physiological cardiovascular regulation by circulating catecholamines, particularly in newborns and young infants, and the role of the neurohumoral response in pHF, triple D therapy (diuretics, digoxin, with fluid restriction, diet) should no longer be first-line use in chronic treatment. It is time to implement age- and disease-specific differentiated β-blocker therapy for all forms of pHF. In addition, ACE-inhibitor treatment promises to work additively and even synergistically with β-blocker therapy; monotherapy in infants, on the other hand, is not recommended [26]. Taking drug-specific contraindications into account, combination therapy is efficient in young patients with symptomatic dilated cardiomyopathy or in HLHS patients in the interstage after S1P, both as a drug to preserve the myocardium and to balance pulmonary to systemic blood flow, and in infants with a hemodynamically relevant ventricular septal defect [27, 28]. The pediatric cardiologist might even take on a pacemaker role by focusing the individualized pHF treatment on the exceptional regenerative potential of the (young) heart, rather than following adult strategies by using diuretics as first-line drugs, particularly for chronic pHF therapy.
References
Weisert M, Su JA, Menteer J, Shaddy RE, Kantor PF. Drug treatment of heart failure in children: gaps and opportunities. Paediatr Drugs. 2022. https://doi.org/10.1007/s40272-021-00485-9 (Epub ahead of print, PMID: 35084696).
Loss KL, Shaddy RE, Kantor PF. Recent and upcoming drug therapies for pediatric heart failure. Front Pediatr. 2021;11(9): 681224. https://doi.org/10.3389/fped.2021.681224 (PMID: 34858897; PMCID: PMC8632454).
Ahmed H, VanderPluym C. Medical management of pediatric heart failure. Cardiovasc Diagn Ther. 2021;11(1):323–35. https://doi.org/10.21037/cdt-20-358 (PMID: 33708503; PMCID: PMC7944205).
Chen S, Dykes JC, McElhinney DB, Gajarski RJ, Shin AY, Hollander SA, et al. Hemodynamic profiles of children with end-stage heart failure. Eur Heart J. 2017;38(38):2900–9. https://doi.org/10.1093/eurheartj/ehx456 (PMID: 29019615).
Bhatt AS, DeVore AD, DeWald TA, Swedberg K, Mentz RJ. Achieving a maximally tolerated β-blocker dose in heart failure patients: is there room for improvement? J Am Coll Cardiol. 2017;69(20):2542–50. https://doi.org/10.1016/j.jacc.2017.03.563 (PMID: 28521892).
Buchhorn R. Beta-blocker therapy in pediatric heart failure: 50 years lost to improve pharmacotherapy of a deadly disease. Ann Pediatr Cardiol. 2021;14(3):341–2. https://doi.org/10.4103/apc.apc_126_21 (Epub 2021 Aug 26, PMID: 34667405; PMCID: PMC8457278).
Schranz D, Voelkel NF. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr. 2016;175(4):445–55. https://doi.org/10.1007/s00431-016-2700-3.
Mienert T, Esmaeili A, Steinbrenner B, Khalil M, Müller M, Akintuerk H, et al. Cardio-vascular drug therapy during interstage after hybrid approach: a single-center experience in 51 newborns with hypoplastic left heart. Pediatr Drugs. 2021;23(2):195–202. https://doi.org/10.1007/s40272-021-00438-2 (Epub 2021 Mar 13, PMID: 33713024).
Schranz D, Recla S, Malcic I, Kerst G, Mini N, Akintuerk H. Pulmonary artery banding in dilative cardiomyopathy of young children: review and protocol based on the current knowledge. Transl Pediatr. 2019;8(2):151–60. https://doi.org/10.21037/tp.2019.04.09 (PMID: 31161082; PMCID: PMC6514280).
Schranz D, Rupp S, Müller M, Schmidt D, Bauer A, Valeske K, et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: a novel therapeutic strategy before heart transplantation. J Heart Lung Transplant. 2013;32(5):475–81.
Schranz D, Akintuerk H, Bailey L. Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: world network report. Circulation. 2018;137(13):1410–2. https://doi.org/10.1161/CIRCULATIONAHA.117.029360.
Schranz D. Pharmacological heart failure therapy in children: focus on inotropic support. Handb Exp Pharmacol. 2020;261:177–92. https://doi.org/10.1007/164_2019_267 (PMID: 31707469).
Conway J, Cantor R, Koehl D, Spicer R, Gupta D, McCulloch M, et al. Survival after heart transplant listing for infants on mechanical circulatory support. J Am Heart Assoc. 2020;9(21):e011890. https://doi.org/10.1161/JAHA.118.011890 (Epub 2020 Oct 20, Erratum in: J Am Heart Assoc. 2020 Dec 15;9(24):e014641, PMID: 33076747; PMCID: PMC7763397).
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA. 2013;110:1446–51.
Yutzey KE. Cytokinesis, beta-blockers, and congenital heart disease. N Engl J Med. 2020;382(3):291–3.
Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35:33–41 (PubMed: 22843448).
Nicin L, Abplanalp WT, Schänzer A, Sprengel A, John D, Mellentin H, et al. Single nuclei sequencing reveals novel insights into the regulation of cellular signatures in children with dilated cardiomyopathy. Circulation. 2021;143(17):1704–19.
Traister A, Patel R, Huang A, et al. Cardiac regenerative capacity is age- and disease-dependent in childhood heart disease. PLoS ONE. 2018;13: e0200342.
Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–9.
Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Pediatric Heart Network I. Enalapril in infants with single ventricle: Results of a multicenter randomized trial. Circulation. 2010;122:333–40 (PubMed: 20625111).
Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29(12):1535–52. https://doi.org/10.1016/j.cjca.2013.08.008.
Rossano JW, Shaddy RE. Update on pharmacological heart failure therapies in children: do adult medications work in children and if not, why not? Circulation. 2014;129(5):607–12. https://doi.org/10.1161/CIRCULATIONAHA.113.003615.
Shaddy R, Canter C, Halnon N, Kochilas L, Rossano J, Bonnet D, Bush C, Zhao Z, Kantor P, Burch M, Chen F. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am Heart J. 2017;193:23–34. https://doi.org/10.1016/j.ahj.2017.07.006 (Epub 2017 Jul 17, PMID: 29129252).
Farris W, Schütz SG, Cirrito JR, et al. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am J Pathol. 2007;171(1):241–51. https://doi.org/10.2353/ajpath.2007.070105.
Collins RT 2nd, Shin AY, Hanley FL. Sacrificing the future for the sake of the present. Ann Surg. 2020;271(2):225–6. https://doi.org/10.1097/SLA.0000000000003432 (PMID: 31425297).
Hansen JE, Brown DW, Hanke SP, Hill G, Anderson JB, et al. Angiotensin-converting enzyme inhibitor prescription for patients with single ventricle physiology enrolled in the NPC-QIC registry. J Am Heart Assoc. 2020;9(10): e014823. https://doi.org/10.1161/JAHA.119.014823.
Schranz D, Krasemann T. A word on netting of angiotensin-converting enzyme inhibitor therapy in hypoplastic left heart syndrome following stage-I. Cardiol Young. 2021;31(8):1323–6. https://doi.org/10.1017/S1047951121002936 (Epub 2021 Jul 28, PMID: 34318741).
Buchhorn R, Ross RD, Hulpke-Wette M, Bartmus D, Wessel A, Schulz R, Bürsch J. Effectivness of low dose captopril versus propanolol therapy in infants with severe congestive failure due to-left-to-right shunts. Int J Cardiol. 2000;76(2–3):227–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
None.
Ethics approval
Ethics approved.
Consent for Participation and publication
Given.
Availability of data and materials
On request.
Code availability
None
Author contribution
Single author, complete responsibility.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schranz, D. Can Pediatric Heart Failure Therapy Be Improved? Yes It Can, But…. Pediatr Drugs 24, 567–571 (2022). https://doi.org/10.1007/s40272-022-00524-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00524-z