Abstract
Viloxazine (QELBREE™), a selective norepinephrine reuptake inhibitor, is being developed by Supernus Pharmaceuticals as a non-stimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD) in pediatric and adult patients. This is a novel formulation of a pharmacological agent formerly marketed in Europe for the treatment of depression in adults. Viloxazine received its first pediatric approval in April 2021 in the USA for the treatment of ADHD in pediatric patients aged 6–17 years. Approval was based on positive results from a series of short-term phase III clinical trials in which viloxazine improved the severity of ADHD symptoms in children and adolescents with diagnosed ADHD. Viloxazine is available as extended-release capsules for once-daily oral administration. This article summarizes the milestones in the development of viloxazine leading to this first pediatric approval for ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.14551284. |
A selective norepinephrine reuptake inhibitor is being developed by Supernus Pharmaceuticals for the treatment of ADHD in pediatric and adult patients |
Received its first pediatric approval on 2 April 2021 in the USA |
Approved for use in ADHD in pediatric patients aged 6–17 years |
1 Introduction
Viloxazine (QELBREE™), a norepinephrine reuptake inhibitor, is being developed by Supernus Pharmaceuticals for the treatment of attention-deficit/hyperactivity disorder (ADHD). This is a novel, extended-release (ER) formulation of an older pharmacological agent for which extensive safety data are available [1]. The original, immediate-release formulation of viloxazine was first marketed in the United Kingdom and several other European countries in the 1970s, as an antidepressant for adults [2, 3]. It was withdrawn from market in the early 2000s for business reasons that were unrelated to the efficacy or safety of the drug [3]. Recent development of viloxazine in ADHD, a chronic neurodevelopmental disorder that often emerges during childhood, was driven by the involvement of norepinephrine transmission in ADHD pathophysiology [2, 4]. As a non-stimulant with low apparent substance abuse liability, viloxazine represents an alternative to classical Schedule II stimulants for the treatment of ADHD [2, 5].
Viloxazine ER capsules were approved in the USA on 2 April 2021 for the treatment of ADHD in pediatric patients aged 6–17 years [6, 7]. Viloxazine is available as 100 mg, 150 mg and 200 mg ER capsules which are intended to be orally administered with or without food [6]. The capsules should be swallowed whole or, alternatively, they may be opened and their entire contents sprinkled over a teaspoonful of applesauce. The applesauce with sprinkled capsule contents should be consumed in its entirety (without chewing) within 2 h and should not be stored for future use [6].
The recommended dosage of viloxazine varies based on age. In children aged 6–11 years, the recommended starting dosage is 100 mg once daily [6]. Depending on clinical response and tolerability, the dosage may be titrated each week by a 100 mg increment to the maximum recommended dosage of 400 mg once daily. In adolescents aged 12–17 years, the recommended starting dosage is 200 mg once daily and, depending on clinical response and tolerability, the dosage may be titrated by a 200 mg increment to the maximum recommended dosage of 400 mg once daily. Treatment of ADHD with viloxazine may be required over an extended period. The long-term use of viloxazine should be re-evaluated periodically and dosage adjustments made if necessary. The US prescribing information for viloxazine carries a boxed warning of suicidal thoughts and behaviours; patients must be closely monitored for the emergence or worsening of suicidal thoughts and behaviours whilst receiving viloxazine (Sect. 2.4) [6].
The concomitant administration of viloxazine and monoamine oxidase inhibitors (MAOI), or the administration of viloxazine within 14 days of discontinuing an MAOI, is contraindicated due to an increased risk of hypertensive crisis [6]. Also contraindicated is the concomitant administration of viloxazine and sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range (Sect. 2.2) [6].
While viloxazine is also currently under phase III evaluation in adults with ADHD in the USA [a recently completed double-blind trial (NCT04016779) demonstrated statistical significance versus placebo [8]; an open-label trial (NCT04143217) is ongoing], this article focuses on its use in pediatric patients. Clinical development of viloxazine in depressive disorders has been discontinued.
1.1 Company Agreements
Supernus Pharmaceuticals entered into a purchase and sale agreement with Rune Healthcare Limited (hereafter referred to as Rune) in June 2006 [9]. Under the terms of this agreement, Supernus Pharmaceuticals obtained from Rune the exclusive worldwide rights to the product concept for viloxazine hydrochloride (SPN-809). Supernus Pharmaceuticals paid Rune an upfront fee. Following the approval to market and sell any product based on this product concept, Supernus Pharmaceuticals is obligated to pay Rune royalties based on net sales worldwide. Unless terminated by either party, Supernus Pharmaceuticals will be obligated to pay Rune royalties on a country-by-country basis until either 10 years from the date of the first commercial sale of a product utilizing the Rune product concept, or the market entry of any product utilizing the Rune product by any entity aside from Supernus Pharmaceuticals or its affiliates or licensees in the given country (whichever is earlier) [9].

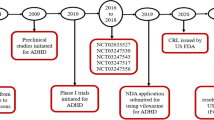
Key milestones in the development of viloxazine, focusing on its use in the treatment of pediatric pts with ADHD. ADHD attention-deficit/hyperactivity disorder, CRL Complete Response Letter, NDA New Drug Application, pts patients
Supernus Pharmaceuticals announced in January 2014 the issuance of a European patent (no. 2341912) and a Canadian patent (no. 2,735,934) covering the use of SPN-812 as a non-stimulant for the treatment of ADHD [1]. These patents will expire no earlier than 2029. At this time, Supernus Pharmaceuticals had additional patent applications for SPN-812 pending in regions such as the USA [1]. US patent protection has since been granted [10].
2 Scientific Summary
2.1 Pharmacodynamics
Viloxazine selectively binds to the norepinephrine transporter [inhibition constant (Ki) = 0.63 µM [6]] and has moderate inhibitory effects on norepinephrine reuptake [half-maximal inhibitory concentration (IC50) = 0.2 µM [6]] [2, 6]. While the mechanism of action through which viloxazine treats ADHD is yet to be fully ascertained [6], targeting norepinephrine transmission has been established to provide a therapeutic benefit in this treatment setting [2]. Viloxazine also modulates serotonin, demonstrating agonistic and antagonist effects on certain serotonin receptor subtypes (5-HT2C and 5-HT2B, respectively) [2]. Results from preclinical studies suggest that viloxazine enhances serotonergic transmission without inhibiting the serotonin transporter [2, 5]. In rats, viloxazine increased extracellular serotonin, norepinephrine and dopamine levels > 5-fold in the prefrontal cortex (an area implicated in ADHD pathophysiology) [2].

Viloxazine chemical structure
Although viloxazine may have the potential to inhibit cardiac sodium channels, a supratherapeutic dose (4.5 times the maximum recommended dose) had no clinically meaningful impact on the QT interval, PR interval or QRS duration in healthy volunteers [6]. Viloxazine can, however, increase heart rate and diastolic blood pressure. In clinical trials of once-daily viloxazine in children aged 6–11 years, a heart rate increase of ≥ 20 beats per minute (bpm) at any time point was experienced by 22% of viloxazine 100 mg recipients (vs 9% of placebo recipients), 31% of viloxazine 200 mg recipients (vs 15%) and 28% of viloxazine 400 mg recipients (vs 23%). In trials in adolescents aged 12–17 years, a heart rate increase of ≥ 20 bpm occurred in 22% of once-daily viloxazine 200 mg recipients (vs 14% of placebo recipients) and 34% of once-daily viloxazine 400 mg recipients (vs 17%). In the latter age group, 25% of once-daily viloxazine 400 mg recipients experienced an increase in diastolic blood pressure of ≥ 15 mmHg at any time during the trial (vs 13% of placebo recipients). Heart rate and blood pressure should be assessed before viloxazine is initiated, and at appropriate intervals during treatment (including following dosage increases) [6].
2.2 Pharmacokinetics
Viloxazine ER capsules exhibit dose-proportional pharmacokinetics over the dosage range of 100–400 mg once daily [6]. With once-daily administration, steady state was achieved after 2 days and there was no accumulation observed. Relative to an immediate-release formulation, the bioavailability of the ER formulation was ≈ 88%. After a single dose of viloxazine 200 mg, the peak plasma concentration (Cmax) was reached in median time of ≈ 5 h (range 3–9 h). When viloxazine ER 200 mg was administered with a high-fat meal, viloxazine Cmax and area under the concentration-time curve (AUC) decreased by ≈ 9% and 8%, respectively, while time to Cmax was extended by ≈ 2 h; when the contents of a capsule were sprinkled on applesauce, Cmax and AUC decreased by ≈ 10% and 5%, respectively. The human plasma protein binding of viloxazine is 76–82% over a blood concentration range of 0.5–10 µg/mL [6].
Viloxazine metabolism is primarily mediated by CYP2D6, UGT1A9 and UGT2B15 [6, 11]. In human plasma analyses, the major metabolite is 5-hydroxy-viloxazine glucuronide. Viloxazine and its metabolites are primarily excreted via renal elimination. Following administration of a single radiolabeled dose of viloxazine, most of the dose (90% [6]) was recovered in urine within the initial 24 h after administration [6, 11]. A negligible proportion of the total dose (< 1%) was recovered in feces [6, 11]. Viloxazine has a mean half-life of 7.02 h [6].
The pharmacokinetics of viloxazine do not meaningfully differ based on race or sex [6]. In children aged 6–11 years, the estimated steady-state Cmax and AUC0–t of viloxazine (at doses of 100–400 mg) and its major metabolite were ≈ 40–50% higher than those in adolescents aged 12–17 years [6]. Abnormal kidney function is associated with increased viloxazine exposure (Cmax and AUC); a dosage reduction is recommended in patients with an estimated glomerular filtration rate of < 30 mL/min/1.73 m2 (starting dosage of 100 mg once daily, with titration in weekly increments of 50–100 mg once daily to a maximum dosage of 200 mg once daily). In patients with hepatic impairment, the pharmacokinetics of viloxazine have not been evaluated and use of viloxazine is not recommended [6].
Certain pharmacokinetic drug-drug interactions are possible with viloxazine. Viloxazine is a strong inhibitor of CYP1A2 [6, 11]. As such, the co-administration of viloxazine and sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range is contraindicated [6]. The co-administration of viloxazine with moderate sensitive CYP1A2 substrates is not recommended and, if commenced, may warrant dose reduction. Viloxazine is a weak inhibitor of CYP2D6 and CYP3A4, and may therefore increase the exposure of CYP2D6 and CYP3A4 substrates when used concomitantly; patients co-administered these drugs should be monitored for adverse reactions and dosages of the CYP2D6/3A4 substrates should be adjusted as clinically indicated [6].
Features and properties of viloxazine
Alternative names | QELBREE; SPN 809; SPN 812; SPN-812 ER; SPN-812V; Viloxazine extended-release—Supernus Pharmaceuticals; Viloxazine hydrochloride extended-release—Supernus Pharmaceuticals |
Class | Antidepressants, Behavioural disorder therapies, Ethers, Morpholines, Small molecules |
Mechanism of Action | Adrenergic uptake inhibitors |
Route of Administration | Oral (extended-release capsules) |
Pharmacodynamics | Binds to norepinephrine transporter to inhibit reuptake of norepinephrine |
Pharmacokinetics | Dose-proportional pharmacokinetics; median time to peak plasma concentration ≈ 5 h; mean half-life 7 h; primarily excreted renally |
Adverse reactions | |
≥ 10% of pediatric pts | Somnolence, headache |
≥ 2 and < 10% of pediatric pts | Decreased appetite, URTI, fatigue, abdominal pain, nausea, vomiting, insomnia, irritability, pyrexia |
ATC codes | |
WHO ATC code | N06A-X09 (Viloxazine) |
EphMRA ATC code | N6A (Anti-Depressants and Mood Stabilisers) |
Chemical name | 2-(2-Ethoxyphenoxymethyl)morpholine hydrochloride |
2.3 Therapeutic Trials
Viloxazine improved the severity of ADHD symptoms in children aged 6–11 years with ADHD when orally administered at lower doses in a randomized, double-blind, placebo-controlled, multicenter, phase III trial (NCT03247530; 812P301) [12]. Patients with a confirmed primary diagnosis of ADHD, an ADHD Rating Scale-5 (ADHD-RS-5) score of ≥ 28 and a Clinical Global Impression—Severity (CGI-S) score of ≥ 4 were randomized to receive once-daily viloxazine ER 100 mg (n = 147 in the intent-to-treat population), viloxazine ER 200 mg (n = 158) or placebo (n = 155) for 6 weeks. Patients were required to refrain from taking any other ADHD medication for ≥ 1 week prior to randomization and throughout the study. Least-squares mean (LSM) changes from baseline in ADHD-RS-5 total score were significantly greater (i.e. improved) in the viloxazine 100 mg and 200 mg groups than in the placebo group at the end of the study (− 16.6 and − 17.7 vs − 10.9; p = 0.0004 and p < 0.0001, respectively) [primary endpoint]. Viloxazine had a fast onset of action; significant (p ≤ 0.0244) improvements in ADHD-RS-5 total score were seen with each viloxazine dose versus placebo after the first week of treatment. At the end of the study, viloxazine 100 mg and 200 mg recipients demonstrated significant (p ≤ 0.002) improvements in Clinical Global Impression-Improvement (CGI-I) score, Conners 3-Parent Short Form (Conners 3-PS) Composite T-score and Weiss Functional Impairment Rating Scale-Parent (WFIRS-P) total average score relative to placebo recipients [12].
Key clinical trials of viloxazine (Supernus Pharmaceuticals, Inc.)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Viloxazine ER (low doses), PL | ADHD in pediatric patients aged 6–11 years | III | Completed | USA | NCT03247530; 812P301 |
Viloxazine ER (low doses), PL | ADHD in pediatric patients aged 12–17 years | III | Completed | USA | NCT03247517; 812P302 |
Viloxazine ER (high doses), PL | ADHD in pediatric patients aged 6–11 years | III | Completed | USA | NCT03247543; 812P303 |
Viloxazine ER (high doses), PL | ADHD in pediatric patients aged 12–17 years | III | Completed | USA | NCT03247556; 812P304 |
Viloxazine ER | ADHD in pediatric patients who participated in a previous blinded study of viloxazine ER | III | Active, not recruiting | USA | NCT02736656; 812P310 |
Viloxazine ER, PL | ADHD in adults | III | Completed | USA | NCT04016779; 812P306 |
Viloxazine ER | ADHD in adults who participated in 812P306 | III | Recruiting | USA | NCT04143217; 812P311 |
Viloxazine ER, PL | ADHD in pediatric patients aged 6–12 years | II | Completed | USA | NCT02633527; 812P202 |
Viloxazine, PL | ADHD in adults | I/IIa | Completed | USA | NCT01107496; 812P201 |
Viloxazine was similarly effective in improving ADHD symptoms in children aged 6–11 years with ADHD when orally administered at higher doses in a randomized, double-blind, placebo-controlled, multicenter, phase III trial (NCT03247543; 812P303) [13]. Patients with a confirmed primary diagnosis of ADHD, an ADHD-RS-5 score of ≥ 28, a CGI-S score of ≥ 4 and a body weight of ≥ 20 kg were randomized to receive once-daily viloxazine ER 200 mg (n = 107 in the intent-to-treat population), viloxazine ER 400 mg (n = 97) or placebo (n = 97) for 8 weeks (including a ≤ 3-week titration period). At the end of the study, LSM changes from baseline in ADHD-RS-5 total score were significantly greater in the viloxazine 200 mg and 400 mg groups than in the placebo group (−17.6 and −17.5 vs −11.7; p = 0.0038 and p = 0.0063, respectively) [primary endpoint]. For both viloxazine doses, improvements in ADHD-RS-5 total score reached statistical significance (p < 0.05 vs placebo) by week five of treatment. At the end of the study, CGI-I scores were significantly (p ≤ 0.0099) improved with both viloxazine doses versus placebo and change from baseline in Conners 3-PS Composite T-score was significantly (p = 0.0064) improved with viloxazine 200 mg (but not viloxazine 400 mg) versus placebo. Treatment groups did not significantly differ with respect to change from baseline in WFIRS-P total average score [13].
Viloxazine was shown to effectively treat ADHD symptoms in adolescents aged 12–17 years with ADHD in a randomized, double-blind, placebo-controlled, multicenter, phase III trial (NCT03247517; 812P302) [14]. In this monotherapy trial, patients with a confirmed diagnosis of ADHD, an ADHD-RS-5 score of ≥ 28 and a CGI-S score of ≥ 4 were randomized to receive once-daily viloxazine ER 200 mg (n = 94 in the intent-to-treat population), viloxazine ER 400 mg (n = 103) or placebo (n = 104) for 6 weeks (including a ≤ 1-week titration period). At the end of the study, viloxazine 200 mg and 400 mg recipients demonstrated significantly greater LSM changes from baseline in ADHD-RS-5 total score than placebo recipients (−16.0 and −16.5 vs −11.4; p = 0.0232 and p = 0.0091, respectively) [primary endpoint]. The improvement in ADHD-RS-5 total score was significantly (p = 0.0085) greater with viloxazine 400 mg than with placebo as early as week one [14]. Both viloxazine groups showed significant (p ≤ 0.0042) improvements in CGI-I score relative to the placebo group, while changes in Conners 3-PS Composite T-score or WFIRS-P total average score did not significantly differ between treatment groups [15].
When administered orally at a high dose, viloxazine did not offer a significant benefit over placebo for the treatment of ADHD symptoms in adolescents aged 12–17 years in a randomized, double-blind, multicenter, phase III trial (NCT03247556; 812P304) [16]. Patients with a confirmed primary diagnosis of ADHD, an ADHD-RS-5 score of ≥ 28, a CGI-S score of ≥ 4 and a body weight of ≥ 35 kg were randomized to receive once-daily viloxazine ER 400 mg (n = 99 in the intent-to-treat population), viloxazine ER 600 mg (n = 97) or placebo (n = 96) for 7 weeks (including a ≤ 3-week titration period). Patients were required to refrain from taking any other ADHD medication for ≥ 1 week prior to randomization and throughout the study. At the end of the study, LSM changes from baseline in ADHD-RS-5 total score were −18.3 and −16.7 with viloxazine 400 mg and 600 mg, respectively, compared with −13.2 with placebo (p = 0.0082 and p > 0.05 vs placebo) [primary endpoint]; due to a sequential gatekeeping testing procedure (in which viloxazine 600 mg was compared with placebo first), neither viloxazine dose could be considered superior to placebo [16].
Viloxazine was first demonstrated to reduce the severity of ADHD symptoms in children aged 6–12 years with ADHD in a randomized, double-blind, placebo-controlled, multicenter, phase II trial (NCT02633527; 812P202) [17]. Patients with a diagnosis of ADHD, an ADHD-RS-IV score of ≥ 26, a CGI-S score of ≥ 4 and a body weight of ≥ 20 kg were randomized to receive once-daily viloxazine ER 100 mg (n = 45 in the intent-to-treat population), viloxazine ER 200 mg (n = 46), viloxazine ER 300 mg (n = 47), viloxazine ER 400 mg (n = 44) or placebo (n = 24) for 8 weeks (including a ≤ 3-week titration period). At the end of the study, LSM changes from baseline in ADHD-RS-IV total score were significantly greater in the viloxazine 200 mg, 300 mg and 400 mg groups versus the placebo group (− 18.4, − 18.6 and − 19.0, respectively, vs − 10.5; p < 0.05 for each comparison). With viloxazine 100 mg, the improvement in ADHD-RS-IV total score did not reach statistical significance relative to that with placebo (− 16.7 vs − 10.5) [17].
2.4 Adverse Events
Viloxazine was generally well tolerated in pediatric patients with ADHD in clinical trials [12,13,14, 16, 17]. In a pooled analysis of safety data from children aged 6–17 years with ADHD participating in randomized, double-blind, placebo-controlled trials (n = 826 and 463 treated with viloxazine 100–400 mg and placebo, respectively), the most common adverse reactions in viloxazine recipients (occurring in ≥ 2% and at a rate greater than in placebo recipients) were somnolence (including lethargy and sedation; 16% with viloxazine vs 4% with placebo), headache (11% vs 7%), upper respiratory tract infection (7% vs 6%), decreased appetite (7% vs 0.4%), fatigue (6% vs 2%), abdominal pain (5% vs 4%), nausea (5% vs 3%), vomiting (4% vs 2%), insomnia (4% vs 1%), irritability (3% vs 1%) and pyrexia (2% vs 0.2%) [6]. Adverse reactions led to discontinuation of viloxazine in few patients (≈ 3%), with somnolence, nausea, headache, irritability, tachycardia, fatigue and decreased appetite being the adverse reactions most often associated with discontinuation [6]. Given that viloxazine has been associated with somnolence and fatigue, patients receiving viloxazine should not perform activities that require mental alertness (e.g. driving) until they know how viloxazine affects them [6].
With respect to the potential effects of viloxazine on body weight, viloxazine recipients aged 6–11 years gained an average of 0.2 kg of body weight (vs 1 kg in placebo recipients of the same age) during the short-term controlled trials (6–8 weeks) [6]. Viloxazine recipients aged 12–17 years lost an average of 0.2 kg (vs a gain of 1.5 kg in placebo recipients of the same age). During an open-label, long-term extension safety study, the mean change from baseline in weight-for-age z-score was − 0.2 in viloxazine recipients evaluated at 12 months (n = 338); it is uncertain whether this weight change can be attributed to treatment with viloxazine [6].
All patients treated with viloxazine should be closely monitored for the emergence or clinical worsening of suicidal thoughts and behaviors (particularly during the first few months of receiving viloxazine and at times of dosage adjustment) [6]. They should also be observed for the emergence of insomnia, irritability or other symptoms that may represent precursors to suicidal ideation or behavior [6]. In the short-term pediatric trials (n = 1019 exposed to viloxazine 100–400 mg), suicidal ideation (n = 6), behavior (n = 1) or both (n = 2) were reported in nine (0.9%) viloxazine recipients. Eight of these recipients reported suicidal ideation or behavior on the Columbia Suicide Severity Rating Scale (C-SSRS). An additional viloxazine recipient reported suicidal behavior but not whilst completing the C-SSRS. Two placebo recipients (0.4%) reported suicidal ideation on the C-SSRS; none reported suicidal behavior. There were no completed suicides during the trials [6].
Viloxazine, as a noradrenergic drug, may induce manic or mixed episodes in patients with bipolar disorder. Patients should be screened for bipolar disorder risk before treatment with viloxazine is initiated [6].
2.5 Ongoing Clinical Trials
The open-label extension study of the blinded phase II and III pediatric trials in ADHD (NCT02736656) is ongoing. With an estimated completion date of June 2024, this study is evaluating the long-term safety and efficacy of viloxazine. Currently, two additional pediatric trials are planned but are yet to begin recruiting. These include an open-label phase IV safety study of viloxazine co-administered with psychostimulants in children aged 6–17 years with ADHD (NCT04786990; 812P412) and a randomized, double-blind, placebo-controlled, multicenter phase IV trial evaluating the efficacy and safety/tolerability of viloxazine ER 100 mg in children aged 4–5 years with ADHD (NCT04781140; 812P401).
3 Current Status
Viloxazine received its pediatric first approval on 2 April 2021 for ADHD in pediatric patients in the USA.
Change history
20 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40272-023-00599-2
References
Supernus Pharmaceuticals. Supernus announces issuance of first use patents protecting SPN-812 as a novel non-stimulant ADHD product [media release]. 2014. http://www.supernus.com.
Yu C, Garcia-Olivares J, Candler S, et al. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285–300.
Faison SL, Fry N, Adewole T, et al. Pharmacokinetics of coadministered viloxazine extended-release (SPN-812) and lisdexamfetamine in healthy adults. J Clin Psychopharmacol. 2021;41(2):155–62.
Pozzi M, Bertella S, Gatti E, et al. Emerging drugs for the treatment of attention-deficit hyperactivity disorder (ADHD). Expert Opin Emerg Drugs. 2020;25(4):395–407.
Cutler AJ, Mattingly GW, Jain R, et al. Current and future nonstimulants in the treatment of pediatric ADHD: monoamine reuptake inhibitors, receptor modulators, and multimodal agents. CNS Spectr. 2020;2020:1–9.
Supernus Pharmaceuticals Inc. QELBREE™ (viloxazine extended-release capsules), for oral use: US prescribing information. 2021. http://www.accessdata.fda.gov/. Accessed 7 May 2021.
Supernus Pharmaceuticals Inc. Supernus announces FDA approval of QelbreeTM (SPN-812) for the treatment of ADHD [media release]. 2021. http://www.supernus.com.
Supernus Pharmaceuticals. SPN-812 ADHD adult study topline results. 2020. http://ir.supernus.com/.
Supernus Pharmaceuticals Inc. Form 10-K (annual report): filed 03/21/14 for the period ending 12/31/13. 2014. http://ir.supernus.com/. Accessed 7 May 2021
Vieira ML, Huang AB, Bhatt PP. United State patent: patent no. US 9,603,853 B2. 2017. http://patents.google.com/. Accessed 7 May 2021
Yu C. Metabolism and in vitro drug-drug interaction assessment of viloxazine. Xenobiotica. 2020;50(11):1285–300.
Nasser A, Liranso T, Adewole T, et al. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended-release) in the treatment of attention-deficit/hyperactivity disorder in school-age children. Clin Ther. 2020;42(8):1452–66.
Nasser A, Liranso T, Adewole T, et al. Once-daily SPN-812 200 and 400 mg in the treatment of ADHD in school-aged children: a phase III randomized, controlled trial. Clin Ther. 2021. https://doi.org/10.1016/j.clinthera.2021.01.027.
Nasser A, Hull JT, Chowdhry FA, et al. Extended-release viloxazine (SPN-812) for the treatment of attention-deficit/hyperactivity disorder (ADHD) in adolescents: topline results of a phase 3, randomized, double-blind, placebo-controlled study (P302) [abstract and poster]. Neurotherapeutics. 2019;16(3):914–5.
Nasser A, Hull JT, Chowdhry FA, et al. A phase 3, randomized, double-blind, placebo-controlled study (P302): efficacy and safety of extended-release viloxazine in adolescents with ADHD [abstract no. 112]. CNS Spectr. 2020;25(2):272–3.
Nasser A, Liranso T, Adewole T, et al. A phase 3 placebo-controlled trial of once-daily 400-mg and 600-mg SPN-812 (viloxazine extended-release) in adolescents with ADHD. Psychopharmacol Bull. 2021;51(2):43–64.
Johnson JK, Liranso T, Saylor K, et al. A phase II double-blind, placebo-controlled, efficacy and safety study of SPN-812 (extended-release viloxazine) in children with ADHD. J Atten Disord. 2020;24(2):348–58.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding. Post-publication open access was funded by Supernus Pharmaceuticals, Inc.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Nicole Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original online version of this article was revised due to retrospective open access request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lamb, Y.N. Viloxazine: Pediatric First Approval. Pediatr Drugs 23, 403–409 (2021). https://doi.org/10.1007/s40272-021-00453-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00453-3