Abstract
Background
Atopic dermatitis (AD) is a chronic inflammatory skin disease common among infants and children. It is associated with a high risk of allergies, asthma, and mental health problems. Attempts have been made to use probiotics in clinical interventions for AD.
Objective
Our objective was to perform an updated meta-analysis of recently published studies to evaluate the effect of probiotics in the prevention and treatment of AD in children and to further understand the role of probiotics in AD interventions in the clinic.
Method
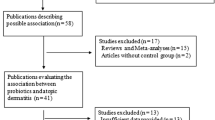
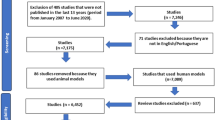
We searched the PubMed/MEDLINE, Embase, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure, and Wanfang databases with prespecified selection criteria from inception of each database to 11 January 2020. No language restrictions were applied.
Results
A total of 25 studies were included in our meta-analysis. Of these, 14 were prevention studies (with 3049 children enrolled) and 11 were treatment studies (with 816 children enrolled). One treatment study was excluded after the sensitivity analysis. From the 14 prevention studies included, the pooled relative risk ratio of AD in those treated with probiotics versus placebo was 0.70 [95% confidence interval (CI) 0.57–0.84; P = 0.0002]. Subgroup analyses showed that only mixed strains of probiotics had a significant effect on lowering the incidence of AD. Probiotics administered solely to infants did not prevent the development of AD, but effects were significant when probiotics were administered to both pregnant mothers and their infants or solely to pregnant mothers. In studies with treatment durations > 6 months, the incidence of AD decreased significantly; a similar effect was achieved when the treatment duration was < 6 months. Meta-analysis of the ten treatment studies showed a significant decrease in the weighted mean difference (WMD) in Scoring Atopic Dermatitis (SCORAD) index values in the probiotics group compared with the control group (WMD, − 7.23; 95% CI − 10.59 to − 3.88; P < 0.0001). Subgroup analyses showed that both single-strain and mixed-strain probiotics had a significant effect on improving SCORAD values. Studies with participants aged < 1 year (P = 0.07) reported no significant results. In studies with treatment periods > 8 weeks, SCORAD values seemed to decrease more than in studies with treatment periods < 8 weeks. However, the subgroup difference was only statistically significant when the analysis was performed according to participant age in prevention studies.
Conclusion
Our updated meta-analysis demonstrates that interventions with probiotics potentially lower the incidence of AD and relieve AD symptoms in children, particularly when treating infants and children aged ≥ 1 year with AD. Interventions with mixed-strain probiotics tended to have better preventive and curative effects. Probiotics administered solely to infants appeared to produce negative preventive effects. Different intervention durations might also affect clinical outcomes. However, given the insignificant subgroup differences, except for treatment by participant age, and the moderate heterogeneity among the studies, these conclusions should be interpreted with caution, and more powerful randomized controlled trials using standardized measurements should be conducted to assess the long-term effects of probiotics.



Similar content being viewed by others
References
Sung M, Lee KS, Ha EG, Lee SJ, Kim MA, Lee SW, et al. An association of periostin levels with the severity and chronicity of atopic dermatitis in children. Pediatr Allerg Immunol. 2017;28(6):543–50.
Boguniewicz M, Schmid-Grendelmeier P, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):40–3.
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22.
Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–57.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am ACad Dermatol. 2014;71(1):116–32.
Lewis MC, Inman CF, Patel D, Schmidt B, Mulder I, Miller B, et al. Direct experimental evidence that early-life farm environment influences regulation of immune responses. Pediatr Allergy Immunol. 2012;23(3):265–9.
Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9.
Zheng H, Liang H, Wang Y, Miao M, Shi T, Yang F, et al. Altered gut microbiota composition associated with eczema in infants. PLoS ONE. 2016;11(11):e166026.
Hong P, Lee BW, Aw M, Shek LPC, Yap GC, Chua KY, et al. Comparative analysis of fecal microbiota in infants with and without eczema. PLoS ONE. 2010;5(4):e9964.
Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39(3):237–8.
Vongbhavit K, Underwood MA. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther. 2016;38(4):716–32.
Binns C, Lee MK. The use of probiotics to prevent diarrhea in young children attending child care centers: a review. J Exp Clin Med. 2010;2(6):269–73.
Wickens K, Barthow C, Mitchell EA, Stanley TV, Purdie G, Rowden J, et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr Allergy Immunol. 2018;29(3):296–302.
Dotterud CK, Storro O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. 2010;163(3):616–23.
Navarro-Lopez V, Ramirez-Bosca A, Ramon-Vidal D, Ruzafa-Costas B, Genoves-Martinez S, Chenoll-Cuadros E, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis a randomized clinical trial. JAMA Dermatol. 2018;154(1):37–433.
Folster-Holst R, Muller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol. 2006;155(6):1256–61.
Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121(1):116–21.
Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356–71.
Panduru M, Panduru NM, Salavastru CM, Tiplica GS. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29(2):232–42.
Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology. 2012;23(3):402–14.
Michail SK, Stolfi A, Johnson T, Onady GM. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101(5):508–16.
Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.
Doege K, Grajecki D, Zyriax BC, Detinkina E, Zu EC, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107(1):1–6.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Atherton J, Bieber T, Bonilazi E, Broberg A, Calza A, et al. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31.
Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–62.
Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122(4):788–94.
Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e386–e393393.
Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130(6):1355–60.
Plummer EL, Chebar LA, Tobin JM, Uebergang JB, Axelrad C, Garland SM, et al. Postnatal probiotics and allergic disease in very preterm infants: sub-study to the ProPrems randomized trial. Allergy. 2020;75(1):127–36.
Ou CY, Kuo HC, Wang L, Hsu TY, Chuang H, Liu CA, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy. 2012;42(9):1386–96.
Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119(1):184–91.
Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YPM, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants—effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy. 2009;39(4):571–8.
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–9.
Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor MB, Garaiova I, et al. Probiotics in the prevention of eczema: a randomised controlled trial. Arch Dis Child. 2014;99(11):1014–9.
Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121(4):e850–e856856.
Niers L, Martin R, Rijkers G, Sengers F, Timmerman H, van Uden N, et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy. 2009;64(9):1349–58.
Zhengchun G, et al. Probiotics have an early preventitive effect on the occurrence of atopic disease in children. Chin J Matern Child Health. 2004;11:120–1.
Nakata J, Hirota T, Umemura H, Nakagawa T, Kando N, Futamura M, et al. Additive effect of Lactobacillus acidophilus L-92 on children with atopic dermatitis concomitant with food allergy. Asia Pac Allergy. 2019;9(2):e18.
Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol. 2010;104(4):343–8.
Yesilova Y, Calka O, Akdeniz N, Berktas M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24(2):189–93.
Kim J, Han Y, Kim B, Ban J, Lee J, et al. Effects of Lactobacillus plantarum CJLP133 on pediatric atopic dermatitis: a double-blind, placebo-controlled trial. Allergy Eur J Allergy Clin Immunol. 2012;67:140–1.
Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90(9):892–7.
Wu YJ, Wu WF, Hung CW, Ku MS, Liao PF, Sun HL, et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: an 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect. 2017;50(5):684–92.
Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010;11(5):351–61.
Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60(4):494–500.
Chernyshov PV. Randomized, placebo-controlled trial on clinical and immunologic effects of probiotic containing Lactobacillus rhamnosus R0011 and L. helveticus R0052 in infants with atopic dermatitis. Microbl Ecol Health Dis. 2009;21(3/4):228–32.
Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113(2):217–26.
Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, Garcia-Romero MT. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr. 2016;170(3):236–42.
Jones CA, Holloway JA, Warner JO. Does atopic disease start in foetal life? Allergy. 2000;55(1):2–10.
Landreth KS. Critical windows in development of the rodent immune system. Hum Exp Toxicol. 2002;21(9–10):493–8.
Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z. Intestinal microbiota in early life and its implications on childhood health. Genom Proteom Bioinform. 2019;17:13–25.
Valles Y, Artacho A, Pascual-Garcia A, Ferrus ML, Gosalbes MJ, Abellan JJ, et al. Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet. 2014;10(6):e1004406.
Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050.
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):e00036-17.
Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44.
Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4):516–20.
Sjögren YM, Jenmalm MC, Böttcher MF, Björkstén B, Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39(4):518–26.
Suzuki S, Kubota N, Kakiyama S, Miyazaki K, Sato K, Harima-Mizusawa N. Effect of Lactobacillus plantarum YIT 0132 on Japanese cedar pollinosis and regulatory T cells in adults. Allergy. 2020;75(2):453–6.
La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA. 2014;111(34):12522–7.
Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74.
Cantey JB, Wozniak PS, Pruszynski JE, Sanchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(10):1178–84.
Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4(1):68.
Passeron T, Lacour JP, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy. 2006;61(4):431–7.
Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156–64.
Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr. 2013;109(Suppl 2):S35–50.
Mah KW, Chin VI, Wong WS, Lay C, Tannock GW, Shek LP, et al. Effect of a milk formula containing probiotics on the fecal microbiota of Asian infants at risk of atopic diseases. Pediatr Res. 2007;62(6):674–9.
Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52.
Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–10.
Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46(6):468–81.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was funded by Major Scientific and Technological Projects for Collaborative Prevention and Control of Birth Defects in Hunan Province (2019SK1012), Major Research and Development Projects in Hunan Province (2018SK2060), and Scientific and Technological Department Projects in Hunan Province (2017SK50802).
Conflict of interest
Wen Jiang, Bin Ni, Zhiyu Liu, Xuan Liu, Wanqin Xie, Irene X.Y. Wu, and Xingli Li have no conflicts of interest that are directly relevant to the content of this review.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author contributions
WJ and XL developed the research question. WX and ZL conducted the literature search. WJ, BN, WX, and XL selected the literature, extracted the data, and assessed the risk of bias. IXYW and WX analyzed the data, and IXYW and WJ interpreted the results. WJ, IXYW, and XL wrote the manuscript. All authors contributed to the interpretation and revision of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, W., Ni, B., Liu, Z. et al. The Role of Probiotics in the Prevention and Treatment of Atopic Dermatitis in Children: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pediatr Drugs 22, 535–549 (2020). https://doi.org/10.1007/s40272-020-00410-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-020-00410-6