Abstract
Background
Treatment preference research can support shared and informed decision making for currently available atopic dermatitis (AD) treatments, and simultaneously guide research and development for future therapies. In this systematic literature review, we aimed to provide an overview of preferences for AD treatments.
Methods
This systematic literature review was conducted in the Medline and Embase (via Ovid) databases, supplemented by manual searching. Quantitative research published from 2010 to September 2023 that investigated preferences for AD treatments were included. Quality assessment was conducted by using the purpose, respondents, explanation, findings, significance checklist, and a checklist developed by the Professional Society for Health Economics and Outcomes Research.
Results
In total, 207 references were screened after removing duplicates and 15 studies were included. Most studies were conducted in the US, followed by European countries. On average, people directly or indirectly affected by AD rate efficacy and treatment-related risk as the most important criteria when choosing an AD therapy. Participants are willing to increase risks in order to have a higher chance of achieving a certain benefit, e.g. reduction in itch or clearer skin. Participants have preferences for different modes of administration. On average, 68% (all full-text studies) and 87% (only discrete choice experiments [DCEs]) of quality criteria per reference were rated as fulfilled. DCEs received generally higher quality assessment scores than non-DCEs.
Conclusions
This review revealed that AD treatment preference research is limited. Diverse study designs hampered comparison and synthesis of the results. We recommend conducting more DCEs in this field to increase the likelihood of AD patients receiving the therapy that best fits their individual needs and preferences.
Clinical Trials Registration
This protocol was published in PROSPERO (ID: CRD42023468757).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
On average, people directly or indirectly affected by atopic dermatitis (AD) rate efficacy and treatment-related risk as the most important criteria when choosing an AD therapy. |
Participants are willing to increase risks in order to have a higher chance of achieving a certain benefit. |
Participants have preferences for different modes of administration. |
1 Introduction
The development and availability of therapies for skin conditions that fulfill patients’ needs is of utmost importance, as these disorders negatively affect patients’ physical and mental well-being [1,2,3,4,5]. Atopic dermatitis (AD) is a chronic inflammatory skin disorder and is one of the most common skin conditions [6]. AD patients can experience, among others, severe itching, skin pain, crusting, erythema, scaling and vesiculation [1, 4]. Furthermore, patients suffer from sleep, depressive, or anxiety disorders caused by stigmatization, lower self-esteem and social isolation [3,4,5]. These circumstances lead to a high burden for patients and can substantially decrease their quality of life [7], and, additionally, are associated with absenteeism and productivity losses [8]. Even though several different therapy options have been developed over time, treatments lack practicability because of time-consuming application, discomfort associated with therapies, or limited respondence [2, 9]. Therefore, it is important to not only make more treatment options available but also to align current treatments to patients’ preferences. Preference research elicits preferences that can improve awareness of dermatologists for their patients’ needs. This way, dermatologists can better take the patient’s and caregivers’ perspective into account when looking for an appropriate treatment. This is essential for making shared and informed decisions [10]. Moreover, the knowledge of patients’ preferences can help Research and Development Departments of manufacturers to develop new and appropriate therapies. Thus, the value of preference research is multidimensional; it supports the appropriate use of currently available therapies, while at the same time can guide the development of new treatments for the future.
In the past, quantitative researches have been conducted to assess preferences in AD. In 2023, Maleki-Yazdi et al. published a systematic review reporting on patient preferences in AD [11]. In that review, the authors included studies about patient and caregiver preferences, values, experiences, perceptions, views, satisfaction, attitudes and experiences regarding AD treatment that were published until March 2022 [11]. While the presented overview filled an important gap in the field, authors only focused on patients and caregivers; however, professionals’ perspectives may also be of interest as these summarize the experiences gained while treating several patients with different needs. Furthermore, no detailed assessment of stated preference studies has been conducted and several additional stated preference studies have been published since March 2022. Therefore, we aimed to develop a systematic literature review and critical appraisal covering not only patients’ and caregivers’ preferences but also professionals’ preferences for AD treatments.
2 Methods
The recommendations of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed during the conduct of this systematic review [12]. This included the publication of a protocol in PROSPERO (ID: CRD42023468757), careful screening of the abstract and full text by two independent reviewers, and quality evaluation of the included articles [12]. Covidence was used to conduct screening and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) was used to support data extraction and quality assessment.
2.1 Literature Search and Study Selection
Only studies published between 2010 and September 2023 were included; we decided to include only studies published in 2010 or later as therapies for AD are improving fast and we wanted to focus on timely discrete choice experiments (DCEs) as their meaning could decrease over time. Furthermore, the development of more advanced therapies in AD started around 2010. The search was conducted in the Medline (via Ovid) and Embase (via Ovid) databases. Additionally, manual searching was performed by reviewing bibliographies of the included studies. A manual search was also performed on Google Scholar. Moreover, experts in the field were requested to review the list of included studies and add any potential missing references. Searches were limited to studies published in English, German or French due to the authors’ restricted language skills. Nonetheless, all relevant articles should have been identified and this language limitation is not assumed to have an impact on the findings of this systematic literature review [13, 14].
The search strategy (see Online Resource 1) was developed with the support of an experienced researcher (CB) and by using terms that included the population, interventions and study design. This is in line with the Centre for Reviews and Dissemination’s (CRD’s) guidance for undertaking reviews in healthcare [15]. Once the literature search was completed, all references were imported into Covidence Software, a web-based collaboration software platform that streamlines the production of systematic and other literature reviews [16]. Duplicates were removed and the inclusion and exclusion criteria that follow the PICOTS (Population, Intervention, Comparator, Outcome, Timing, Setting/Study Design) framework [17], and which are presented in Table 1, were applied to guide study selection. Only treatment preference research was considered in the sense that studies that solely investigate experiences or satisfaction with, for example, certain treatments were not included. Although the first intention was to consider qualitative and quantitative research, in the final review only quantitative work was included. During the conduct of the review, it became clear that there is enough quantitative work, including stated preference methods research, and also, for example, surveys with quantitative data available. Furthermore, comparability and data synthesis is more valuable when research methods are more aligned. Based on the inclusion and exclusion criteria, two independent reviewers (KH and CB) screened the articles for eligibility, firstly based on title and abstract, and secondly based on full-text. In cases of disagreement, a third reviewer (MH) was consulted. Reasons for exclusion of articles during the full-text screening were collected.
2.2 Data Extraction
Data extraction was performed on a standardized data extraction form that was predefined and reviewed by the research team. This form was informed by former research articles [18, 19]. The data extraction form was pretested on two studies by two reviewers. Data extraction was then performed by one reviewer (KH) and checked by a second reviewer (CB). The following data were extracted for any included papers: authors, year of publication, availability of full text or abstract, funding, study design, country, population of interest, number of participants, investigated preference, mean age, percentage of males, and disease severity of interest. For DCEs, characteristics of attribute selection process, attributes investigated, choice set generation, experimental design and DCE analysis model were also extracted. Results included the most preferred attributes identified, main insights regarding preferences, and results of subgroup analyses. In cases where neither relative importance nor coefficients were reported, the corresponding author of the respective paper was contacted and asked for provision. Additional data extracted for non-DCE studies included selection process of questions, survey administration method, topics covered by questions and methods used for data analysis.
2.3 Quality Assessment
The quality of the included articles was assessed by using two checklists: the Purpose, Respondents, Explanation, Findings, Significance (PREFS) checklist that consists of five criteria [20], and a checklist that was developed by the Professional Society for Health Economics and Outcomes Research (ISPOR) that includes 10 criteria with three questions each [21]. Both checklists use 0 and 1 to rate each question [20, 21]. The sum of each vote yields the quality result. An independent reviewer (KH) rated all included studies according to these two checklists. In cases of doubt, another researcher (MH) was consulted. References that were not available as full texts were excluded from quality assessment to avoid bias in the quality assessment results. No quality assessment was performed for references not available as full texts due to a paucity of information.
2.4 Data Synthesis
To facilitate the presentation of results, a summary was created in which attributes investigated in DCEs were categorized and subclassified. Moreover, classification of attributes revealed how often certain attribute categories were tested in AD DCEs thus far and how many of them turned out to be significant. Attributes were classified by KH, and in case of doubt, a second researcher (CB or MH) was consulted. The conditional relative importance scores of all attributes within a study was compared and the most important attributes were identified. If the conditional relative importance was not available, it was calculated when coefficients of attributes/levels were available. If no coefficients were available, the corresponding author of the respective study was contacted to attain essential data. Preference insights of DCEs and non-DCEs were synthesized by a subsequent summary and comparison of results to provide an holistic overview of the current scientific knowledge about treatment preferences in AD.
3 Results
3.1 Study Selection
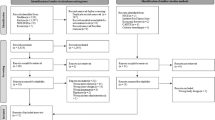
A total of 274 references were identified via the predefined search strategy. Thereof, 67 duplicates were directly removed, resulting in 207 references that underwent screening. Another 170 records were removed after title and abstract screening, and thus 37 references were screened based on full texts. A total of 24 of these were removed afterwards, resulting in the final inclusion of 13 references. Online Resource 2 contains a list of excluded studies, with respective reasons. Citation searching led to two more hits and therefore a total of 15 references underwent data extraction. The corresponding PRISMA flow chart is shown in Fig. 1.
3.2 Study Characteristics
Details regarding the general study characteristics are presented in Table 2. Ten of 15 included studies were available as full texts [22,23,24,25,26,27,28,29,30,31]. The other five references were three letters [32,33,34] and two abstracts [35, 36]. Six studies were DCEs [22, 23, 25,26,27,28], eight studies were surveys [24, 30,31,32,33,34,35,36] and one study was a randomized, investigator-blind, prospective study that compared ointment versus cream [29]. Seven references focused on the US [22, 25, 26, 29, 34,35,36], two on Denmark [30, 31], two on Japan [27, 33], one each on China [24], Poland [32], and Spain [23], and one reference included the UK, Spain and France [28]. Nine studies solely focused on adult patients [22, 25, 26, 28, 30, 31, 33, 35, 36], one study included adolescent patients in addition to adult patients [27], one study incorporated only children [24], two references explored caregivers’ perspectives [32, 34], one study looked exclusively at physicians’ point of view [23], and one study included adolescent and adult patients as well as physicians [27]. All kinds of severity levels were considered in the included studies.
3.3 Methodology of DCEs
Table 3 provides an overview of the methodology of the six DCEs. Three DCEs relied solely on literature and data when selecting attributes and levels [25, 26, 28], and the remaining three included DCEs that additionally consulted people, e.g. experts or physicians [22, 23, 27]. On average, 7.5 attributes were investigated per DCE, while the smallest number was 5 and the highest number was 10. Attributes were grouped into categories (outcomes, process and costs) and subcategories. Outcomes include efficacy and adverse events; process attributes consist of mode, frequency and place of administration, dose modifications, further medications and need for monitoring and costs only contain treatment costs. The number of attributes covered per subcategory are depicted in Fig. 2. Efficacy attributes were most commonly integrated in the choice tasks, followed by adverse events. All DCEs except for one used mode, frequency, or place of administration as an attribute [22, 23, 26, 28]. Only one DCE included costs as an attribute [27]. Fifty percent of the DCEs incorporated three alternatives per choice task, while the third alternative was an opt-out option [23, 28], and 50% included two alternatives and forced participants to make a choice [22, 25]. On average, participants were given 11.5 choice tasks. Two references stated that a fractional factorial design was used [23, 25]. The remaining DCEs did not provide information. All DCEs were administered online. Most DCEs provided information about the methods used to test participants’ comprehension [22, 25, 27, 28]. To analyze participants’ choices, four DCEs used a random parameters or mixed multinomial logit model [22, 25,26,27], one used a conditional logit model [23], and one used a multinomial logit model [28]. Heterogeneity of preferences was taken into account by four DCEs [22, 26,27,28]. The remaining two DCEs did not provide any information [23, 25]. Online Resource 3 presents additional information about the selection process of attributes and levels, the exact attributes and levels included, the methods used to test comprehension, and the topics covered in addition to the DCE questions.
Significance and conditional relative importance of attributes. Total attributes depict the number of all attributes of the six included DCEs of a specific attribute category. An attribute was considered significant when at least one attribute level was significant (at a 5% level). Okubo et al. [27] did not report coefficients for frequency of administration, therefore it was counted as not significant in this figure. Myers et al. [26] and Okubo et al. [27] did not report underlying data and therefore correctness of calculations of conditional relative importance could not be checked. Okubo et al. reported the top three most important attributes for patients and physicians separately; therefore, the top three attributes were included twice in this figure. Thus, the sum of the top three attributes is 21, not 18. DCE discrete choice experiment
3.4 Methodology of Other Study Designs
Nine of the included studies were non-DCEs, i.e. seven were surveys [24, 31,32,33,34,35,36], one was a cross-sectional study, [30] and one was a randomized, investigator-blind, prospective study [29]. Table 4 presents a detailed overview of the methodology of these studies. Most of the studies did not provide information about the selection process of the questions [24, 29, 30, 32,33,34,35]. One study conducted a targeted literature review [36] and another study took literature as well as people’s opinions into account when developing questions [31]. While all six DCEs were conducted online, this was only the case for three of the non-DCEs [30, 31, 34]. Two surveys were performed in-person [24, 33], one study used a combination of telephone and online [32], and three references did not provide information [29, 35, 36]. Overall, in non-DCEs, less treatment characteristics were investigated per study than was the case in DCEs. Topics covered in non-DCE surveys were, for instance, mode and frequency of administration [24, 29, 35, 36], specific features of a product [31, 32, 34], or whether recommendations play a role in the choice of a treatment [31, 32].
3.5 Main Treatment Preference Insights
Online Resource 4 provides an overview of the main insights regarding the treatment preferences of all included studies. This table emphasizes that study results are diverse, because study objectives and designs were diverse.
3.5.1 Significance and Conditional Relative Importance of Attributes
Figure 2 provides an overview of the investigated attribute categories, attribute significance, and attributes considered most important of the six included DCEs. For this purpose, attributes were categorized into attribute classes. Overall, most attributes, i.e. 38/45 (84%) turned out to be significant. Therefore, all included attribute categories seemed to be important for participants. Efficacy and adverse event attributes were, by far, incorporated most often and almost all attributes turned out to be significant, which indicates that these attribute categories play an essential role for participants when choosing an AD treatment. However, one cannot conclude from this finding that efficacy and adverse events are more important than other categories, as significance only indicates that these attributes are considered in decision making. To investigate which attributes are most important, conditional relative importance has to be considered. Figure 2 shows how often an attribute of a certain attribute category was part of the top three most important attributes. More than half of the 21 top-three most important attributes were efficacy and one-third were risk attributes, indicating that efficacy and risk were decisive factors for participants when choosing an AD treatment. Mode, frequency, and place of administration accounted for only 10%. This underlines that this attribute category should not go unnoticed and that it is also not the most important for participants.
3.5.2 The Importance of Efficacy and Risk Attributes
Itch relief and clear skin seem to be the most important efficacy attributes to participants [22, 25, 26, 28, 30]; participants were even willing to accept increased risks in order to improve these two attributes [25, 26]. One study reported that almost clear skin was even more important to participants than achieving complete clear skin [30]. According to another reference, best effect based on own experience was considered the most important reason to choose a certain product [31]. Despite the differences in included studies, it became clear that efficacy of treatments plays a significant role in AD treatment preferences.
Even though fewer studies identified risk attributes as most important when choosing an AD treatment, risk still seems to be very important [22, 27, 28]. As an example, risk of malignancy, serious infection, and mild adverse effects were rated high by participants [22, 27, 28]. Physicians as well as patients were willing to accept higher probabilities of risk attributes in order to increase the chances of achieving certain efficacy attributes [23, 25, 26]. For example, respondents of one study were willing to accept an increased risk of more than 6% for serious infection and more than 5% for additional risk of pain, burning, and/or stinging to increase the probability of clear or almost clear skin within 3–4 months of treatment [26].
3.5.3 The Role of Mode, Frequency, and Place of Administration
Although efficacy and risk are usually most important in the choice of a treatment, mode of administration should not be neglected [28] (see Fig. 2). Several studies found that participants preferred daily oral pills over bi-weekly injectables [22, 35, 36]; however, one research revealed that this preference might depend on the efficacy of treatment [35]. One reference identified ease in dosing and knowing when and how to take the dose as reasons for preferring a pill [36]. Not having to remember every day to take a pill and not having to worry about forgetting to take the pill were advantages of injections instead of pills [36]. Generally, patients seem to want to stop treatment when the disease is under control [35]. One study stated that a topical cream applied twice daily was the most preferred mode of administration when offering an oral pill every day, bi-weekly self-injection, or topical cream twice daily [26]. Another study found that even though a cream was easier to apply, ointment was preferred [29]. Furthermore, according to one reference, patients feel that although different treatments at hospitals or clinics are good for their skin, they do not want to visit the doctor on a regular basis [33].
3.5.4 Further Insights
One study described the ideal emollient as a non-fragrant white cream that needs to be applied two to three times per day [24], while another reference stated that it should be delivered in a bottle with a pump, personally tailored, without preservatives and allergens, and with an hydrating activity [32].
Only two studies assessed physician recommendation, which is why a general statement about the importance of this attribute is not justifiable. Nevertheless, these two studies found that recommendations play a minor part, but if a product is recommended by a physician, patients preferred it [31, 32].
One reference identified factors that are associated with opting for a new treatment [27]. Patients with high costs, low needle fear, low treatment satisfaction, and a full-time job, as well as physicians who work at facilities certified for the use of biologics, seemed to be more likely to prefer a new treatment [27].
Some references reported the impacts of certain characteristics on preferences. One study found that female physicians seem to be more risk-averse than male professionals and try to minimize the risks of adverse events [23]. Another study reported that patients with a lower self-assessed disease burden seem to care more about mode of administration than more severely affected people and would even accept greater risks in order to use an emollient instead of a self-injection [26]. Furthermore, according to one study, patients with prior self-injection experience seemed to be more willing to use injections than people without this experience, while at the same time people over 50 years of age seemed to care about receiving a pill instead of an injection [28].
When comparing physicians’ perspectives with patients’ preferences, differences are rather small. For physicians, efficacy and risk attributes are also the most important [23, 27]. Physicians seemed to be less risk averse, and according to one study, the question of add-on or replacement therapy seems to matter to them more than to patients [27]. How caregivers’ perspectives differ was difficult to assess because their preferences were not assessed in a DCE. The ideal emollient was described differently by patients and caregivers but this could also be due to the fact that different attributes were assessed in these two studies, e.g. one study including color and the other study including packaging.
3.6 Quality of Studies
Online Resource 5 and Online Resource 6 contain the quality assessment sheets for each included study. All studies that were available as full-texts were rated according to the PREFS checklist, which allows a maximum score of five in case all quality requirements are fulfilled. The average score for these 10 included full-text studies was 3.4 (68%), with 4 being the highest sum and 1 the lowest. This indicates that the average quality was satisfying, but by far, not outstanding. It was noticeable that the second item on the PREFS checklist, which asked whether respondents were similar to non-respondents, was never fulfilled because information was missing in all 10 studies. Non-DCEs in particular lacked an explanation of the methods used for preference assessment. DCEs had, on average, a higher score than non-DCEs, yielding 3.8 (76%), and therefore were of good quality with regard to the PREFS checklist. DCEs were additionally rated according to ISPOR checklist. The maximum achievable score of this list is 30. On average, the DCEs fulfilled 26.3 of all items (87.7%), with 25 being the lowest and 29 the highest score. This underlines the results of the PREFS checklist that the identified DCEs are of high quality. The main reason for not fulfilling an item was missing justification. Sampling strategy was not appropriately justified by five of six DCEs. One study provided too less information for all three statistical analyses items. Other than that, there was no pattern in non-fulfillment of items.
4 Discussion
This systematic review aimed to provide an overview of available quantitative treatment preference research of those directly or indirectly affected by AD, i.e. patients, caregivers and professionals. In total, 15 studies were rated eligible and were included in this review. The included studies were characterized by their diversity with regard to study design and participants, and research questions investigated. Synthesis of results showed that, on average, people directly or indirectly affected by AD rate efficacy and treatment-related risk most important when choosing a therapy to treat their disease. Itch and clear skin seemed to be the most important efficacy aspects. This review also shows that several studies found there is a substantial willingness to increase risks in order to have a higher chance of achieving a certain benefit, such as a reduction in itch or a clearer skin. While mode of administration was also relevant for participants, it was less important than efficacy and risk attributes, indicating that to achieve individual treatment aims, participants are willing to accept different modes of administration.
The identified studies were mainly conducted in the US (47%) [22, 25, 26, 29, 34,35,36], followed by one-third that covered a total of five European countries [23, 28, 30,31,32] and 20% that explored Asian countries [24, 27, 33]. Currently, no research is available that investigates AD treatment preferences in, for instance, Canada, Korea, Germany, or Italy, and large European countries such as the UK and France have only been explored once thus far. Cultures and healthcare systems differ between countries, which could also mean that treatment preferences are not the same in all countries. Although our study did not show large differences between countries, transferability of preferences between countries remains uncertain. Therefore, it is important that further countries are included in future AD treatment preference research, and it would even be of more value if preferences of different countries were assessed in one study.
Only six DCEs were identified in the course of this review [22, 23, 25,26,27,28]. While this may sound sufficient, several countries have not been covered thus far. Preference may differ between cultures and countries and therefore it is important to individually investigate preferences. Furthermore, no DCEs have explored caregivers’ perspectives thus far and only two studies took physicians’ preferences into account [23, 27]. Additionally, the identified DCEs mainly focused on pills and injections as the mode of administration. Only one study included emollients [26], but that application form could be of particular interest when investigating the preferences of people affected by mild-to-moderate AD and should therefore be further explored in future research. This emphasizes that there is need for further DCEs, especially to learn more about trade-offs affected people are willing to make between different treatment attributes and about which treatment characteristics are most important for different groups directly or indirectly affected by AD. Furthermore, the methodology of DCE offers the possibility to investigate the importance of different attributes in relation to each other. In surveys, this can be asked for the most important attributes, but in DCEs this can be tested as participants are asked to make a decision and not just state their preference. Although patients express their preference in a hypothetical setting and might decide differently in real life [37], a DCE can elicit preferences much more accurately than a survey can. Therefore, performing more DCEs will help physicians, patients, and caregivers to make shared decisions that best represent patients’ needs and lead to better adherence and outcomes [38].
The 15 included studies investigated different perspectives. While the majority of research questions focused solely on adult AD patients, there were also studies that investigated children’s, adolescents’, caregivers’ and physicians’ perspectives. It is however difficult to make comparisons between these different participant groups as the investigated attributes differed. As an example, studies that included adolescents, children, and caregivers focused on the constitution of a product, while studies that investigated adults incorporated specific efficacy and risk attributes. One study included both patients and physicians and found that physicians seem to be more willing to opt into new treatments than patients. This might be because physicians have more knowledge about clinical research than patients and thus could be less scared of new treatments. Another reason could be that physicians decide for other people, which leads to less risk averseness because it is not them taking the risk; however, both patients and physicians are to some extent willing to accept greater risk to increase the chance for efficacy.
Caution is advised when interpreting the results of the included studies and also of this review, as results of preference research are highly dependent on the choice of attributes investigated. An attribute that is not investigated can never become most or least important. Therefore, it is of utmost importance that study questions and investigated attributes are carefully selected based on literature and interviews. With respect to this, it is even worse that 47% of the included studies did not report how questions were selected. However, as several studies concluded that a certain characteristic is most or very important to participants, it can be assumed that this aspect is indeed relevant in the choice of treatment.
4.1 Strengths and Limitations
This review had several strengths. We carefully followed guidelines and standards for the good conduct of literature reviews, including the investigation of at least two databases and an exhaustive manual search. We included data from abstracts and letters to ensure the inclusion of all available evidence even though abstracts are not peer reviewed and despite a slight deviation from our protocol in which we planned to only include peer-reviewed articles. While this procedure helped to enrich the review, it is simultaneously a limitation as, in the absence of information, quality could not be adequately assessed. This review accounted for diverse study designs and tailored data synthesis and results presentation to individual demands of study designs; however, this hampered comparison of results of these different study types. In the absence of a sufficient amount of studies, this systematic review could not focus on a single study type. Another limitation was the language restriction in the search strategy. Additionally, quality assessment had shortcomings. In the absence of more suitable quality assessment tools for DCEs, the PREFS checklist was appraised as being the best to assess the quality of the 10 manuscripts available as full texts. This tool might not have been completely suitable for non-DCEs, as the item asking for significance tests was not applicable to them; however, all non-DCEs fulfilled this criterion and thus no harm has resulted from this decision. Furthermore, the final conduct of the review deviated slightly from the published protocol. Our first intention was to include both qualitative and quantitative research; however, sufficient quantitative work was available to answer the research question, and thus in the final review, only quantitative research was considered. An advantage of this procedure was that comparability and synthesis of results is of more value when research methods are more similar to each other.
4.2 Impact of the Results
Although shared decision making must include individuals’ preferences and perspectives, the results of this review can help dermatologists to discuss important patient considerations and thus find an adequate treatment for each individual patient. For instance, physicians could offer options with an increased risk of an adverse event that give the chance of better outcomes, or therapies with modes of administrations that could subjectively be assumed to be less comfortable for patients, as this research showed that for several patients, these treatments could be the preferred treatment options. With regard to the development of new treatments, Research and Development Departments of pharmaceutical companies could invest less capacities to adapt a therapy to a certain mode of administration but focus on reducing itch and achieving a clearer skin. While risks are important for patients in their therapy choice and should therefore be kept within limits, certain increases in risks seem to be acceptable for new and more beneficial therapies.
5 Conclusions
This systematic literature review showed that reduction in itch, better skin clearance, and less risks are decisive factors in the choice of AD treatments. Several identified studies underlined that participants were willing to accept greater risk of adverse events for an increased chance of achieving benefits. Mode of administration played a role but was not as relevant as efficacy and risks. This review revealed that the availability of treatment preference research is limited. In particular, DCEs are missing and several countries are not covered, or are only covered once, by current research. The limitation of studies as well as the diverse study designs, including the difference in investigated attributes, hampered comparison and synthesis of the results. It is important to consider that the results of preference research are highly dependent on the attributes investigated. Attributes not investigated cannot be rated by their importance even though they might be essential for affected people. To improve shared decision-making processes of physicians and AD patients, as well as to better inform research and development of new AD therapies, more preference research, especially DCEs, are needed. This could increase the likelihood of AD patients receiving the therapy that best fits their individual needs and preferences.
References
Williams HC. Atopic dermatitis: the epidemiology, causes and prevention of atopic eczema. Cambridge: Cambridge University Press; 2000.
Love I, White K. Atopic dermatitis/atopic eczema: disease landscape & forecast. Decision Resources Group; 2020.
Senra MS, Wollenberg A. Psychodermatological aspects of atopic dermatitis. Br J Dermatol. 2014;170(Suppl 1):38–43. https://doi.org/10.1111/bjd.13084.
Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119:548-552.e3. https://doi.org/10.1016/j.anai.2017.09.076.
Cheng C-M, Hsu J-W, Huang K-L, Bai Y-M, Su T-P, Li C-T, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60–5. https://doi.org/10.1016/j.jad.2015.02.025.
Avail Dermatology. Top 5 most common skin conditions. 2018. https://availdermatology.com/common-skin-conditions/. Accessed 18 Dec 2022.
Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340–7. https://doi.org/10.1016/j.anai.2018.07.006.
Ariëns LFM, van Nimwegen KJM, Shams M, de Bruin DT, van der Schaft J, van Os-Medendorp H, de Bruin-Weller M. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762–8. https://doi.org/10.2340/00015555-3212.
National Institute for Health and Care Excellence. Dupilumab for treating moderate to severe atopic dermatitis: technology appraisal guidance [TA534]. 2018. https://www.nice.org.uk/guidance/TA534. Accessed 18 Dec 2022.
van Overbeeke E, Janssens R, Whichello C, Schölin Bywall K, Sharpe J, Nikolenko N, et al. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol. 2019;10:1395. https://doi.org/10.3389/fphar.2019.01395.
Maleki-Yazdi KA, Heen AF, Zhao IX, Guyatt GH, Suzumura EA, Makhdami N, et al. Values and preferences of patients and caregivers regarding treatment of atopic dermatitis (eczema): a systematic review. JAMA Dermatol. 2023;159:320–30. https://doi.org/10.1001/jamadermatol.2022.6045.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. https://doi.org/10.1136/bmj.n71.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44. https://doi.org/10.1017/S0266462312000086.
Dobrescu AI, Nussbaumer-Streit B, Klerings I, Wagner G, Persad E, Sommer I, et al. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: a systematic review. J Clin Epidemiol. 2021;137:209–17. https://doi.org/10.1016/j.jclinepi.2021.04.012.
Centre for Reviews and Dissemination. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009.
Veritas Health Innovation. Covidence systematic review software. 2023. http://www.covidence.org. Accessed 25 Nov 2023.
Nelson HD. Systematic reviews to answer health care questions. Philadelphia: Wolters Kluwer Health; 2014.
Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. The Patient. 2017;10:553–65. https://doi.org/10.1007/s40271-017-0235-y.
Sharma P, Kularatna S, Abell B, Eagleson K, Vo LK, Halahakone U, et al. Preferences in the design and delivery of neurodevelopmental follow-up care for children: a systematic review of discrete choice experiments. Patient Prefer Adher. 2023;17:2325–41. https://doi.org/10.2147/PPA.S425578.
Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JFP. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31:877–92. https://doi.org/10.1007/s40273-013-0089-7.
Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–13. https://doi.org/10.1016/j.jval.2010.11.013.
Boeri M, Sutphin J, Hauber B, Cappelleri JC, Romero W, Di Bonaventura M. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatol Treat. 2022;33:1449–58. https://doi.org/10.1080/09546634.2020.1832185.
Carrascosa Carrillo JM, Baselga Torres E, Gilaberte Calzada Y, Jurgens Martínez YN, Roustan Gullón G, Yanguas Bayona JI, et al. Quantifying physician preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. Dermatol Ther (Heidelb). 2022;12:1197–210. https://doi.org/10.1007/s13555-022-00723-z.
Hon K-LE, Wang SS, Pong NH, Leung TF. The ideal moisturizer: a survey of parental expectations and practice in childhood-onset eczema. J Dermatolog Treat. 2013;24:7–12. https://doi.org/10.3109/09546634.2012.672713.
Kwatra SG, Lio P, Weidinger S, Calimlim B, Ladizinski B, Vigna N, et al. Patient preferences for atopic dermatitis treatments: a discrete choice experiment. J Dermatol Treat. 2023;34:2222201. https://doi.org/10.1080/09546634.2023.2222201.
Myers K, Silverberg JI, Parasuraman S, Pierce A, Eichenfield LF, Poulos C. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J Dermatol Treat. 2023;34:2215356. https://doi.org/10.1080/09546634.2023.2215356.
Okubo Y, Ho K-A, Fifer S, Fujita H, Oki Y, Taguchi Y. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatol Treat. 2020;31:821–30. https://doi.org/10.1080/09546634.2019.1623860.
Thomas C, Raibouaa A, Wollenberg A, Capron J-P, Krucien N, Karn H, Tervonen T. Patient preferences for atopic dermatitis medications in the UK, France and Spain: a discrete choice experiment. BMJ Open. 2022;12: e058799. https://doi.org/10.1136/bmjopen-2021-058799.
Cadmus SD, Sebastian KR, Warren D, Hovinga CA, Croce EA, Reveles LA, et al. Efficacy and patient opinion of wet-wrap dressings using 0.1% triamcinolone acetonide ointment vs cream in the treatment of pediatric atopic dermatitis: a randomized split-body control study. Pediatr Dermatol. 2019;36:437–41. https://doi.org/10.1111/pde.13830.
Egeberg A, Thyssen JP. Factors associated with patient-reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol. 2019;81:943–9. https://doi.org/10.1016/j.jaad.2019.06.018.
Joergensen KM, Jemec GBE. Use of moisturizers among Danish atopic dermatitis patients-which perceived product characteristics associate with long-term adherence? J Dermatol Treat. 2018;29:116–22. https://doi.org/10.1080/09546634.2017.1358803.
Kunkiel K, Natkańska A, Nędzi M, Zawadzka-Krajewska A, Feleszko W. Patients’ preferences of leave-on emollients: a survey on patients with atopic dermatitis. J Dermatol Treat. 2022;33:1143–5. https://doi.org/10.1080/09546634.2020.1772452.
Ito K, Imafuku S, Nakayama J. Therapeutic preferences are different in psoriatic and atopic dermatitis patients: a questionnaire-based study. J Dermatol. 2013;40:292–4. https://doi.org/10.1111/1346-8138.12094.
Johnson MC, Heron CE, Feldman SR. Caregiver preferences for the treatment of childhood atopic dermatitis. J Cutan Med Surg. 2021;25:336–8. https://doi.org/10.1177/1203475420988866.
Barrera T, Margolis D, Fuxench ZCC. 555 Treatment preferences in adult patients with atopic dermatitis. J Investig Dermatol. 2023;143:S95. https://doi.org/10.1016/j.jid.2023.03.561.
Prajapati VH, Eichenfield LF, Schuttlelaar ML, Ladizinski B, Vigna N, Chen N, et al. Preference for oral versus injectable treatment in adults with moderate-to-severe atopic dermatitis: results from the phase 3b heads up clinical trial. In: 4th annual revolutionizing atopic dermatitis (RAD) conference 9–11 April 2022; Baltimore, MD.
Laba T-L, Brien J, Fransen M, Jan S. Patient preferences for adherence to treatment for osteoarthritis: the MEdication Decisions in Osteoarthritis Study (MEDOS). BMC Musculoskelet Disord. 2013;14:160. https://doi.org/10.1186/1471-2474-14-160.
Blaiss MS, Steven GC, Bender B, Bukstein DA, Meltzer EO, Winders T. Shared decision making for the allergist. Ann Allergy Asthma Immunol. 2019;122:463–70. https://doi.org/10.1016/j.anai.2018.08.019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for conducting this study.
Conflicts of interest
Katja C. Heinz, Charlotte Beaudart and Mickaël Hiligsmann have no competing interests to declare that are relevant to the contents of this article. Damon Willems is also an employee of UCB Pharma, but UCB Pharma had no role in the design, conduct, and analysis of the study, or in the writing/reviewing of this manuscript.
Availability of data and material
All data generated and/or analyzed during this study are included in this published article and its Online Resource files.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by all authors. The first draft of the manuscript was written by KH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Heinz, K.C., Beaudart, C., Willems, D. et al. Treatment Preference Research in Atopic Dermatitis: A Systematic Review of Quantitative Studies. Patient (2024). https://doi.org/10.1007/s40271-024-00698-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s40271-024-00698-3