Abstract
Objectives
Collection of patient-reported outcome (PRO) measures is critical to fully understand chronic obstructive pulmonary disease (COPD) management and progression, as the impact on health-related quality of life is not well understood by objective measures alone. Electronic PROs (ePROs) are increasingly used because of their advantages over paper data collection, including elimination of transcription errors, increased accuracy and data quality, real-time data reporting, and increased compliance. The objective of this study was to characterize how patients with COPD prefer to use various types of technology to report disease symptoms, and their preferences for ePRO design and display.
Methods
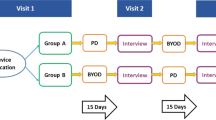
The sample consisted of subjects with COPD (N = 103) who completed in-person surveys on their ePRO preferences.
Results
The majority of subjects prefer to use a form of electronic media over paper to report their disease symptoms. Of these electronic methods, subjects most often prefer to use a smartphone provided by their physician. Subjects were also interested in ePRO features, such as knowing estimated PRO completion time at the outset, tracking their progress in real time as they complete a questionnaire, seeing the data that they report in order to track their health status, being encouraged to complete their diary if they fall behind by positive messaging, and being thanked for their completion of a daily diary.
Conclusions
Investigators should consider including these preferences when designing ePRO assessments. Incorporating patient preferences for ePRO design can ultimately help reduce patient burden and increase engagement, compliance, and improve data quality.



Similar content being viewed by others
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because all data are proprietary to eResearch Technology (ERT), but are available from the corresponding author on reasonable request.
References
Burden of COPD. In: World Health Organization: chronic respiratory diseases: chronic obstructive pulmonary disease (COPD). http://www.who.int/respiratory/copd/burden/en/index.html. Accessed 15 May 2019.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. 2019. https://goldcopd.org/gold-reports/. Accessed 15 May 2019.
Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59–63.
Gensheimer SG, Wu AW, Snyder CF, PRO-HER Users’ Guide Steering Group, PRO-HER Users’ Guide Working Group. Oh, the places we’ll go: patient-reported outcomes and electronic health records. Patient. 2018;11(6):591–8.
Chronic obstructive pulmonary disease: use of the St. George’s Respiratory Questionnaire as a PRO assessment tool: guidance for industry. 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chronic-obstructive-pulmonary-disease-use-st-georges-respiratory-questionnaire-pro-assessment-tool. Accessed 15 May 2019.
Wolpin S, Nguyen HQ, Donesky-Cuenco D, Carrieri-Kohlman V, Doorenbos A. Effects of automated prompts for logging symptom and exercise data on mobile devices in patients with chronic obstructive pulmonary disease. Comput Inform Nurs. 2011;29(2):75–80.
Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S, et al. Standardizing measurement of COPD exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183(3):323–9.
Shah SA, Velardo C, Gibson OJ, Rutter H, Farmer A, Tarassenko L. Personalized alerts for patients with COPD using pulse oximetry and symptom scores. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:3164–7.
Kulich K, Keininger DL, Tiplady B, Banerji D. Symptoms and impact of COPD assessed by an electronic diary in patients with moderate-to-severe COPD: psychometric results from the SHINE study. Int J Chron Obstruct Pulmon Dis. 2015;10:79–94.
Mazze RS, Shamoon H, Pasmantier R, Lucido D, Murphy J, Hartmann K, et al. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984;77(2):211–7.
Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ. 2002;324:1193–4.
Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004;107(3):213–9.
Dale O, Hagen KB. Despite technical problems personal digital assistants outperform pen and paper when collecting patient diary data. J Clin Epidemiol. 2007;60(1):8–17.
Given JE, O’Kane MJ, Bunting BP, Coates VE. Comparing patient-generated blood glucose diary records with meter memory in diabetes: a systematic review. Diabet Med. 2013;30(8):901–13.
Patient-reported outcome measures: use in medical product development to support labeling claims: guidance for industry. 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed 15 May 2019.
Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. European Medicines Agency; 2005. Doc. Ref. EMEA/CHMP/EWP/139391/2004.
Smith HS, Criner AJ, Fehrle D, Grabianowski CL, Jacobs MR, Criner GJ. Use of smartphone/tablet-based bidirectional telemedicine disease management program facilitates early detection and treatment of COPD exacerbation symptoms. Telemed J E-Health. 2016;22(5):395–9.
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health. 2009;12(4):419–29.
CDER patient-focused drug development. 2018. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development. Accessed 28 June 2019.
Coons SJ, Eremenco S, Lundy JJ, O’Donohoe P, O’Gorman H, Malizia W. Capturing patient-reported outcome (PRO) data electronically: the past, present, and promise of ePRO measurement in clinical trials. Patient. 2015;8:301–9.
Rose M, Bezjak A. Logistics of collecting patient-reported outcomes (PROs) in clinical practice: an overview and practical examples. Qual Life Res. 2009;18(1):125–36.
Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Caner J Clin. 2012;62:336–47.
Jose N, Langel K. ePRO vs. paper. Appl Clin Trials Online. 2010. http://www.appliedclinicaltrialsonline.com/epro-vs-paper. Accessed 15 May 2019.
Ring A, Cheong KA, Watkins CL, Meddis D, Cella D, Harper PG. A randomized study of electronic diary versus paper and pencil collection of patient-reported outcomes in patients with non-small cell lung cancer. Patient. 2008;1(2):105–13.
Hollen P, Gralla RJ, Jones RA, Thomas CY, Brenin DR, Weiss GR, et al. A theory-based decision aid for patients with cancer: results of feasibility and acceptability testing of DecisionKEYS for cancer. Support Care Cancer. 2013;21(3):889–99.
Allena M, Cuzzoni MG, Tassorelli C, Nappi G, Antonaci F. An electronic diary on a palm device for headache monitoring: a preliminary experience. J Headache Pain. 2012;13(7):537–41.
Greenwood MC, Hakim AJ, Carson E, Doyle DV. Touch-screen computer systems in the rheumatology clinic offer a reliable and user-friendly means of collecting quality-of-life and outcome data from patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):66–71.
Stinson JN, Petroz GC, Stevens BJ, Feldman BM, Streiner D, McGrath PJ, et al. Working out the kinks: testing the feasibility of an electronic pain diary for adolescents with arthritis. Pain Res Manag. 2008;13(5):375–82.
Bushnell DM, Reilly MC, Galani C, Martin ML, Ricci JF, Patrick DL, et al. Validation of electronic data capture of the irritable bowel syndrome-quality of life measure, the work productivity and activity impairment questionnaire for irritable bowel syndrome and the EuroQol. Value Health. 2006;9(2):98–105.
Araujo L, Jacinto T, Moreira A, Castel-Branco MG, Delgado L, Costa-Pereira A, et al. Clinical efficacy of web-based versus standard asthma self-management. J Investig Allergol Clin Immunol. 2012;22(1):28–34.
Gwaltney C, Coons SJ, O’Donohoe P, O’Gorman H, Denomey M, Howry C, et al. “Bring Your Own Device” (BYOD): the future of field-based patient-reported outcome data collection in clinical trials? Ther Innov Regul Sci. 2015;49(6):783–91.
Vervloet M, Linn AJ, van Weert JCM, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systemic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704.
Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21(1):3–13.
Kaptein AA, Fischer MJ, Scharloo M. Self-management in patients with COPD: theoretical context, content, outcomes, and integration into clinical care. Int J Chron Onstruct Pulmon Dis. 2014;9:907–17.
Acknowledgements
This work was supported by eResearch Technology (ERT). The authors would like to acknowledge the subjects who participated in the study.
Author information
Authors and Affiliations
Contributions
Author KMD analyzed the data and wrote the manuscript. Authors ND, LK, and ST contributed to data analysis. Authors BW and CJE performed participant interviews and edited the manuscript. Author SMD contributed to study design and edited the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed involving human participants were in accordance with the ethical standards of the national research committee (Copernicus Group IRB) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by eResearch Technology (ERT).
Conflict of Interest
Authors KMD, ND, LK, STG, and SMD are employees of eResearch Technology (ERT), which funded this research, and authors CE and BW are employees of Endpoint Outcomes, which performs services for ERT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dumais, K.M., Dias, N., Khurana, L. et al. Preferences for Use and Design of Electronic Patient-Reported Outcomes in Patients with Chronic Obstructive Pulmonary Disease. Patient 12, 621–629 (2019). https://doi.org/10.1007/s40271-019-00376-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-019-00376-9