Abstract
Damoctocog alfa pegol (BAY 94-9027, Jivi®), is a site-specifically PEGylated, extended half-life recombinant factor VIII (FVIII) that is approved in several European and non-European countries for on-demand treatment and prophylaxis of bleeding in previously treated patients aged ≥ 12 years with hemophilia A. Reliable measurements can be obtained using most one-stage and chromogenic FVIII assays over a wide concentration range. The efficacy, safety and pharmacokinetics (PK) of damoctocog alfa pegol have been studied extensively in the PROTECT VIII clinical trials, and its long-term safety and effectiveness profile is continuing to build through observational and interventional real-world studies. The PK of damoctocog alfa pegol was shown to be improved as compared with that of sucrose-formulated rFVIII (rFVIII-FS, Kogenate®), and was also demonstrated to be non-inferior to and, for some variables, more favorable than rFVIII-Fc fusion protein, efmoroctocog alfa (Elocta®; NCT03364998), rurioctocog alfa pegol (BAX 855, Adynovate®/Adynovi®; NCT04015492), and antihemophilic factor (recombinant) plasma/albumin-free method (rAHF-PFM, Advate®; NCT02483208). Damoctocog alfa pegol was generally well tolerated and none of the patients in any of the clinical trials, including the PROTECT VIII clinical program, HEM-POWR, or ongoing single-center studies, developed FVIII inhibitors. Efficacy for perioperative hemostasis has been demonstrated. Low bleeding rates were achieved across the studies, with twice weekly, every 5-day and every 7-day prophylaxis offering patients ≥ 12 years and their clinicians the chance to tailor treatment to individual needs and lifestyles, while maintaining long-term protection from bleeds and their consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This product review article provides a comprehensive overview of the safety, pharmacokinetics, and efficacy of damoctocog alfa pegol for the treatment of people living with hemophilia A. Damoctocog alfa pegol is well tolerated and is effective for prophylaxis, on-demand treatment of bleeding episodes, and perioperative management. The data support the use of damoctocog alfa pegol for tailored prophylaxis to accommodate individual needs and lifestyle while maintaining good bleed protection. |
1 Introduction
Hemophilia A is a congenital bleeding disorder caused by deficiency of clotting factor VIII (FVIII). Disease severity is defined based on residual FVIII coagulation activity (FVIII:C) in plasma [1]. In untreated patients with the severe phenotype, the majority of bleeds tend to recur into joints leading to chronic irreversible arthropathy [1, 2].
Replacement therapy with FVIII concentrates is the long-established standard of care for patients with hemophilia A; it is used to control acute bleeds, avoid their recurrence, and prevent joint damage [3, 4]. FVIII prophylaxis is the gold-standard treatment aiming to prevent the vast majority of bleeds, but its accomplishment is burdened by the need for frequent intravenous infusions. The advent of modified extended half-life (EHL) recombinant FVIII (rFVIII) products facilitated tailored treatment, as EHL products can maintain high levels of protection with less frequent infusions when compared with standard half-life (SHL) products [5]. In fact, individualized prophylaxis is the optimal treatment approach because it considers individual patients’ clinical needs, lifestyles and pharmacokinetic (PK) performance, to provide more convenient dosing that improves adherence, greater cost efficiency and superior outcomes compared with fixed prophylactic regimens [4, 6].
Damoctocog alfa pegol (BAY 94-9027, Jivi®) is an EHL rFVIII product [7] approved in several European and non-European countries worldwide for on-demand treatment and prophylaxis of bleeding in previously treated patients (PTPs) aged ≥ 12 years with hemophilia A [8,9,10,11]. This product review of damoctocog alfa pegol aims to provide a comprehensive summary of relevant published literature (based on PubMed searches for ‘Jivi’, ‘BAY 94-9027’ and ‘damoctocog alfa pegol’) at the time of writing. In Europe, damoctocog alfa pegol can be used according to three prophylactic regimens: 30–40 IU/kg twice weekly (2×W), 45–60 IU/kg every 5 days (E5D) or 60 IU/kg every 7 days (E7D). Efficacy and safety of damoctocog alfa pegol in PTPs with hemophilia A aged ≥ 12 years has been demonstrated in the PROTECT VIII trial (ClinicalTrials.gov identifier: NCT01580293) and its extension [12, 13] and studied in previously treated children aged < 12 years in the PROTECT VIII Kids study (ClinicalTrials.gov identifier: NCT01775618) and its extension [14, 15].
2 Damoctocog Alfa Pegol Chemistry and Manufacturing
The half-life of wild-type FVIII is around 12 h [7, 16]. Current ways to extend FVIII half-life include PEGylation and Fc fusion [17]. PEGylation reduces the binding of FVIII to clearance receptors, including the low-density lipoprotein receptor-related protein-1 (LRP1) [7, 18].
Damoctocog alfa pegol is a site-specifically PEGylated B-domain deleted recombinant human coagulation FVIII, conjugated with a 60 kDa PEG (a double-branched 30 kDa PEG; PEG-60) moiety [7]. The amino acid sequence of FVIII has been purposely modified by the insertion of a cysteine residue at position 1804 within the A3 domain to favor site-specific attachment of PEG, via a maleimide linker at K1804C [7]. Such modification does not impinge upon the mechanism of FVIII activation, which is the same as wild-type FVIII (Fig. 1) [7, 19].
For damoctocog alfa pegol, PEGylation was selected as the technology to enhance FVIII PK properties based on > 20 years of PEGylated therapeutics and the established use of PEG within consumer products [18]. A recent study of nonacog beta pegol, another PEGylated factor replacement product, found no PEG-related safety concerns in children followed for up to 8 years [20]. The BDD-K1804C-rFVIII (Fig. 1) variant was selected as it has high PEGylation efficiency, and 60 kDa (2 × 30 kDa) PEG-BDD-K1804C-rFVIII retained its specific activity after PEGylation. It also has a dissociation rate for von Willebrand factor (vWF) similar to full-length unmodified rFVIII [7].
2.1 Manufacturing
The damoctocog alfa pegol manufacturing process is shown in Fig. 2. It is expressed in baby hamster kidney (BHK) cells, as used in the manufacture of other rFVIII products, and the cell culture supernatant is harvested via continuous filtration. Expressed damoctocog alfa pegol is collected via an anion exchange membrane. The protein is eluted in sucrose stabilized solution and frozen down for storage (− 30 °C) [21]. Any potential impurities left over from the host cells are cleared via filtration.
The harvested protein is then PEGylated using site-specific PEGylation as described above [7, 22]. Site-specific PEGylation controls the molar ratio between rFVIII molecules and PEG moieties, which avoids accumulation and overload [22]. The purification process includes cation-exchange chromatography to separate out the PEGylated molecule from other products, and the PEGylated rFVIII is concentrated and ultra-filtered before being blast frozen to − 60 °C [21]. The average purity of a sample of 29 batches was found to be 98% [21]. The final product is thawed, pooled, and diluted to achieve specific concentrations and different dosages, followed by sterilization and packaging into 2.5 mL vials. No human- or animal-derived raw materials are added to the cell culture medium, consequently reducing the risk of pathogen transmission. The drug product remains stable for 24 months at 5 °C and 25 °C and when cycled from 5 to 25 °C for 6 months [9].
3 Assay Performance
Damoctocog alfa pegol can be measured reliably by most one-stage and chromogenic FVIII assays over a wide concentration range [23, 24]. An international comparative laboratory field study, including laboratories in North America (n = 25), Europe (n = 26) and Israel (n = 1) compared FVIII:C assay results obtained with damoctocog alfa pegol and a conventional unmodified rFVIII. Three concentrations of samples were provided (low, medium, and high), and laboratories analyzed the samples using one-stage and chromogenic assays routinely used in clinical practice. Accurate FVIII measurements were obtained at all concentrations for both products using chromogenic assays and most of the commonly used one-stage reagents, including ellagic acid- and silica-based reagents (Fig. 3) [23]. Two specific silica-based reagents, APTT-SP and PTT-A, underestimated FVIII recovery by 25% and 18%, respectively (Fig. 3) [23]. This was consistent with previous findings that PTT-A and APTT-SP underestimated FVIII by 60% and < 10%, respectively [24]. Overall, the results were more reliable with chromogenic assays as compared with one-stage assays. Recommended and unrecommended assays for damoctocog alfa pegol are shown in Table 1.
4 Preclinical Studies
The safety profile of damoctocog alfa pegol was explored preliminarily in rats and rabbits in which a dose of 2250 IU/kg administered every other day (comparable with 30 times the highest human dose of 60 IU/kg) for 2 weeks was well tolerated [25]. In juvenile rats, no adverse effects were seen with 2×W dosing of 200 and 1000 IU/kg [25]. In these studies, no histological changes (e.g. cellular vacuolation) or detectable PEG in organs and tissues were observed [25]. In a chronic 26-week toxicity study, adverse effects were not induced at 40, 400 or 1200 IU/kg administered 2×W, and no tissue vacuolation was observed [25].
PK studies in rats demonstrated the existence of excretion processes for eliminating the PEG-60 moiety [26]. Six months after the administration of a single dose of radiolabeled or nonlabeled PEG-60 moiety equivalent to the human lifetime dose, rats had excreted 68.4% of radioactivity via urine and 13.8% of the radioactivity in feces [26]. There was no evidence of irreversible binding to tissues, retention in organs, or crossing the blood–brain barrier [26]. These results suggested that patients re-treated with damoctocog alfa pegol would have very low steady-state concentrations of 60 kDa PEG and that excretion processes are in place for high molecular weight PEG molecules [26].
5 Pharmacokinetic Studies
In a phase I, prospective, non-randomized, open-label, parallel-group study (ClinicalTrials.gov identifier: NCT01184820) evaluating the PK and safety of damoctocog alfa pegol, results from 14 patients (mean [range] age 36.1 [21–58] years) demonstrated improved PK profile as compared with that of sucrose-formulated rFVIII (rFVIII-FS, Kogenate®) (Table 2) [27].
5.1 Post Hoc Pharmacokinetic (PK) Analyses
In a post hoc analysis of phase I (n = 14), PROTECT VIII (n = 22) and PROTECT VIII Kids (n = 34) studies (mean [range] age 37.0 [12–62] years), PK improvements were observed in adults, adolescents and children [28]. In line with other FVIII products, PK characteristics of damoctocog alfa pegol were age-dependent [28,29,30]. PK characteristics were similar between adolescents (12 to < 18 years of age) and adults (≥18 years of age) but differed in children (< 12 years of age), who showed somewhat faster clearance (Fig. 4) [13, 14, 27]. The PK parameters observed for adults and adolescents in the PROTECT VIII study were comparable with the 60 IU/kg dose cohort data in the phase I study. PK parameters were also similar after single- and multiple-dose infusions [28]. In addition, post hoc analysis of pooled data from adult patients in the phase I (n = 13) and PROTECT VIII (n = 21) studies noted a significant positive correlation between baseline vWF antigen levels and FVIII half-life, and significant negative correlation with clearance rate [28, 31,32,33].
Geometric mean plasma concentration–time profiles after a 60 IU/kg infusion in the phase I (60 IU/kg dose cohort only), PROTECT VIII and Kids studies† [28]. †Data are expressed as geometric mean ± geometric SD. LLOQ lower limit of quantitation, SD standard deviation
5.2 Crossover PK Studies
In an intraindividual, across-study comparison of PK results from 15 patients who participated in the LEOPOLD I [34, 35] and PROTECT VIII studies, damoctocog alfa pegol demonstrated an improved PK profile over octocog alfa and rFVIII-FS [35]. Damoctocog alfa pegol showed longer geometric mean half-life (17.1 h vs. 14.0 h and 12.1 h, respectively) and greater AUC (AUCnorm 66.8 vs. 32.5 and 27.1 h*kg/dL, respectively). Predicted time to a FVIII threshold concentration of 1 IU/dL was longer with damoctocog alfa pegol (119.7 h vs. 93.5 h and 81.2 h, respectively) following a single 50 IU/kg infusion using a population PK (popPK) approach [35].
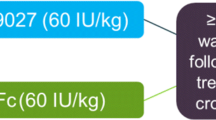
Two randomized, crossover, head-to-head studies were conducted comparing PK parameters of a single infusion of damoctocog alfa pegol with single infusions of rFVIII-Fc fusion protein, efmoroctocog alfa (Eloctate®/Elocta®; NCT03364998), and rurioctocog alfa pegol (BAX 855, Adynovate®/Adynovi®; NCT04015492) using non-compartmental analyses that demonstrated the superior PK profile of damoctocog alfa pegol over these two products (Table 3) [36, 37].
In a post hoc analysis of seven patients who had each participated in the same two crossover studies, damoctocog alfa pegol demonstrated a greater geometric mean AUCnorm (IU*h/dL per IU/kg) over efmoroctocog alfa (50.0 vs. 39.3; NCT03364998), octocog alfa (35.7; BAY 81-8973; NCT02483208), and antihemophilic factor (recombinant) plasma/albumin-free method (25.9; rAHF-PFM, Advate®; NCT02483208) [38]. Damoctocog alfa pegol also showed a longer geometric mean half-life compared with efmoroctocog alfa, octocog alfa, and rAHF-PFM (16.2 h vs. 14.5 h, 14.3 h and 11.0 h, respectively).
5.3 Population PK studies
A popPK model was developed based on PK data from the phase I, PROTECT VIII and PROTECT VIII Kids studies: this model has been used to characterize the interindividual variability of the damoctocog alfa pegol PK profile and its relationship to patient characteristics [39]. Data from the model indicated that the PK of damoctocog alfa pegol can be described adequately using a one-compartment model. Lean body weight and vWF level were identified as the determinants of interindividual variability [39].
Based on popPK modeling, a longer median time was needed to reach a 1 IU/dL FVIII threshold after a single infusion with damoctocog alfa pegol when compared with efmoroctocog alfa and rurioctocog alfa pegol (Fig. 5) [36, 37]. In a simulation reported by Berntorp et al. with 40 IU/kg damoctocog alfa pegol 2×W, it is possible to maintain FVIII levels above 20% for approximately half of the week [40].
Real-world PK data are limited. However, in a Canadian observational, retrospective, intrapatient comparison study using real-world data from 22 patients, damoctocog alfa pegol exhibited prolonged median (Q1; Q3) terminal half-life (15.28 h [13.79; 19.15] vs. 13.24 h [12.00; 16.97]), greater median (Q1; Q3) AUC (20,928 IU h/L [15,360; 24,520] vs. 11,097 IU h/L [8339; 12,998]), slower median (Q1; Q3) clearance (0.13 L/h [0.11; 0.14] vs. 0.21 [0.18; 0.24]), and slower median (Q1; Q3) time to the 1% FVIII threshold (116.4 h [105.2; 151.8] vs. 91.90 h [81.80; 118.20]) when compared with octocog alfa [41].
6 Damoctocog Alfa Pegol Clinical Evaluation
Safety and efficacy of damoctocog alfa pegol have been evaluated extensively in the PROTECT VIII clinical program that included multicenter, international studies whose designs have been described previously (Fig. 6) [12,13,14,15].
Summary of study designs for the PROTECT VIII clinical trial program. †In the PROTECT VIII main study, patients receiving prophylaxis were allowed to switch their prophylaxis treatment regimen at any time, and those receiving on-demand treatment could switch to prophylaxis in the extension study. In the PROTECT VIII extension and PROTECT VIII Kids main study and extension, investigators were asked to begin treatment with the least infusion schedule appropriate for the patient. Patients were then allowed to switch doses and dosing frequency at any time. ‡Four patients discontinued prophylaxis during the 10-week run-in period. §2×W arm included all patients experiencing > 1 breakthrough bleed in the run-in period (n = 13) or ≤1 bleed who completed the run-in when the E5D and E7D arms were full (n = 11). ‖121/126 patients completing the PROTECT VIII main study enrolled in the extension. ¶20 patients enrolled in the PROTECT VIII main study underwent 26 major surgeries during the trial and were included in the PROTECT VIII main study Part B. ††59/61 patients completing the PROTECT VIII Kids main and expansion studies were enrolled in the extension. 2×W twice weekly, E5D every 5 days, E7D every 7 days, ED exposure days, OD on demand, Var variable frequency group
6.1 Study Population
Overall, 222 PTPs were enrolled in the PROTECT VIII clinical program, of whom 149 were aged ≥ 12 years and 73 were aged < 12 years [13, 14]. Of these, 195 received at least one dose of damoctocog alfa pegol during the program, 123 patients were treated for ≥ 3 years, 94 were treated for ≥ 4, and 75 were treated for ≥ 5 years [12, 15].
6.2 Efficacy
6.2.1 Prophylaxis
In the main study, 134 patients (median age 35.9 years) received treatment with damoctocog alfa pegol [13]. Most (n = 97, 88%) patients had ≤1 breakthrough bleed during the run-in period and were eligible for 1:1 randomization to the E5D and E7D prophylactic regimens (Fig. 6). The remaining 37 patients received damoctocog alfa pegol 2×W, including 11 patients who would have been eligible for randomization based on bleeding frequency but were not randomized as that arm of the study was full. All patients achieved a reduction in their total annualized bleeding rate (ABR) compared with their respective prestudy ABRs. A summary of ABRs, including available prestudy ABR data, are presented in Table 4.
During the study, 11 patients (26%) originally randomized in the E7D arm moved to a more frequent dosing regimen (E5D: n = 8; 2×W: n = 3) with an improvement in bleeding control as noted by the reduction in median (Q1; Q3) total ABR from 16.9 (6.5; 25.2) to 4.7 (0.0; 14.7) after changing regimen. In patients who were ineligible for randomization and therefore continued their 2×W regimen, median (Q1; Q3) total ABR reduced from 17.4 (14.3; 26.0) during the run-in period to 4.1 (2.0; 10.6) during Weeks 11–36 (Table 4) [13].
A post hoc analysis of the E5D group (n = 43) in the main study showed that the best responders (ABR = 0; n = 19) were older (mean age 36.0 vs. 31.1 years), had experienced fewer bleeds (median 2.0 vs. 10.0), and had fewer target joints (57.9% vs. 70.8%) in the 12 months prior to study enrollment [42].
A post hoc analysis of the 126 patients who completed the main study showed that most patients receiving prophylaxis (n = 104) had a stabilized treatment regimen in the last 90 days of the study, with low ABRs [43]. Median (Q1; Q3) total and joint ABRs were 0.0 (0.0; 4.1) for E5D (n = 46) and E7D (n = 31) patients and 0.0 (0.0; 8.1) for 2×W patients (n = 27). Median (Q1; Q3) spontaneous ABRs were 0.0 (0.0; 4.1) across all prophylactic regimens [43].
Of those 126 patients, 121 (96%) enrolled in the extension study [12], where prophylaxis patients (n = 107) accumulated a median (range) time in the study of 3.2 (0.1–6.3) years, with 211 (9–621) exposure days (EDs). ABRs were comparable with those in the main study and mostly remained low (Table 4). Marked improvements in median ABRs were observed in the 22 patients who switched from their on-demand regimen prestudy to damoctocog alfa pegol prophylaxis; median total ABRs reduced from prestudy (33.0) to the main study (3.3) and continued to improve during extension (1.3). Similarly, median joint ABRs reduced from 22.0 prestudy to 2.1 in the main study and 0.6 in the extension.
In an interim post hoc analysis of data from the main and extension studies (n = 82), a high rate of target joint resolution was observed with long-term (approximately 4 years) prophylaxis across all prophylactic regimens (Table 5). Overall, at least one target joint was resolved in over 90% of patients [44].
Data from the main and extension studies indicated long-term efficacy of prophylactic treatment with damoctocog alfa pegol in adolescents and adults. At completion of the extension, median (range) total time in the main and extension studies was 3.9 (0.8–7.0) years, with 223 (23–698) EDs [12]. In total, 71 patients were treated for ≥ 3 years, 53 were treated for ≥ 4 years, 36 were treated for ≥ 5 years, and 22 were treated for ≥ 6 years. For the 36 patients who had ≥ 5 years of prophylaxis treatment with damoctocog alfa pegol, median (Q1; Q3) total ABR was 1.14 (0.43; 2.10); spontaneous and joint ABRs were 0.53 (0.09; 1.41) and 0.87 (0.36; 1.70), respectively. In the last 6 months of the extension, 61.1% of patients had zero total bleeds and 63.9% of patients had zero joint bleeds [12]. In the 22 patients who received damoctocog alfa pegol prophylaxis for ≥ 6 years, prestudy total and joint ABRs reduced during the extension (median [Q1; Q3] 3.0 [1.0; 15.0] to 0.9 [0.4; 2.2] and 3.0 [0.0; 12.0] to 0.6 [0.4; 1.7], respectively). The median (range) time spent in the extension was 5.6 (5.3–6.3) years. [45].
The impact of comorbidities on the efficacy of damoctocog alfa pegol treatment has been investigated using data from prophylaxis patients in the main and extension studies [46]. Patients with comorbidities of interest included those with HIV infection, hepatitis B or C infection, and risk factors for cardiovascular disease (hypertension and hyperlipidemia). The mean (standard deviation [SD]) age of these patients (n = 34) at enrollment was 49.4 (6.2) years. Nineteen (55.9%) patients presented with ≥ 1 target joints at baseline, ranging from one to six target joints, with a mean (median) of 1.5 (1.0) per patient. All patients presenting with a target joint at baseline had ≥ 1 resolved target joint by the end of the extension study. Of these, four pre-existing target joints in four patients were unresolved. Seven patients developed a new target joint during the study. Of these, three were in the E7D/Var group and all three resolved during the extension. Two were in the E5D/E5D group and one was E7D/E7D. One patient was in the 2×W/2×W group and their joint also resolved during the extension. Prestudy median (Q1; Q3) total ABR for all 34 patients was 6.0 (0.0; 15.0). During the main study and extension, median total ABRs were 2.1 (0.0; 5.8) and 2.2 (0.6; 6.0), respectively. No discontinuations due to adverse events (AE) or serious AEs (SAEs) occurred in either the main or extension studies in this population.
Overall, 73 patients were enrolled in the PROTECT VIII Kids main and expansion studies (the expansion study increased the size of the group aged < 6 years), of whom 61 completed the studies [14]. In the main study, 61 patients received at least one dose of damoctocog alfa pegol. Median (range) age of patients aged < 6 years (n = 32) and 6 to < 12 years (n = 29) was 3.0 (2.0–5.0) years and 9.0 (6.0–11.0) years, respectively. Median total ABR in study completers (n = 53) was < 3.0 and similar in both age cohorts; median joint and spontaneous ABRs were ≤2.5 (Table 4). Of 53 patients who completed the main study, 44 (72%) remained on their initial dosing regimen. Of 9 patients who switched, 8 switched from E7D to a more frequent dosing regimen. Improvements in ABR for total, joint, spontaneous, and trauma bleeds were observed in these 8 patients following the switch (preregimen switch median [Q1; Q3] 18.3 [12.3; 29.2], 4.8 [1.0; 12.9], 8.9 [0.0; 16.3], and 6.4 [0.0; 18.4] vs. post-regimen switch 2.6 [0.7; 5.3], 0.0 [0.0; 0.7], 0.0 [0.0; 1.0], and 1.7 [0.0; 2.6], respectively). This marked improvement in bleed control was reflected in a comparative analysis of all bleeds in the first 90 days versus the last 90 days of treatment in those who completed the study (n = 53); median (Q1; Q3) total ABR reduced from 4.1 (0.0; 4.1) to 0.0 (0.0; 4.1) in patients aged < 6 years, and from 2.0 (0.0; 8.1) to 0.0 (0.0; 4.1) in those aged 6 to < 12 years. Of the 12 patients enrolled in the expansion, 67% (n = 8) completed the study [14].
Of the 61 patients who completed the main or expansion studies, 59 (97%) continued into the extension [15]. Median (range) age of patients at the end of the extension was 9.0 (3.0–12.0) years for those aged < 6 years (n = 32) and 15.0 (10.0–18.0) for those aged 6 to < 12 years (n = 27). Most patients (n = 57, 97%) enrolled completed the extension, with a median (Q1; Q3) total ABR of 1.6 (0.52; 3.07). Low median joint and spontaneous ABRs (< 1.0) were also observed (Table 4). Of those enrolled, most (n = 50, 85%) were on the same dosing regimen at the start and end of the extension, and 47 (80%) patients never varied their regimens. Nine patients switched their regimens: 8 (13.6%) switched from E5D (n = 6) and E7D (n = 2) to a 2×W regimen, and one patient decreased their dosing frequency (E5D to E7D). Most patients who completed the extension received long-term treatment with damoctocog alfa pegol: 52 patients completed ≥ 3 years of treatment, 41 completed ≥ 4 years, and 39 completed ≥ 5 years [15].
During the PROTECT VIII Kids main and extension studies, 46 patients were followed up for ≥ 1 year. Within each patient’s final 12 months of observation, 100% of new and historic target joints had resolved (Table 5).
6.3 Treatment of Bleeds
A total of 1518 bleeding events were reported in the PROTECT VIII clinical program, 702 in the main study [12, 13] and 816 in the pediatric study [14, 45]. In adolescent/adult patients, the mean (range) dose to treat bleeds was 33.7 (14–62) IU/kg/infusion. Most bleeds (81.1%) were treated with one infusion of damoctocog alfa pegol; 9.5% required two infusions and 9.4% required ≥ 3 infusions. Where a bleed required a second infusion, the mean length of time between the first and second infusion was 2.1 days. Most patients (72.4%) reported good or excellent response to the treatment of bleeds [13].
In children, 93% of bleeds during the main study were treated with one or two infusions and the mean (range) dose to treat bleeds was 46.8 (21–71) IU/kg/infusion [14]. Response to treatment was rated by patients/caregivers as good or excellent for 85.7% of bleeding events [14]. In the expansion, all bleeds (n = 9) were treated with a single infusion [15]. In the extension, the number of bleeds in the < 6 years and 6 to < 12 years age cohorts were similar (323 bleeds and 344 bleeds, respectively); most of these bleeds were treated with ≤2 infusions and achieved a good or excellent response-to-treatment rating for the majority of bleeding events (87.4%) [15].
6.4 Perioperative Management
Damoctocog alfa pegol proved effective in preventing postoperative bleeding complications during major and minor surgeries.
PROTECT VIII Part B enrolled 22 patients who underwent 26 major surgeries [47]. Most surgeries (81%) were orthopedic, including 14 joint replacements. Median dose on the day of surgery was 77.57 (42.9–136.4) IU/kg, in a median of two infusions. Median (range) preoperative dose was 52.9 (41–64) IU/kg. Intraoperative blood loss was within the expected ranges (median [range] 50 mL [0–1000 mL]). Hemostatic efficacy of damoctocog alfa pegol during major surgery was reported to be ‘good’ (65%) or ‘excellent’ (35%) in all cases, as assessed by the surgeon. Three patients required blood transfusion during knee surgery [47].
Interim analysis of 41 patients (36 adolescents and adults, 5 pediatric patients) who underwent minor surgeries showed that hemostasis was rated as ‘good’ (56.1%) or ‘excellent’ (43.9%) during 57 of the 69 procedures [48]. No bleeding complications were observed during the intra- or postoperative period with minimal blood loss reported. Of the 69 minor surgeries performed, most were dental procedures (n = 45; 65.2%), 7 were orthopedic (10.1%), 7 were dermatologic (10.1%), 4 were ophthalmologic (5.8%), and 6 were classified as ‘other’ (8.7%). Only 9 surgeries required ≥ 1 infusion [48].
In the PROTECT VIII Kids extension, no major surgeries were performed (as per the study protocol) [15]. A total of 17 minor surgeries were performed in 14 patients, most (n = 16; 94.0%) being elective. Based on available data, hemostatic control was rated ‘good’ or ‘excellent’ in all cases except for one that received a moderate rating [15].
6.5 Damoctocog Alfa Pegol Utilization
A summary of FVIII utilization in the PROTECT VIII studies is presented in Table 6. During the PROTECT VIII extension, the number of infusions per year received by patients treated with E7D prophylaxis (median [range] 53.0 [48.7–67.6]) was marginally higher than those who received treatment on-demand (median [range] 38.0 [18.6–108.0]) [12]. Nevertheless, E7D prophylaxis resulted in marked improvement in ABRs compared with the on-demand group [12]. Median (range) total number of infusions per year for those receiving prophylaxis during the PROTECT VIII extension was 75.0 (48.7–143.9). For those who completed ≥ 5 years of damoctocog alfa pegol treatment, median (Q1; Q3) FVIII utilization and dose per infusion for those receiving prophylaxis were 3332 (3144; 3991) IU/kg/year and 51 (44; 58) IU/kg/infusion, respectively, indicating that utilization of damoctocog alpha pegol was quite consistent over the years [12].
In the PROTECT VIII Kids expansion study, eight patients had a total mean ± SD dose of 4933 ± 931 IU/kg/year [14]. In the PROTECT VIII Kids extension, the median dose per year was 4062.4 IU/kg and 4160.1 IU/kg in patients who had completed ≥ 5 years of treatment. Median dose per infusion for all patients was 49.0 IU/kg, and the median number of infusions per year was 77.5 [15].
6.6 Safety
None of the patients in the PROTECT VIII clinical program developed FVIII inhibitors [8, 12,13,14,15]. Three patients in the PROTECT VIII Kids extension were transiently positive for low-titer anti-FVIII antibodies; no patient showed any clinical evidence of inhibitor activity [15]. A positive result for anti-FVIII antibodies at a low-titer (1.7 BU/mL) was reported in one adult following surgery [8]. Follow-up inhibitor test results were negative in all patients [8, 15].
In the PROTECT VIII main and extension studies, study drug-related AEs were experienced by 12 patients and 10 patients, respectively (Table 7) [13]. Of these, two patients in the extension study experiencing study drug-related AEs reported SAEs, leading to study drug discontinuation (Table 7) [12]. All other study drug-related AEs were mild or moderate, including increased alanine aminotransferase, mild urine β2 microglobulin increase, bone marrow edema, arthralgia, meniscal degeneration, and moderate osteoarthritis [12]. In the PROTECT VIII Kids main and extension studies, study-drug related AEs were experienced by 13 patients and 4 patients, respectively (Table 7) [14, 15].
During the PROTECT VIII Kids main study, 11 patients aged < 6 years discontinued treatment due to loss of efficacy (n = 8) or hypersensitivity reactions (n = 3) during the first four EDs. Of these, four patients developed transient anti-PEG antibodies of immunoglobulin (Ig) M class. In fact, all IgM immune responses resolved upon switch to prestudy treatment product without Ig class switch. No other loss of efficacy, hypersensitivity reaction, or anti-PEG antibody detection after the first four EDs was reported in patients completing the PROTECT VIII Kids main and extension studies [15].
In the adult and adolescent study population, five patients in the PROTECT VIII main study and seven patients in the extension study were transiently positive at different timepoints for low-titer anti-PEG antibodies [12, 13]. None of these patients tested positive for anti-PEG antibodies at two consecutive visits; all of these patients tested negative at the final visit. In the extension study, an additional patient also tested positive for low-titer anti-PEG antibodies at the final visit; the positive/transient nature of these could not be confirmed, as no further testing was possible under the study protocol. No patient had any anti-PEG antibody-associated immunogenicity and/or clinical symptoms in the PROTECT VIII clinical program after the first four EDs [12,13,14,15].
Most patients in the PROTECT VIII clinical program had no detectable levels of PEG in the plasma (lower limit of quantification [LLOQ], 0.1 mg/L). Of 121 patients in the PROTECT VIII extension, 3 (2%) had low-level plasma PEG just above the LLOQ that was not detected at subsequent visits, and one patient had low-level plasma PEG at the final visit [12]. In the PROTECT VIII Kids extension, 11 (19%) of 59 patients had low-level plasma PEG. PEG was detected at a single visit only for six of these patients [15]; for three of those six patients, PEG detection occurred during the last visit and it was not possible to confirm that PEG detection was transient.
During major surgeries, AEs were noted in 19 patients [47]. In three of these patients, AEs were deemed by the investigator to be study drug-related. One patient had hematoma in the surgery site, while the other two patients had low-titer anti-FVIII antibodies in the blood sample collected immediately before surgery. However, one of these patients had 0.5 BU/mL anti-FVIII antibody noted at study enrollment (this patient was eligible to take part in the study as the protocol excluded only those with anti-FVIII antibody titers ≥ 0.6 BU/mL), and the other patient tested negative in a follow-up test [47].
6.7 Patient-Reported Outcomes
Patient-reported outcomes, including the Work Productivity and Activity Impairment (WPAI) and Haemophilia specific Quality of Life for Adults (Haemo-QoL-A) questionnaires captured in the PROTECT VIII study indicated reduced treatment burden with damoctocog alfa pegol and improved QoL. At all explored time points (baseline, Week 10 and Week 36), patients receiving prophylaxis had improved levels of activity compared with those receiving on-demand treatment. Improvements in all Haemo-QoL-A scores were observed in patients who switched from on-demand treatment to prophylaxis in the PROTECT VIII study (Fig. 7), with the highest increase in QoL noted in the E7D regimen. This is in line with the marked improvements in ABRs noted for this subgroup of patients [12].
Improvement† in Haemo-QoL-A scores from baseline to Week 36 during the PROTECT VIII trial in a the total prophylaxis population and b patients previously treated with on-demand rFVIII. †Data are mean changes from baseline, higher scores reflect better QoL. Haemo-QoL-A Haemophilia-specific health-related quality-of-life questionnaire for adults, PPX prophylaxis, QoL quality of life
Based on WPAI, at the end of the PROTECT VIII main study, there was no activity impairment in adults receiving prophylaxis (median [Q1; Q3] 0.0% [0.0; 20.0]; n = 110) vs. 30.0% (0.0; 50.0) in those treated on-demand (n = 20). During prophylaxis, improvement was observed in the E7D regimen (n = 42): mean (±SD) change from baseline was − 9.3% (±19.9). These changes were similar to those observed in the 2×W eligible but not randomized group (n = 11). A substantial improvement was observed in patients who switched from on-demand to prophylaxis. Mean (±SD) change in impairment score from baseline in the on-demand (prestudy) to prophylaxis switch group (n = 22) was − 15.9 (±23.0) [median (Q1; Q3) − 10.0 (−20.0 to 0.0)] versus − 4.9 (±18.6) [median (Q1; Q3) 0.0 (–20.0; 0.0)] in the prophylaxis (prestudy) to prophylaxis switch group (n = 86).
Based on Haemo-QoL-A, patients previously treated on-demand who switched to prophylaxis (2×W or E7D) during the study demonstrated improvements in total score and physical functioning subscore. In the 2×W eligible but not randomized group (n = 4), median changes of five and nine points in the total score and physical functioning subscale score were noted, respectively. In the E7D regimen (n = 5), these changes were much more pronounced, with median changes of 14 and 13 points in the total score and physical functioning subscale score, respectively.
At the end of the PROTECT VIII extension study, a qualitative exit interview was conducted with 10 physicians and 16 patients to gather views on infusion frequency and the potential benefits of reduced infusion frequency with damoctocog alfa pegol [49, 50]. The majority (n = 11, 69%) of patients who participated in the exit interview were receiving an E5D regimen [49], while the remainder were receiving an E7D regimen (n = 3) or 2×W regimen (n = 2). The interviews highlighted less frequent administration compared with conventional FVIII replacement therapies [49]. These attributes were associated with increased ability to participate in physical activities, reduced time to schedule and administer FVIII, reduced impact on work, and improved emotional wellbeing [49]. Based on a post hoc analysis comparing patients’ and physicians’ views from the exit interviews, both groups were in concordance on the experience with damoctocog alfa pegol [50]. Both physicians (80%) and patients (88%) reported that damoctocog alfa pegol led to an improvement in bleed frequency compared with the patients’ previous FVIII therapy [50]. Physicians reported improved confidence, which was supported by a statistically significant (p = 0.04) improvement on the Haemo-QoL-A emotional impact subscale from baseline to the end of the main study [50].
7 Post-Approval Studies, Trials, and Analyses
Since its approval in 2018 [8,9,10,11], several studies have further investigated the efficacy, PK, and utilization of damoctocog alfa pegol as compared with other FVIII products, all with similar outcomes. An interventional, postmarketing study of damoctocog alfa pegol prophylaxis in patients with severe hemophilia A was recently completed [51, 52].
7.1 Real-World Evidence
The effectiveness and safety of damoctocog alfa pegol beyond the PROTECT VIII clinical program is being further evaluated by ongoing interventional and observational studies that will capture long-term and real-world data in a broad population (Table 8), including HEM-POWR, a multinational, multicenter, non-interventional, prospective, postmarketing cohort study, and HA-SAFE, a multinational, long-term observation study of the safety of damoctocog alfa pegol in clinical practice. [51,52,53,54]. In addition, the planned JOIHA study will obtain real-world evidence (RWE) on the effectiveness of damoctocog alfa pegol in the protection of joints in clinical practice. JOIHA will look at changes from baseline in joint status and the association between joint status and joint bleeds, target joints, pain, and physical activity in adult patients with hemophilia A [55].
The first independent RWE data for damoctocog alfa pegol was obtained from a retrospective analysis of 10 patients switching from on-demand treatment (n = 2 patients with moderate hemophilia A) or prophylaxis (n = 8 patients with severe hemophilia A) with SHL FVIII to damoctocog alfa pegol prophylaxis at a Portuguese hemophilia reference center, with follow-up after 5–9 months [56]. Pre-switch, 5 patients (50%) had arthropathies and 2 (20%) had previous FVIII inhibitors. Patients received individualized prophylaxis with damoctocog alfa pegol at a dose of approximately 29 IU/kg (ranging from 16–37 IU/kg, 2×W, every 4 days, E5D, or E7D). Post-switch, monthly dosing frequency decreased by 33.3% and bleeding rate decreased from 1 to 0.25 for all previous SHL FVIII prophylaxis patients (n = 8). No FVIII inhibitors were evident in the 2 patients with previous inhibitors, no observed spontaneous joint bleeds, and 80% of patients had zero bleeds [56]. A further single-center study is ongoing at Bonn, Germany [57], and another is planned at Aarhus, Denmark.
7.2 Matching Adjusted Indirect Comparisons
Matching adjusted indirect comparisons (MAICs) are a validated methodology used to statistically compare the efficacy of two or more products in the absence of a randomized controlled trial [58,59,60,61]. Two MAICs have demonstrated similar mean ABRs following damoctocog alfa pegol treatment, but with lower utilization, compared with efmoroctocog alfa, rurioctocog alfa pegol, octocog alfa (Table 9) [58], and turoctocog alfa pegol (N8-GP, Esperoct®) [62]. Mean ABRs for damoctocog alfa pegol versus turoctocog alfa pegol were 4.10 versus 3.70 (adjusted treatment difference [95% confidence interval {CI}] 1.11 [0.85–1.44]), but with a significant reduction of 26.7% in FVIII utilization (mean [95% CI] 3546.9 [3536.2–3557.6] IU/kg/year vs. 4845.0 [4127.2–5562.8] IU/kg/year) [62].
This MAIC-reported reduction in FVIII utilization with damoctocog alfa pegol is supported by a popPK model that used PK data from 49 patients who participated in head-to-head crossover PK studies of damoctocog alfa pegol versus rFVIII-FS [28], efmoroctocog alfa [36], and rurioctocog alfa pegol [37]. The model predicted that damoctocog alfa pegol reduces FVIII utilization by 33–58% compared with these rFVIII products, when targeting and maintaining a FVIII threshold of 3% on a 2×W prophylaxis regimen [63].
8 Future Perspectives
Further studies are ongoing (Table 8). Alfa-PROTECT (ClinicalTrials.gov identifier: NCT05147662) is a phase III, single-arm study to assess the suitability of damoctocog alfa pegol for use in young patients with hemophilia A aged ≥ 7 years [64, 65]. The PREDICT study (ClinicalTrials.gov identifier NCT05036278) is assessing a new patient scoring system, based on the best known predictive, phenotypic variables that are expected to predict the optimal regimen for a favorable outcome when switching from SHL FVIII products to damoctocog alfa pegol [66].
9 Conclusions
The efficacy, safety and PK of damoctocog alfa pegol have been studied extensively in the PROTECT VIII clinical trials, and its long-term safety and effectiveness profile is continuing to build via a range of both observational and interventional real-world studies. The low bleeding rates and maintenance of factor thresholds demonstrated with 2×W, E5D, and E7D damoctocog alfa pegol prophylaxis offer patients ≥ 12 years and their clinicians the freedom to select an individualized regimen to suit patients’ needs and lifestyles, while maintaining long-term, comprehensive hemophilia A management.
Data Availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
Mahlangu JN, et al. BAY 81–8973, a full-length recombinant factor VIII for the treatment of hemophilia A: product review. Ther Adv Hematol. 2018;9(7):191–205.
Centers for Disease Control and Prevention (CDC). Hemophilia Homepage. 2018. Available at: https://www.cdc.gov/ncbddd/hemophilia/facts.html. Accessed 9 Jul 2024
Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17(7):493–508.
Srivastava A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158.
Lieuw K. Many factor VIII products available in the treatment of hemophilia A: an embarrassment of riches? J Blood Med. 2017;8:67–73.
Petrini P, et al. Individualizing prophylaxis in hemophilia: a review. Expert Rev Hematol. 2015;8(2):237–46.
Mei B, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270–9.
European Medicines Agency. Jivi, INN- damoctocog alfa pegol. 2020 [cited 2021 July]; Available at: https://www.ema.europa.eu/en/documents/product-information/jivi-epar-product-information_en.pdf. Accessed 9 Jul 2024
US Food and Drug Administration. JIVI Package Insert. 2021 [cited 2021 Jul]; Available at: https://www.fda.gov/files/vaccines,%20blood%20%26%20biologics/published/Package-Insert-JIVI.pdf. Accessed 9 Jul 2024
Government of Canada Regulatory decision summary - Jivi - Health Canada. 2018.
Pharmaceuticals and Medical Devices Agency Review report (Jivi, Damoctocog alfa pegol). 2018.
Reding MT, et al. Confirmed long-term safety and efficacy of prophylactic treatment with BAY 94-9027 in severe haemophilia A: final results of the PROTECT VIII extension study. Haemophilia. 2021;27:e347–e356.
Reding MT, et al. Safety and efficacy of BAY 94–9027, a prolonged-half-life factor VIII. J Thromb Haemost. 2017;15(3):411–9.
Santagostino E, et al. PROTECT VIII Kids: BAY 94–9027 (PEGylated Recombinant Factor VIII) safety and efficacy in previously treated children with severe haemophilia A. Haemophilia. 2020;26(3):e55–65.
Mancuso, M.E., et al. PROTECT VIII kids extension study: Long-term safety and efficacy of BAY 94-9027 (damoctocog alfa pegol) in children with severe haemophilia A. Haemophilia. 2021;27:434–444.
Graf L. Extended half-life factor VIII and factor IX preparations. Transfusion Med Hemother. 2018;45(2):86–91.
Dumont JA, et al. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119(13):3024–30.
Ivens IA, et al. PEGylated therapeutic proteins for haemophilia treatment: a review for haemophilia caregivers. Haemophilia. 2013;19(1):11–20.
Saenko EL, et al. Factor VIII—novel insights into form and function. Br J Haematol. 2002;119(2):323–31.
Walsh KS, et al. Nonacog beta pegol prophylaxis in children with hemophilia B: safety, efficacy, and neurodevelopmental outcomes for up to 8 years. Res Pract Thromb Haemost. 2024;8(2): 102341.
Whetstone, P., et al. BAY 94-9027: a long-acting, site-specifically PEGylated, B-domain–deleted recombinant factor VIII product of high and consistent purity for prolonged protection in patients with hemophilia A. In: THSNA. 2018.
Dozier JK, Distefano MD. Site-Specific PEGylation of Therapeutic Proteins. Int J Mol Sci. 2015;16(10):25831–64.
Church N, et al. Factor VIII activity of BAY 94–9027 is accurately measured with most commonly used assays: Results from an international laboratory study. Haemophilia. 2018;24(5):823–32.
Meijer P, et al. Inter-Laboratory Evaluation of the Recovery of Bay 94–9027 [Jivi®] with One-Stage Clotting and Chromogenic Assays. Blood. 2019;134(Supplement 1):1124.
Ivens IA, et al. Nonclinical Safety Assessment of a Long-Acting Recombinant PEGylated Factor Eight (BAY 94–9027) With a 60 kDa PEG. Toxicol Pathol. 2019;47(5):585–97.
Baumann A, et al. Pharmacokinetics, excretion, distribution, and metabolism of 60-kDa polyethylene glycol used in BAY 94–9027 in rats and its value for human prediction. Eur J Pharm Sci. 2019;130:11–20.
Coyle TE, et al. Phase I study of BAY 94–9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12(4):488–96.
Shah A, et al. BAY 94–9027, a PEGylated recombinant factor VIII, exhibits a prolonged half-life and higher area under the curve in patients with severe haemophilia A: comprehensive pharmacokinetic assessment from clinical studies. Haemophilia. 2018;24(5):733–40.
US Food and Drug Administration. Adynovate (antihemophilic factor [recombinant], pegylated). Full Prescribing Information; Shire, Westlake Village, CA. 2017. Available at: https://www.drugs.com/pro/adynovate.html#s-34090-1. Accessed 9 Jul 2024
US Food and Drug Administration. Eloctate (antihemophilic factor [recombinant], Fc fusion protein). Full Prescribing Information, Bioverativ, Cambridge, MA. 2017. Available at: https://www.drugs.com/pro/eloctate.html. Accessed 9 Jul 2024
Lalezari S, et al. Correlation between endogenous VWF: Ag and PK parameters and bleeding frequency in severe haemophilia A subjects during three-times-weekly prophylaxis with rFVIII-FS. Haemophilia. 2014;20(1):e15-22.
Konkle BA, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–85.
Powell JS, et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119(13):3031–7.
Saxena K, et al. Efficacy and safety of BAY 81–8973, a full-length recombinant factor VIII: results from the LEOPOLD I trial. Haemophilia. 2016;22(5):706–12.
Solms A, Delesen H, Maas Enriquez M, Kenet G, Lalezari S. Intra‐individual across‐study comparison of pharmacokinetics of rFVIII‐FS, bay 81‐8973 and bay 94‐9027 in patients with severe haemophilia A (P043 EAHAD 2020). Haemophilia. 2020;26. https://doi.org/10.1111/hae.13911
Shah A, et al. Direct comparison of two extended-half-life recombinant FVIII products: a randomized, crossover pharmacokinetic study in patients with severe hemophilia A. Ann Hematol. 2019;98(9):2035–44.
Solms A, et al. Direct comparison of two extended half-life PEGylated recombinant FVIII products: a randomized, crossover pharmacokinetic study in patients with severe hemophilia A. Ann Hematol. 2020;99(11):2689–98.
Shah A, et al. Improved pharmacokinetics with BAY 81–8973 versus antihemophilic factor (Recombinant) plasma/albumin-free method: a randomized pharmacokinetic study in patients with severe hemophilia A. Clin Pharmacokinet. 2017;56(9):1045–55.
Solms A, et al. Favorable pharmacokinetic characteristics of extended-half-life recombinant factor VIII BAY 94–9027 enable robust individual profiling using a population pharmacokinetic approach. Clin Pharmacokinet. 2020;59(5):605–16.
Berntorp E, et al. Optimising prophylaxis in haemophilia A: The ups and downs of treatment. Blood Rev. 2021;50: 100852.
Matino D, et al. Canadian clinical experience on switching from standard half-life recombinant factor VIII (rFVIII), octocog alfa, to extended half-life rFVIII, damoctocog alfa pegol, in persons with haemophilia A >/= 12 years followed in a Comprehensive Hemophilia Care Program in Canada. Haemophilia. 2024;30(2):345–54.
Santagostino E, et al. Characteristics of Bleed-Free Patients on Every-5-Day Dosing in the Protect VIII (BAY 94–9027) Study. Blood. 2018;132(Supplement 1):2486–2486.
Liu TRM, et al. Bleeding Outcomes During the Last 90 Days of Treatment with BAY 94–9027: Results From a Post-hoc Analysis of the PROTECT VIII Study. Haemophilia. 2019;25(S2):3–77.
Reding, M.T., et al. Target joint resolution in patients with haemophilia A receiving long-term prophylaxis with BAY 94-9027. 2020. 26(1365-2516 (Electronic)):3.
Mancuso ME, et al. Efficacy and safety of Bay 94–9027 prophylaxis is sustained for ≥ 6 years: Outcomes in 22 patients in the PROTECT VIII extension study. Haemophilia. 2021;27(S2):18–181.
Reding MT, et al. Efficacy and safety of damoctocog alfa pegol prophylaxis in patients ⩾40 years with severe haemophilia A and comorbidities: post hoc analysis from the PROTECT VIII study. Ther Adv Hematol. 2023;14:20406207231166780.
Santagostino E, et al. Safety and efficacy of BAY 94–9027, an extended-half-life factor VIII, during surgery in patients with severe hemophilia A: results of the PROTECT VIII clinical trial. Thromb Res. 2019;183:13–9.
Santagostino E. et al. Safety and efficacy of BAY 94-9027, an extended-half-life factor VIII, during minor surgical procedures in patients with severe haemophilia A. Haemophilia (under review). 2021;27:e559–e562.
Wells JR, et al. Exploring the impact of infusion frequency in hemophilia A: exit interviews with patients participating in BAY 94–9027 extension studies (PROTECT VIII). Patient. 2019;12(6):611–9.
Lalezari S, et al. Comparing physician and patient perspectives on prophylactic treatment with BAY 94–9027 for severe haemophilia A: a post hoc analysis. Adv Ther. 2020;37(6):2763–76.
ClinicalTrials.gov. Study to Gain More Information on How Safe and Effective Jivi Works in Patients With Severe Hemophila A (Post-marketing Investigation). 17 May 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04085458.
Holme PA, et al. Safety and efficacy of BAY 94–9027 prophylaxis in patients with severe haemophilia A: interim results of a post-marketing interventional study. Haemophilia. 2021;27(Suppl 2):125.
Sanabria M, et al. Design of the HEM-POWR study: a prospective, observational study of real-world treatment with damoctocog alfa pegol in patients with haemophilia A. BMJ Open. 2021;11: e044997.
ClinicalTrials.gov. Study to Learn More About the Safety of Drug Jivi Over a Long Period of Time in Previously Treated Patients With Hemophilia A (Bleeding Disorder Resulting From a Lack of FVIII) Who Are Receiving Jivi Regularly at Their Treating Doctors to Prevent Bleeding (HA-SAFE). 17 May 21. Available at: https://clinicaltrials.gov/ct2/show/NCT04461639.
Di Minno G. A prospective, observational, italian study to assess long-term effectiveness of damoctocog alfa pegol prophylaxis on joint health in adults with haemophilia A (JOIHA) PO024. Haemophilia. 2022;28:25–126.
Campanico S, et al. Prophylaxis with damoctocog alfa pegol: real world usage and effectiveness in patients with Hemophilia A of a Portuguese hemophilia Reference Centre PO110. Haemophilia. 2022;28:25–126.
Mulders G. POSTER ABSTRACTS. Haemophilia. 2023;29(S1):24–202.
Batt K, et al. Matching-adjusted indirect comparisons of annualized bleeding rate and utilization of BAY 94–9027 versus three recombinant factor VIII agents for prophylaxis in patients with severe hemophilia A. J Blood Med. 2019;10:147–59.
Signorovitch JE, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–45.
Phillippo, D., et al. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. NICE. 2016.
Signorovitch JE, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7.
Vashi P, et al. Indirect treatment comparison of damoctocog alfa pegol versus turoctocog alfa pegol as prophylactic treatment in patients with hemophilia A. J Blood Med. 2021;12:935–43.
Solms, A., et al. Predicted FVIII consumption of BAY 94-9027 compared with standard- and extended-half-life FVIII products in patients with severe hemophilia A [abstract]. Res Pract Thromb Haemost. 2021;5:423–424.
ClinicalTrials.gov. A Study to Learn How Safe the Study Treatment BAY94-9027 is and How it Affects the Body in Previously Treated Children Aged 7 to Less Than 12 Years With Severe Hemophilia A, a Genetic Bleeding Disorder That is Caused by the Lack of a Protein Called Clotting Factor 8 (FVIII) in the Blood. 2022 [cited 4 May 2022]. Available at: https://clinicaltrials.gov/ct2/show/NCT05147662.
Jonathan Ducore, et al. Alfa-PROTECT: a phase 3, open-label study to evaluate the safety of damoctocog alfa pegol in previously treated children aged 7 to < 12 years with severe hemophilia A in THSNA. 2022.
Young G, et al. PREDICT: A multicenter, prospective, open-label, clinical study using a new risk score approach to assess the most appropriate prophylaxis regimen to reach favorable outcomes in hemophilia A, when switching from standard-half-life products to damoctocog alfa pegol. Blood. 2021;138(Suppl 1):2115.
ClinicalTrials.gov. Evaluating Effectiveness and Long Term Safety of Damoctocog Alfa Pegol in Patients Who Have Been Diagnosed With Hemophilia A (HEM-POWR). 2019 [cited 24 Aug 2022]. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03932201.
ClinicalTrials.gov. Prophylaxis Regimen for Hemophilia A Patients (PREDICT). 2022 [cited 24 Aug 2022]. Available at: https://clinicaltrials.gov/ct2/show/NCT05036278.
Acknowledgments
This review was funded by Bayer. The authors are grateful for the contributions of all PROTECT VIII and other study investigators and site staff. The authors thank Graeme Baldwin and Lianne Holloway of Darwin Healthcare Communications (Oxford, England) for providing medical writing support, which was fully funded by Bayer, in accordance with Good Publication Practice (GPP) guidelines.
Funding
This article was funded by Bayer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
MTR, SL, GK, GDM, JD, AS, AS, PAH, LHP, KM, MS, and MEM contributed to data selection and interpretation, and were involved in the review design. All authors contributed to the development of the manuscript, reviewed and commented on each draft, and approved the final draft.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have consented to the publication of this review.
Conflict of interest
Mark T. Reding has received grants and research support from Bayer and BioMarin; honoraria and consultation fees from Bayer, CSL Behring, Novo Nordisk, Sanofi Genzyme and Takeda; and participates in a company-sponsored speakers bureau for Bayer, CSL Behring, Sanofi Genzyme, and Takeda. Shadan Lalezari has participated in advisory boards for Bayer; and has received honoraria or consultation fees from Bayer, Teva Pharmaceuticals, Takeda, PI Healthcare, Roche, and Pfizer. Gili Kenet has no disclosures to declare. Giovanni Di Minno has participated in speakers bureaus for Bayer, CSL Behring, Novo Nordisk, Pfizer, Sobi and Takeda; and has received consultancy or speakers fees from Bayer, Novo Nordisk, Pfizer, Sanofi Aventis, and Sobi. Jonathan Ducore has received consultancy fees from Bayer and HEMA Biologics and has participated in speakers bureaus for Bayer. Alexander Solms is an employee and shareholder of Bayer AG. Anita Shah is an employee of Bayer and owns Bayer stocks. Pål André Holme has received grant/research support, paid to his institution, from Bayer, Octapharma, Pfizer, Shire, and Sobi, and is a consultant for Bayer, Biomarin, CSL, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and Sobi. Lone H. Poulson has received grant/research support from Pfizer, and congress support from Bayer, Novo Nordisk, Octapharma, Pfizer, and Sobi. Karina Meijer has no disclosures to declare. Mindy Simpson has received institutional research grants from Bayer, Bioverativ/Sanofi, Daichii-Sankyo, Octapharma, Novo Nordisk, Baxalta (Shire, Takeda), and Roche (Genentech), and has received honoraria and consultation fees from Bayer, CSL Behring, Octapharma, Novo Nordisk, Roche, and Takeda/Shire. Maria Elisa Mancuso has received honoraria and consultation fees from Bayer, Bioverativ, Catalyst, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche, BioMarin, Shire/Takeda and Sobi, and participates in a company-sponsored speakers bureau for Bayer, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche, BioMarin, Shire/Takeda and Sobi.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Reding, M.T., Lalezari, S., Kenet, G. et al. Damoctocog Alfa Pegol, a PEGylated B-domain Deleted Recombinant Extended Half-life Factor VIII for the Treatment of Hemophilia A: A Product Review. Drugs R D (2024). https://doi.org/10.1007/s40268-024-00481-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s40268-024-00481-7