Abstract
Background and Objectives
New acute pain medications are needed that provide effective analgesia while minimizing side effects and opioid exposure. Clinical trials of co-crystal of tramadol-celecoxib (CTC) have demonstrated an improved benefit/risk profile versus tramadol or celecoxib alone. We pooled data from two phase 3 clinical trials to evaluate the efficacy of CTC 200 mg twice daily (BID) in acute moderate-to-severe pain.
Methods
Efficacy data were pooled from STARDOM1 [acute pain following oral surgery (NCT02982161)] and ESTEVE-SUSA-301 [acute pain following bunionectomy (NCT03108482)]. The primary efficacy outcome was sum of pain intensity difference from 0 to 48 h (SPID0–48).
Results
A total of 344 patients received CTC 200 mg BID, 342 received tramadol 50 or 100 mg four times a day, 181 received celecoxib 100 mg BID, and 172 received placebo. The least-squares mean difference in SPID0–48 was −21.8 (p = 0.002) for CTC versus tramadol and −72.8 (p < 0.001) for CTC versus placebo. A similar pattern of SPID0–48 was observed with CTC versus comparator whether patients had moderate or severe pain at baseline. Reduction in pain intensity was faster and reached mild intensity earlier with CTC versus comparators. Patients were significantly (p ≤ 0.005) less likely to receive rescue medication within 4 or 48 h with CTC compared with tramadol or placebo.
Conclusions
This pooled analysis reinforces the efficacy profile of CTC versus tramadol and, given that CTC permits lower daily tramadol dosing and thereby reduces unnecessary opioid use, this highlights its improved benefit/risk profile and its potential for the management of moderate-to-severe pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We evaluated the efficacy of co-crystal of tramadol-celecoxib [CTC 200 mg twice daily (BID)] in a pooled analysis of trials in acute moderate-to-severe pain. Formal comparisons were conducted versus tramadol and placebo, but descriptive analyses were used for celecoxib as it was only included as a comparator in one of the studies. |
Compared with tramadol and placebo, CTC 200 mg BID provided rapid and effective analgesia, while also reducing the need for rescue medication. These results were mirrored in descriptive comparisons with celecoxib. |
Exposure to opioids, either from tramadol dosing or use of opioid rescue medication, was reduced in patients receiving CTC versus tramadol. CTC 200 mg BID provides an improved benefit/risk profile versus tramadol. |
1 Introduction
Acute pain, described as a pain of sudden onset resulting from a specific injury or disease, is highly prevalent in prehospital (~ 40–70%) [1,2,3] and emergency department settings (~ 60–90%) [2, 4,5,6], and is thought to affect millions of people each year [2]. Indeed, moderate-to-severe pain represents a primary driver for attendance at the emergency department [7]. Unfortunately, the treatment of pain has been reported to be inadequate in the emergency department, at discharge, and after surgery [7,8,9,10]. Given that unrelieved acute pain can negatively impact health and quality of life and may develop into chronic pain [11, 12], there is a need to improve its management. Unfortunately, current single-agent analgesics, such as opioids and non-steroidal anti-inflammatory drugs (NSAIDs), are hampered by side effects [13,14,15], and opioids also have a potential for dependence [15,16,17]. The adverse effects of opioids appear to be dose related [18, 19] and the speed at which plasma concentrations are achieved may also promote drug liking [20]. Consequently, there is a need for new pain medications that provide effective analgesia with fewer side effects [21], while also reducing doses of opioids when managing postoperative pain, in hospital and after discharge.
Multimodal therapy allows the opportunity to provide effective analgesia at lower doses of opioids and thereby mitigate the potential for dose-dependent opioid-related adverse events [22, 23]. Consequently, multimodal therapy is recommended by guidelines for acute pain management [2]. The co-crystal of racemic tramadol hydrochloride and celecoxib (CTC) not only represents a multimodal approach, but it also adjusts the pharmacokinetic profiles of its constituents. CTC targets multiple pain pathways, both central and peripheral [24]. Tramadol is a centrally acting synthetic atypical opioid analgesic with multiple actions, having agonist properties at the µ-opioid receptor from both the parent drug and its primary metabolite, (+)-O-desmethyl-tramadol (M1), and an ability to inhibit noradrenaline and serotonin reuptake, enhancing the inhibitory effects on pain transmission in the spinal cord [25]. Celecoxib is a NSAID selective for cyclooxygenase-2 that is also effective for the treatment of acute pain [26]. CTC is formulated as 100 mg orally administered, immediate-release tablets (44 mg rac-tramadol hydrochloride and 56 mg celecoxib). It was approved in the USA in 2021 [27] and has received European regulatory approval (in Spain) in 2023 [28].
Co-crystals combine two or more active pharmaceutical ingredients in a single entity form, and are distinct from combinations of the individual active pharmaceutical ingredients: co-crystallization of active pharmaceutical ingredients can modify their physicochemical properties, optimizing bioavailability and pharmacokinetics and permitting synchronous release [24, 29]. In the case of CTC, co-crystallization has been shown to confer optimized pharmacologic and pharmacokinetic profiles of tramadol and celecoxib compared with those observed with either agent alone or when administered concomitantly, and this may provide clinical benefits beyond that seen with combinations of the individual products [24, 30,31,32,33]. Indeed, by targeting multiple complementary pain pathways and optimizing pharmacokinetics, analgesic efficacy may be improved and the daily tramadol dosage decreased, thereby reducing the potential for adverse effects or development of opioid dependence.
Phase 3 clinical trials have demonstrated that CTC provides a better benefit/risk profile compared with either tramadol or celecoxib alone in adults with acute moderate-to-severe pain [34,35,36,37]. Moreover, CTC permitted lower cumulative daily dosing of tramadol in adults with acute moderate-to-severe pain [36].
To further evaluate the use of CTC we conducted a formal pooled analysis (to strengthen and validate analyses from the individual trials alone) of the two phase 3 clinical trials of CTC in models of acute moderate-to-severe somatic pain. Through use of a pooled analysis, and the inherent larger sample size, the power of efficacy analyses is increased, improving the precision of efficacy estimates and allowing for more robust analysis of subgroups. We also provide additional information through number needed-to-treat (NNT) calculations based on data from both studies.
2 Methods
2.1 Study Design: ESTEVE-SUSA-301
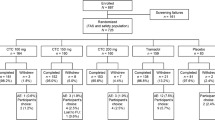
The ESTEVE-SUSA-301 study design has been described in full previously [37]; key aspects are shown in Fig. 1. In brief, ESTEVE-SUSA-301 was a phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial conducted at centers in the USA (NCT03108482). The study enrolled patients aged ≥ 18 years with moderate-to-severe pain [pain intensity rating score of 5–9 on the Numerical Pain Rating Scale (NPRS) 0–10] within 8 h of stopping local anesthesia (continuous local anesthetic popliteal sciatic nerve block infusion) following primary unilateral first metatarsal osteotomy, with internal fixation and no additional collateral procedure.

Adapted from Co-crystal of tramadol-celecoxib (CTC) for acute moderate-to-severe pain, Langford R. et al., Current Medical Research and Opinion, 2024, reprinted by permission of the publisher Informa UK Limited trading as Taylor & Francis Ltd, http://www.tandfonline.com
Summary of the study design and features of the ESTEVE-SUSA-301 and STARDOM1 phase 3 clinical trials of CTC. BID twice daily, CTC co-crystal of tramadol-celecoxib, QID four times daily, SPID sum of pain intensity difference.
Patients (N = 637) were randomized 2:2:2:1 to receive oral CTC 200 mg twice daily (BID) (n = 184), tramadol 50 mg four times daily (QID) (n = 183), celecoxib 100 mg BID (n = 181), or placebo QID (n = 89). Randomization was stratified by study center and baseline pain score: moderate (NPRS 5–6) or severe (NPRS 7–9). After a ≤ 28-day screening period, patients were admitted to the study center on the day of surgery and remained there for three nights, during which rescue medication could be administered, and returned for a follow-up visit 5–9 days post-surgery. The treatment period was 48 h. Rescue medication for pain could be administered after initiation of study medication. First-line rescue medication was intravenous acetaminophen 1 g every 4–6 h as needed, up to 4 g in 24 h; second-line rescue medication was oxycodone 5 mg immediate-release tablets every 4–6 h as needed, up to 30 mg in 24 h.
The primary efficacy endpoint was the sum of pain intensity differences (SPID) from 0 to 48 h (SPID0–48). SPID was calculated as a time-weighted sum of pain intensity difference values at each follow-up timepoint (difference between starting pain intensity and pain intensity at the given assessment timepoint) multiplied by time (hours) since the last nonmissing assessment. SPID is a measurement of analgesic effectiveness, which quantifies overall pain relief over a given period [35].
2.2 Study Design: STARDOM1
The STARDOM-1 study design has been described in full previously [36]; key aspects are shown in Fig. 1. STARDOM1 was a phase 3 randomized, double-blind, active- and placebo-controlled trial conducted at sites in Canada, Germany, Hungary, Italy, Poland, and Spain (NCT02982161; EudraCT number: 2016-000592-24). Eligible patients were aged ≥ 18 years who, within 28 days of screening, had undergone an elective oral surgical procedure (extraction of ≥ 2 impacted third molars, including ≥ 1 mandibular molar) that required bone removal and occurred without immediate complication, and were experiencing acute pain of moderate-to-severe intensity [defined as a rating of ≥ 45 mm on a 100-mm Pain Intensity Visual Analog Scale (PI-VAS) measured within 6 h of the procedure] as a result of the surgery.
Patients (N = 726) were randomized 2:2:2:2:1 to receive oral CTC 100 mg BID (n = 164), CTC 150 mg BID (n = 160), CTC 200 mg BID (n = 160), tramadol 100 mg QID (n = 159), or placebo QID (n = 83); the treatment period was 72 h. Randomization was stratified by baseline pain intensity score: moderate (PI-VAS ≥ 45 and < 70 mm) or severe (PI-VAS ≥ 70 mm). Oral acetaminophen QID up to a maximum of 4000 mg daily was permitted as rescue medication.
The primary endpoint was the sum of pain intensity difference over 0–4 h (SPID0–4). SPID was defined as the weighted difference (baseline pain minus current pain) using PI-VAS.
2.3 Pooled Analysis
A systematic pooled analysis of efficacy was conducted using integrated data from ESTEVE-SUSA-301 and STARDOM1. Since the two studies used different pain intensity scales, the PI-VAS 0–100 mm scores from STARDOM1 were normalized and converted to a 0–10 scale comparable with the NPRS scale used in ESTEVE-SUSA-301 to harmonize the two datasets. The primary objective was to assess the analgesic efficacy of CTC 200 mg BID versus Total Tramadol 50/100 mg QID (i.e., patients receiving tramadol 50 or 100 mg QID) and versus placebo for the management of moderate-to-severe pain by evaluating SPID0–48. Secondary objectives were assessments of analgesic efficacy of CTC 200 mg BID versus Total Tramadol and versus placebo on SPID0–4, 30% and 50% responder rates at 4 and 48 h, total pain relief (TOTPAR; time-weighted sum of pain relief at each timepoint that is the area under the time–analgesic effect curve for pain relief) up to 4 h (TOTPAR0–4) and up to 48 h (TOTPAR0–48), and rescue medication use.
A subgroup analysis was also conducted by baseline pain intensity [moderate (NPRS ≤ 6) versus severe (NPRS > 6)]. Since celecoxib was included as a comparator in ESTEVE-SUSA-301 only, no formal hypothesis testing of celecoxib was performed.
For the primary analysis, missing pain assessments within the first 48 h after administration of study treatment were imputed by linear interpolation (i.e., using a time-weighted average of the previous and next available values). A windowed last observation carried forward (LOCF) approach was used to account for rescue medication use, where the last pain assessment before rescue medication was used for the expected duration of rescue medication (4 h). If subjects did not complete pain intensity assessments for the full 48-h period, the SPID for the primary analysis was calculated up to the last available timepoint and later timepoints were not imputed. A similar approach was used for calculating TOTPAR. Both SPID0–48 and SPID0–4 were analyzed using an analysis of covariance (ANCOVA) model with treatment and study as fixed effects and baseline pain intensity as covariate, comparing CTC versus placebo, CTC versus Total Tramadol, and Total Tramadol versus placebo. The probability of being a 30% or 50% responder was analyzed using a logistic regression model with treatment and study as fixed effects, and baseline pain intensity as covariate. A Cox regression analysis was used to analyze time to first rescue medication use.
All statistical analyses were performed using Statistical Analysis Systems (SAS®) Software version 9.4. All statistical tests were based on a two-sided test at a significance level of 0.05 and 95% confidence interval (CI).
2.4 Study Oversight
Both studies were conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines and received ethical approval. All patients provided written informed consent.
3 Results
3.1 Baseline Characteristics
A total of n = 344 patients received CTC 200 mg BID, n = 342 received tramadol (50 or 100 mg QID), n = 181 received celecoxib 100 mg BID, and n = 172 received placebo. Approximately three-quarters of the pooled population were female and over 90% of patients were < 65 years of age. Baseline pain severity among the respective treatment groups was evenly distributed between the moderate (NPRS ≤ 6; 47.5–56.4%) and severe (NPRS > 6; 43.6–52.5%) pain subgroups (Table 1). There were no relevant differences between treatment groups in terms of distribution by key baseline and demographic characteristics (Table 1).
3.2 SPID
The mean pain intensity values from baseline to 48 h in the overall population are shown in Fig. 2. The reduction in severity of pain intensity was faster and occurred to a greater extent in subjects treated with CTC 200 mg BID compared with those treated with Total Tramadol, celecoxib 100 mg BID, or placebo. In the CTC treatment group, a mean pain intensity reduction of one point occurred approximately 1 h post-treatment, and a two-point reduction was observed after approximately 4 h. In the Total Tramadol group, a mean pain intensity reduction of one point occurred approximately 2 h post-treatment, and a two-point reduction was observed after approximately 8 h. Patients treated with CTC reached levels of mild pain severity (NPRS < 4) at ~ 6 h. This was compared with the achievement of mild pain severity at ~ 12 h with Total Tramadol, > 24 h with celecoxib, and ~ 30 h with placebo. Although there was a slight increase in pain intensity at 12 h in CTC-treated subjects, a reduction of greater than two points (NPRS) was maintained with respect to baseline.
In general, patients in the CTC group experienced greater reductions in pain intensity versus the Total Tramadol group, and versus the placebo and celecoxib groups. In the primary analysis, the least-squares mean (LSM; 95% CI) SPID0–48 was −163.3 (−173.2, −153.5) for CTC 200 mg BID, −141.5 (−151.4, −131.6) for Total Tramadol, and −90.5 (−104.5, −76.6) for placebo. LSM differences (95% CI) were −21.8 (−35.8, −7.9; p = 0.002) and −72.8 (−89.8, −55.7; p < 0.001) for CTC 200 mg BID versus Total Tramadol and placebo, respectively, and −50.9 (−68.0, −33.9; p < 0.001) for Total Tramadol versus placebo (Fig. 3a). A subgroup analysis by baseline pain severity demonstrated a similar pattern of differences in pain intensity with these treatments (Fig. 3b, c).
Least-squares mean difference (95% CI) in SPID0-48 in the a overall population, and in patients with b moderate pain intensity at baseline, and c severe pain intensity at baseline (FAS). BID twice daily, CI confidence interval, CTC co-crystal of tramadol-celecoxib, FAS full analysis set, LSM least-squares mean, SPID0–48 sum of pain intensity difference from 0 to 48 h
The LSM (95% CI) SPID0–4 was −5.9 (−6.8, −5.0) for CTC 200 mg BID, −3.2 (−4.1, −2.3) for Total Tramadol, and 0.2 (−1.1, 1.5) for placebo. LSM differences (95% CI) were −2.7 (−4.0, −1.4; p < 0.001) and −6.1 (−7.7, −4.6; p < 0.001) for CTC 200 mg BID versus Total Tramadol and placebo, respectively, and −3.5 (−5.1, −1.9; p < 0.001) for Total Tramadol versus placebo (Fig. 4).
3.3 The 30% and 50% Responder Rates
A greater proportion of patients achieved a 30% or 50% response at 4 or 48 h with CTC 200 mg BID versus Total Tramadol or placebo (Fig. 5a, b).
Responder rates at 4 and 48 h for a a 30% reduction and b a 50% reduction in pain intensity (FAS). Two-sided p values for treatment comparisons are reported from a logistic regression model with treatment and study as fixed effects, and baseline pain intensity as covariate. BID twice daily, CTC co-crystal of tramadol-celecoxib, FAS full analysis set
A total of 41.3% and 28.2% of patients achieved a 30% reduction in pain intensity at 4 and 48 h, respectively, with CTC 200 mg BID, compared with 24.9% and 21.3%, respectively, with Total Tramadol, and 10.5% and 11.0%, respectively, with placebo. The odds ratio (OR; 95% CI) to achieve a 30% response significantly favored CTC 200 mg BID versus Total Tramadol [4 h: OR 2.1 (1.5, 3.0), p < 0.001; 48 h: OR 1.5 (1.0, 2.1), p = 0.031] and versus placebo [4 h: 6.1 (3.6, 10.5), p < 0.001; 48 h: OR 3.5 (2.0, 5.9), p < 0.001], and Total Tramadol versus placebo [4 h: OR 2.9 (1.7, 5.0), p < 0.001; 48 h: OR 2.3 (1.3, 4.0), p = 0.003].
After 4 h, 34.3% of patients treated with CTC 200 mg BID achieved a 50% reduction in pain intensity compared with 20.2% with Total Tramadol and 6.4% with placebo. The OR [95% CI] of achieving a 50% response at 4 h also favored CTC 200 mg BID versus Total Tramadol and placebo [2.1 (1.5, 3.0); p < 0.001 and 7.9 (4.1, 15.1); p < 0.001, respectively], and Total Tramadol versus placebo [3.8 (1.9, 7.3), p < 0.001]. These response rates translate to a NNT of four for CTC 200 mg BID compared with seven for Total Tramadol.
After 48 h, 27.9% of patients achieved a 50% reduction in pain intensity with CTC 200 mg BID, compared with 20.8% with Total Tramadol and 11.0% with placebo. The ORs (95% CI) to achieve a 50% response for CTC 200 mg BID versus Total Tramadol and placebo at 48 h were 1.5 (1.1, 2.2), p = 0.024, and 3.4 (2.0, 5.8), p < 0.001, respectively, and to achieve a 50% response at 48 h for Total Tramadol versus placebo was 2.2 (1.3, 3.9), p = 0.004. These response rates translate to an NNT of six for CTC 200 mg BID compared with ten for Total Tramadol.
3.4 TOTPAR
Table 2 reports LSM TOTPAR0–48 and TOTPAR0–4 for the placebo, Total Tramadol, and CTC groups along with between-group LSM differences. LSMs TOTPAR0–48 and TOTPAR0–4 scores were greater with CTC than with Total Tramadol and placebo. The LSM difference (95% CI) in TOTPAR0–48 significantly favored CTC 200 mg BID versus placebo [26.9 (17.8, 36.1); p < 0.001] and Total Tramadol versus placebo [20.6 (11.5, 29.8); p < 0.001]. LSM difference in TOTPAR0–4 significantly favored CTC 200 mg BID versus Total Tramadol [1.6 (1.0, 2.2); p < 0.001] and versus placebo [3.2 (2.5, 4.0); p < 0.001]. LSM difference in TOTPAR0–4 also significantly favored Total Tramadol versus placebo [1.6 (0.9, 2.4); p < 0.001].
3.5 Rescue Medication
A lower proportion of patients in the CTC 200 mg BID group received any rescue medication within 4 h (44.5%) or within 48 h (65.7%) compared with patients in the Total Tramadol group (58.8% and 75.1%, respectively) or the placebo group (76.7% and 87.8%, respectively) (Table 3). Moreover, numerically fewer doses of rescue medication were required by 4 or 48 h for patients in the CTC 200 mg BID group versus the Total Tramadol and placebo groups. The proportion of patients receiving rescue medication within 4 or 48 h in the Total Tramadol group was lower than in the placebo group, and patients in the Total Tramadol group also required fewer doses of rescue medication versus placebo.
Logistic regression confirmed that fewer patients in the CTC 200 mg BID group took rescue medication versus Total Tramadol within 4 h [OR (95% CI) 0.6 (0.4, 0.8); p < 0.001] and 48 h [0.6 (0.4, 0.9); p = 0.005] and versus placebo within 4 h [0.2 (0.2, 0.4); p < 0.001] and 48 h [0.2 (0.1, 0.4); p < 0.001]. Use of rescue medication was also lower in the Total Tramadol group versus placebo within 4 h [0.4 (0.3, 0.6); p < 0.001] and 48 h [0.4 (0.2, 0.7); p < 0.001].
The time to first rescue medication is shown in Fig. 6. The median (95% CI) time to rescue medication was 6.9 h (4.2, 8.4) for the CTC 200 mg BID group, 2.3 h (1.9, 3.2) for the Total Tramadol group, and 1.5 h (1.2, 1.9) for the placebo group. Cox regression analysis confirmed that time to first rescue medication use was significantly shorter for both Total Tramadol and placebo versus CTC 200 mg BID (p < 0.001 for both comparisons).
4 Discussion
This pooled analysis of two clinical trials assessed the analgesic efficacy of CTC 200 mg BID versus tramadol alone and versus placebo in patients with moderate-to-severe acute somatic pain. The results of the analysis demonstrated that CTC 200 mg BID reduced pain intensity over 4 h and over 48 h, and significantly increased the probability of achieving a 30% or 50% reduction in pain intensity versus tramadol and versus placebo. A 50% reduction in pain intensity was achieved in 34.3% of patients at 4 h and 27.9% at 48 h with CTC, compared with 20.2% and 20.8%, respectively, with Total Tramadol, and 6.4% and 11.0%, respectively, with placebo. This translated to smaller NNTs for a 50% response with CTC (NNT4h = 4; NNT48h = 6) than with tramadol (NNT4h = 7; NNT48h = 10). In addition to providing effective analgesia, fewer patients treated with CTC required rescue medication. Moreover, when rescue medication was used, it occurred later during the treatment course, and fewer doses were required with CTC than for patients receiving tramadol or placebo. Achievement of analgesia also appeared to be rapid, with reductions in pain intensity occurring faster than with comparators. Indeed, response rates and achievement of total pain relief during the first 4 h were greater with CTC than with tramadol. The delivery of early reductions in pain intensity to a mild level that were sustained and better than seen with tramadol would seem to be a desirable trait for the clinical control of acute moderate-to-severe pain.
CTC represents a multimodal approach to analgesia that targets central and peripheral pain pathways [24]. The co-crystallization of celecoxib and tramadol allows the synchronous release of the individual components and also optimizes their pharmacokinetics [30, 32, 33]. Pharmacokinetics studies have noted that the maximum plasma concentration for tramadol and celecoxib with CTC were lower than those observed with equivalent doses of the individual components alone [30, 32, 33]. Furthermore, the time to achieve the maximum plasma concentrations with CTC was slower than tramadol alone and faster than celecoxib alone [30, 32, 33]. The modified pharmacokinetic profile achieved by co-crystallization of tramadol and celecoxib may contribute to the clinical benefits observed with CTC, such as improved efficacy and rapid onset of effect. In addition, the modified pharmacological profile might also help to explain why numerical reductions in adverse events have been noted with CTC versus tramadol alone or tramadol and celecoxib in combination [30, 32, 33, 35, 36].
Opioid analgesics have an important role in the management of postoperative pain. However, they are associated with numerous side effects, such as nausea, vomiting, constipation, and somnolence [15, 38], as well as a potential for misuse and dependence [39]. Side effects associated with opioid use may prolong length of stay after surgery and impact patient satisfaction [39]. Consequently, the importance of minimizing opioid use is recognized in Enhanced Recovery After Surgery (ERAS) protocols [39]. In addition to improving tolerability and patient satisfaction, it is hoped that minimizing opioid exposure by dose and/or duration will also reduce the risk of dependence.
Guidelines recognize the benefits of a multimodal treatment as a strategy for reducing opioid use in many postoperative settings [2, 40,41,42]. In our analysis we noted that CTC offers the potential for lower daily dosing of opioids compared with tramadol alone, with patients randomized to CTC receiving the equivalent of 176 mg of tramadol per day, whereas patients receiving tramadol alone received either 200 or 400 mg daily. In addition, we showed that use of rescue medication (typically acetaminophen but could also include oxycodone in the ESTEVE-SUSA-301 trial) was lower in the CTC group, with fewer patients needing rescue medication, fewer doses being used, and rescue medication being administered later compared with patients in the tramadol and placebo groups. A reduction in the use of oxycodone as a rescue medication has been reported previously in patients receiving CTC versus tramadol, celecoxib, or placebo in the ESTEVE-SUSA-301 trial [37]. Oxycodone is associated with adverse events, such as constipation, nausea, and vomiting, that may impact quality of life [43], and it appears to have a greater potential for misuse than tramadol [17]. Therefore, treatment options that help to limit exposure to oxycodone or other opioid rescue medication may be useful. In our analysis, the overall benefit/risk profile of CTC was supported by its ability to provide effective analgesia while also reducing the overall amount of opioid analgesia received after surgery. This reduced exposure to opioids with CTC may be reflected in the reports from the original trials of fewer treatment-related adverse events compared with tramadol, including events typically associated with opioids, such as nausea, vomiting, and constipation [36, 37].
Our pooled analysis has various strengths and limitations that should be considered when interpreting the data. Pooling of data provides more of an overall picture of efficacy across a broader patient population, while also increasing the number of patients, and thus the statistical power and potential to identify sources of variation. The trials pooled in our analysis also employed standard models of acute somatic pain that were different yet complementary, resulting in moderate-to-severe acute pain with a predictable trajectory and sharing a complex pathophysiology involving tissue damage and inflammation resulting from a painful bone removal procedure. Trials assessing the efficacy of medications to treat acute pain need to be validated, reliable, and highly sensitive, and the postoperative bunionectomy and molar impaction models meet these requirements [44]. By demonstrating consistent effects across different models, it is thought that the efficacy of treatments may be extrapolated to a wider acute pain population [44]. It is therefore reassuring that the efficacy of CTC was demonstrated in a pooled analysis population reflecting these two different models, suggesting that the results may be generalizable to patients with moderate-to-severe acute pain. In addition to pooling data, this analysis supplements the previous studies by providing NNT data to aid interpretation. Finally, another potential strength of our analysis is its consistency with the findings presented in the primary analyses [36, 37].
Potential limitations that should be considered include the lack of efficacy analyses of pooled CTC versus celecoxib, due to a celecoxib arm not being included in one of the two studies. However, descriptive data were included for celecoxib on responder rates, change in pain intensity, and rescue medication use. Our analysis also pooled data from two different dose regimens for the Total Tramadol group, and it has been suggested that the 50 mg QID dose may have been lower than the full therapeutic dose [37]. Nevertheless, the 50 mg QID dose of tramadol provides effective analgesia and was considered by the US Food and Drug Administration to be a suitable and fair comparator [37]. However, it should be noted that when administered as a free combination, the dissolution and absorption of tramadol and celecoxib interact. Indeed, the maximum serum concentration of celecoxib is reduced when used in a free combination with tramadol, possibly due to the effects of co-administration on the dissolution and absorption profile of celecoxib, which normally has low solubility [30, 32]. This has been replicated in other pharmacokinetic studies [33]. Consequently, a free combination of tramadol and celecoxib was not included as a comparator in these studies. Another potential limitation of our analysis is its generalizability to a broader postoperative or acute pain patient population, as the majority of patients were female, which reflected the inclusion of a population of patients seeking treatment for a bunionectomy, and most were < 65 years of age, which reflected the inclusion of patients undergoing third molar extraction. However, both tramadol and celecoxib are well-established treatments for acute pain, and it would be reasonable to extrapolate that CTC efficacy would translate to a broader population of patients with acute moderate-to-severe pain. This has resulted in an indication for CTC in adults with acute pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate [27]. However, it should be noted that the studies used for our analysis excluded patients using NSAIDs or other pain relief medications prior to enrolment and patients with inadequate pain relief from tramadol, celecoxib, or acetaminophen [36, 37].
Further considerations regarding our pooled analysis include differences between the studies in terms of pain scales used, primary endpoints, and the original approaches for imputing missing data. Although the pain scales used in each study were different and required conversion before integration for analysis, the scales were validated and considered to be standard, and the conversion was simple. The original primary endpoints of ESTEVE-SUSA-301 (SPID0–48) and STARDOM1 (SPID0–4) were used as primary and secondary endpoints, respectively, in the pooled analysis. The choice of SPID0–48 as a primary endpoint is in line with regulatory guidance, which recommends the assessment of SPID [45] over a timescale that includes the duration of drug effect and possibly beyond [46]. Since the SPID assessment reflects a cumulative response to the therapeutic intervention but does not provide information on temporal aspects of the analgesia, we also included SPID0–4 as an assessment to understand the analgesia provided in the shorter term, and because this timepoint may be considered of interest when using tramadol as a comparator in the acute pain setting. The time to onset of rescue medication and the descriptive data showing change in pain intensity add further support to our understanding of the speed of analgesic onset, and the NNTs, responder rates, and rescue medication use provide additional clinically relevant context to the data.
Finally, the individual studies employed different approaches to impute missing values. The STARDOM1 trial in patients with impacted third molar extraction used a LOCF approach when PI-VAS scores were missing or from the last available value before receiving rescue medication [36]. In contrast, the ESTEVE-SUSA-301 study in patients with bunionectomy performed pain intensity assessments upon each request for rescue medication, with the score replacing timed assessments over the subsequent 4 h [37]. The present pooled analysis employed the windowed LOCF approach used in the ESTEVE-SUSA-301 trial since single imputation methods may not be totally reliable in multidose studies, and a windowed LOCF approach provides advantages in that data were imputed only during periods when they were “contaminated” by the presence of rescue medication and not when rescue medication had worn off [47].
5 Conclusions
Our analysis pooled data from two phase 3 trials of CTC in patients with acute somatic postoperative pain to increase the statistical power for efficacy assessments and strengthen conclusions beyond those observed in the individual studies. In patients with moderate-to-severe acute pain, CTC 200 mg BID provided superior and more rapid analgesic efficacy versus placebo and versus tramadol alone, and reduced the need for rescue medication, permitting lower exposure to opioid-containing medications. In the acute pain setting, co-crystallization of tramadol and celecoxib in CTC modifies the pharmacokinetics of both tramadol and celecoxib to provide an improved benefit/risk profile over tramadol alone, by inducing rapid analgesia despite decreasing exposure to tramadol, and reducing the need for rescue medication in general, and opioids in particular. In this pooled analysis of efficacy data, CTC appears to have potential benefits for the management of moderate-to-severe acute pain.
References
Berben SA, Schoonhoven L, Meijs TH, van Vugt AB, van Grunsven PM. Prevalence and relief of pain in trauma patients in emergency medical services. Clin J Pain. 2011;27(7):587–92. https://doi.org/10.1097/AJP.0b013e3182169036.
European Society for Emergency Medicine. Guidelines for the management of acute pain in emergency situations. https://eusem.org/images/EUSEM_EPI_GUIDELINES_MARCH_2020.pdf. Accessed 6 Aug 2021.
Galinski M, Ruscev M, Gonzalez G, et al. Prevalence and management of acute pain in prehospital emergency medicine. Prehosp Emerg Care. 2010;14(3):334–9. https://doi.org/10.3109/10903121003760218.
Berben SA, Meijs TH, van Dongen RT, et al. Pain prevalence and pain relief in trauma patients in the accident & emergency department. Injury. 2008;39(5):578–85. https://doi.org/10.1016/j.injury.2007.04.013.
Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165–9. https://doi.org/10.1053/ajem.2002.32643.
Guéant S, Taleb A, Borel-Kühner J, et al. Quality of pain management in the emergency department: results of a multicentre prospective study. Eur J Anaesthesiol. 2011;28(2):97–105. https://doi.org/10.1097/EJA.0b013e3283418fb0.
Mura P, Serra E, Marinangeli F, et al. Prospective study on prevalence, intensity, type, and therapy of acute pain in a second-level urban emergency department. J Pain Res. 2017;10:2781–8. https://doi.org/10.2147/jpr.S137992.
Todd KH, Ducharme J, Choiniere M, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007;8(6):460–6. https://doi.org/10.1016/j.jpain.2006.12.005.
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40. https://doi.org/10.1213/01.Ane.0000068822.10113.9e.
Butti L, Bierti O, Lanfrit R, et al. Evaluation of the effectiveness and efficiency of the triage emergency department nursing protocol for the management of pain. J Pain Res. 2017;10:2479–88. https://doi.org/10.2147/JPR.S138850.
Meissner W, Coluzzi F, Fletcher D, et al. Improving the management of post-operative acute pain: priorities for change. Curr Med Res Opin. 2015;31(11):2131–43. https://doi.org/10.1185/03007995.2015.1092122.
Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98. https://doi.org/10.2147/jpr.S144066.
Chou R, Griffin JC, Blazina I, Schwarz E, Atchison C, Mauer K. Systematic review on treatments for acute pain: surveillance report 1: literature update period: August 2020 through October 2021. In: Treatments for Acute Pain: A Systematic Review: Surveillance Reports. Rockville: Agency for Healthcare Research and Quality (US); 2020.
Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012;35(12):1127–46. https://doi.org/10.2165/11633470-000000000-00000.
Edinoff AN, Kaplan LA, Khan S, et al. Full opioid agonists and tramadol: pharmacological and clinical considerations. Anesth Pain Med. 2021;11(4): e119156. https://doi.org/10.5812/aapm.119156.
Dunn KE, Bergeria CL, Huhn AS, Strain EC. A systematic review of laboratory evidence for the abuse potential of tramadol in humans. Front Psychiatry. 2019;10:704. https://doi.org/10.3389/fpsyt.2019.00704.
Reines SA, Goldmann B, Harnett M, Lu L. Misuse of tramadol in the United States: an analysis of the national survey of drug use and health 2002–2017. Subst Abuse. 2020;14:1178221820930006. https://doi.org/10.1177/1178221820930006.
Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–6. https://doi.org/10.1345/aph.1H386.
Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 Suppl):6s–11s. https://doi.org/10.1002/j.1875-9114.2012.01177.x.
Marsch LA, Bickel WK, Badger GJ, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299(3):1056–65.
Gan TJ, Epstein RS, Leone-Perkins ML, Salimi T, Iqbal SU, Whang PG. Practice patterns and treatment challenges in acute postoperative pain management: a survey of practicing physicians. Pain Ther. 2018;7(2):205–16. https://doi.org/10.1007/s40122-018-0106-9.
Raffa RB, Pergolizzi JV Jr, Tallarida RJ. The determination and application of fixed-dose analgesic combinations for treating multimodal pain. J Pain. 2010;11(8):701–9. https://doi.org/10.1016/j.jpain.2009.12.010.
Varrassi G, Hanna M, Macheras G, et al. Multimodal analgesia in moderate-to-severe pain: a role for a new fixed combination of dexketoprofen and tramadol. Curr Med Res Opin. 2017;33(6):1165–73. https://doi.org/10.1080/03007995.2017.1310092.
Almansa C, Mercè R, Tesson N, Farran J, Tomàs J, Plata-Salamán CR. Co-crystal of tramadol hydrochloride–celecoxib (CTC): a novel API–API co-crystal for the treatment of pain. Cryst Growth Des. 2017;17(4):1884–92. https://doi.org/10.1021/acs.cgd.6b01848.
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. https://doi.org/10.2165/00003088-200443130-00004.
Cheung R, Krishnaswami S, Kowalski K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: a single-dose, two-center, randomized, double-blind, active- and placebo-controlled study. Clin Ther. 2007;29(Suppl):2498–510. https://doi.org/10.1016/j.clinthera.2007.12.008.
US Food and Drug Administration. Prescribing information, SELGENTIS. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213426s000lbl.pdf. Accessed 15 Jan 2024.
Agencia Española de Medicamentos y Productos Sanitos. Velyntra Spanish regulatory approval. https://cima.aemps.es/cima/dochtml/ft/89051/FT_89051.html. Accessed 23 Jan 2024.
Almansa C, Frampton CS, Vela JM, Whitelock S, Plata-Salamán CR. Co-crystals as a new approach to multimodal analgesia and the treatment of pain. J Pain Res. 2019;12:2679–89. https://doi.org/10.2147/jpr.S208082.
Cebrecos J, Carlson JD, Encina G, et al. Celecoxib-tramadol co-crystal: a randomized 4-way crossover comparative bioavailability study. Clin Ther. 2021;43(6):1051–65. https://doi.org/10.1016/j.clinthera.2021.04.002.
Port A, Almansa C, Enrech R, Bordas M, Plata-Salamán CR. Differential solution behavior of the new API–API co-crystal of tramadol–celecoxib (CTC) versus its constituents and their combination. Cryst Growth Des. 2019;19(6):3172–82.
Videla S, Lahjou M, Vaqué A, et al. Single-dose pharmacokinetics of co-crystal of tramadol-celecoxib: results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br J Clin Pharmacol. 2017;83(12):2718–28. https://doi.org/10.1111/bcp.13395.
Videla S, Lahjou M, Vaqué A, et al. Pharmacokinetics of multiple doses of co-crystal of tramadol-celecoxib: findings from a four-way randomized open-label phase I clinical trial. Br J Clin Pharmacol. 2018;84(1):64–78. https://doi.org/10.1111/bcp.13428.
Langford R, Morte A, Sust M, et al. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: a randomized, double-blind, phase 3 trial (STARDOM2). Eur J Pain. 2022;26(10):2083–96. https://doi.org/10.1002/ejp.2021.
Langford R, Margarit C, Morte A, et al. Co-crystal of tramadol-celecoxib (CTC) for acute moderate-to-severe pain. Curr Med Res Opin. 2024;40(3):455–68. https://doi.org/10.1080/03007995.2023.2276118.
Langford R, Pogatzki-Zahn EM, Morte A, et al. Co-crystal of tramadol-celecoxib versus tramadol or placebo for acute moderate-to-severe pain after oral surgery: randomized, double-blind, phase 3 trial (STARDOM1). Adv Ther. 2024;41(3):1025–45. https://doi.org/10.1007/s12325-023-02744-2.
Viscusi ER, de Leon-Casasola O, Cebrecos J, et al. Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: a phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial. Pain Pract. 2023;23(1):8–22. https://doi.org/10.1111/papr.13136.
Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7(5):R1046–51. https://doi.org/10.1186/ar1782.
Echeverria-Villalobos M, Stoicea N, Todeschini AB, et al. Enhanced recovery after surgery (ERAS): a perspective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. 2020;36(3):219–26. https://doi.org/10.1097/ajp.0000000000000792.
Adams TJ, Aljohani DM, Forget P. Perioperative opioids: a narrative review contextualising new avenues to improve prescribing. Br J Anaesth. 2023;130(6):709–18. https://doi.org/10.1016/j.bja.2023.02.037.
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–57. https://doi.org/10.1016/j.jpain.2015.12.008.
Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107(2):e70–80. https://doi.org/10.1002/bjs.11477.
Anastassopoulos KP, Chow W, Ackerman SJ, Tapia C, Benson C, Kim MS. Oxycodone-related side effects: impact on degree of bother, adherence, pain relief, satisfaction, and quality of life. J Opioid Manag. 2011;7(3):203–15. https://doi.org/10.5055/jom.2010.0063.
Cooper SA, Desjardins PJ, Turk DC, et al. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain. 2016;157(2):288–301. https://doi.org/10.1097/j.pain.0000000000000375.
European Medicines Agency. Guideline on the clinical development of medicinal products intended for the treatment of pain. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-medicinal-products-intended-treatment-pain-first-version_en.pdf. Accessed 20 Feb 2024.
US Food and Drug Administration. Development of non-opioid analgesics for acute pain: guidance for industry. https://www.fda.gov/media/156063/download. Accessed 2 Feb 2024.
Singla NK, Meske DS, Desjardins PJ. Exploring the interplay between rescue drugs, data imputation, and study outcomes: conceptual review and qualitative analysis of an acute pain data set. Pain Ther. 2017;6(2):165–75. https://doi.org/10.1007/s40122-017-0074-5.
Acknowledgments
We thank all patients and investigators involved in the ESTEVE-SUSA-301 and STARDOM1 studies. Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact checking, and referencing, was provided by Daniel Binks, PhD at Aspire Scientific Limited (Bollington, UK), funded by ESTEVE Pharmaceuticals S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by ESTEVE Pharmaceuticals S.A.
Conflict of Interest
R.L. reports consulting and speaker fees from Avenue Therapeutics, BioQ Pharma, Camarus, Compass, Eli Lilly, Grünenthal GmbH, Grünenthal Ltd, GSK, Heron Therapeutics, MedinCell, Mundipharma, Pfizer, and Sintetica. E.R.V. reports consulting fees from ESTEVE Pharmaceuticals, Heron Therapeutics, Merck, Orion Pharmaceuticals, Salix Pharmaceuticals, and Vertex. A.M., J.C., M.S., and J.M.G.A. are employees of ESTEVE Pharmaceuticals. O.d.L.-C. reports personal fees for advisory board membership from ESTEVE Pharmaceuticals during the conduct of the study, and from ESTEVE Pharmaceuticals, Merck, and Stimgenics-Medtronic outside the submitted work.
Ethical Approval
This was a pooled analysis of two previously reported clinical trials. Both studies were conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines and received ethical approval.
Consent to Participate/Consent for Publication
All patients provided written informed consent.
Code Availability
Not applicable.
Availability of Data and Material
ESTEVE Pharmaceuticals S.A. will consider requests for de-identified patient-level data and supporting study documents from qualified external researchers. Approval of requests will be at the discretion of ESTEVE Pharmaceuticals S.A. and will depend on the scientific merit of the proposed research and intended use of the data. If approval is granted, a Data Sharing Agreement must be signed and access to data will be provided only if ESTEVE Pharmaceuticals S.A. has legal authority to provide the data and there are no contradictory requirements relating to regulatory filings or reviews. Proposals should be sent to esteve@esteve.com.
Author Contributions
R.L., E.R.V., A.M., J.C., M.S., and O.d.L.-C. conceived or designed the studies. J.C. contributed to data collection. R.L., E.R.V., A.M., J.C., M.S., J.M.G.A., and O.d.L.-C. contributed to data analysis and/or interpretation. All authors drafted, edited, and/or reviewed drafts of the manuscript, and all approved the final version, and agree to be accountable for the accuracy and integrity of the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Langford, R., Viscusi, E.R., Morte, A. et al. Efficacy of Co-Crystal of Tramadol-Celecoxib (CTC) in Patients with Acute Moderate-to-Severe Pain: A Pooled Analysis of Data from Two Phase 3 Randomized Clinical Trials. Drugs R D (2024). https://doi.org/10.1007/s40268-024-00469-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s40268-024-00469-3