Abstract
Background
The FLT3/ITD mutation exists in many acute myeloid leukemia (AML) patients and is related to the poor prognosis of patients. In this study, we attempted to evaluate the antitumor activity of simvastatin, a member of the statin class of drugs, in vitro and in vivo models of FLT3/ITD AML and to identify the potential mechanisms.
Methods
Cell Counting Kit-8 (CCK-8) and Annexin V/propidium iodide (PI) staining kits were used to detect cell viability and apoptosis, respectively. Subsequently, Western blot and rescue experiment were applied to explore the potential molecular mechanism. In vivo anti-leukemia activity of simvastatin was evaluated in xenograft mouse models.
Results
In vitro experiments revealed that simvastatin inhibited AML progression in a dose- and time-dependent manner, while in vivo experiments showed that simvastatin significantly reduced tumor burden in FLT3/ITD xenograft mouse models. After simvastatin treatment of FLT3/ITD AML cells, intracellular Rap1 was downregulated and the phosphorylation levels of its downstream targets MEK, ERK and p38 were significantly inhibited. The rescue experiment showed that mevalonate, an intermediate product of the metabolic pathway of mevalonate, and its downstream geranylgeranyl pyrophosphate (GGPP) played a key role in this process. Finally, we demonstrate that simvastatin can induce apoptosis of primary AML cells, while having no effect on peripheral blood mononuclear cells from normal donors.
Conclusions
Simvastatin can selectively and effectively eradicate FLT3/ITD AML cells in vitro and in vivo, and its mechanism may be related to the disruption of the HMG-CoA reductase pathway and the downregulation of the MEK/ERK and p38-MAPK signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study showed that simvastatin can selectively and effectively eradicate FLT3/ITD acute myeloid leukemia (AML) cells in vitro and in vivo. |
Simvastatin inhibited the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) on the mevalonate pathway in the FLT3/ITD AML cells. Intermediate products of the mevalonate pathway, mevalonate and GGPP, acted as the facilitators, whereby simvastatin exerted its influence. |
The antileukemia activity of simvastatin is associated with the disruption of the HMG-CoA reductase pathway and downregulation of the MER/ERK and p38-MAPK signaling pathways. |
1 Background
Acute myeloid leukemia (AML) is a type of clonal malignant proliferative disease of hematopoietic myeloid blasts. The prognosis remains poor in pediatric and adult patients, with 5-year survival rates approximating 66–68% and 10–20%, respectively [1,2,3]. FMS-like tyrosine kinase 3 (FLT3), a tyrosine kinase type III receptor, is normally present in hematopoietic stem and progenitor cells and plays an important role in early proliferation, differentiation and formation of hematopoietic cells [4]. FLT3 mutation is one of the most frequently mutated genes in AML, which can lead to constitutive activation of FLT3 in nearly 30% of AML patients [5,6,7]. The most frequent FLT3 mutation is internal tandem repeat mutations in the proximal membrane domain, known as FLT3-ITD, which happens in about 20–25% of AML patients [8]. FLT3 mutants can induce the activation of many intracellular signaling pathways, including PI3K/AKT/mTOR, RAS/MAPK and JAK/STAT5 signaling, which provided a proliferation/survival privilege to cancer cells [9, 10].
Previous studies have confirmed that FLT3 mutation in AML patients is associated with increased white blood cell count, higher risk of recurrence and poor prognosis, and is one of the important factors for risk stratification [11,12,13]. Although the introduction of tyrosine kinase inhibitors (TKIs) has yielded positive clinical responses in FLT3-mutant AML, several issues that limit the anti-leukemia activity, e.g. the short-lived partial responses, off-target effects, and drug resistance, as well as the high affordability costs, cannot be neglected [14, 15]. Thus, safe and effective new treatment strategies are sought to achieve better clinical response in patients with FLT3-mutated AML.
As we all know, the development of new drugs is a process that requires a lot of time, investment and manpower. Therefore, identifying new indications from existing drugs can greatly shorten the development cycle and reduce the investment, which is a new strategy for drug development. Developed originally as lipid-lowering agents, statins exhibit multiple effects via inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which is the first rate-controlling step of the mevalonate pathway, responsible for generating low-density lipoprotein receptor (LDLR) feedback to prevent cholesterol formation, supporting plasma cholesterol internalization and disrupting protein prenylation. The increase of mevalonate synthesis is beneficial to the growth of tumor cells. The imbalance of cellular cholesterol homeostasis has been confirmed to protect AML cells from the cytotoxic effect of chemotherapy. The cholesterol levels are increased in AML cells after exposure to chemotherapy drugs, which may reduce the cytotoxicity of these drugs. Therefore, interfering with cholesterol metabolism by using statins to inhibit cholesterol synthesis has been proposed as a method to improve the anti-leukemia treatment strategy [16,17,18,19,20]. Currently, it has been reported that statins exhibit cytotoxicity to a variety of human AML cells, such as in primary CD34 + AML, where the combination of simvastatin and tipifarnib enhances the inhibitory effect on AML cells [21,22,23,24,25]. Moreover, statins also play an important role in FLT3/ITD AML. Studies have shown that statins can induce cell death in FLT3/ITD AML by inhibiting FLT3 glycosylation, leading to a loss of surface expression [26]. These studies provided a great support for re-positioning statins as anti-leukemia drugs. Several other studies also reported that the use of statins significantly reduced cancer-related risks and prolonged patients’ survival rates in gastric, colorectal, and breast cancers [27,28,29].
In light of the above observations, we sought to evaluate the antitumor activity of simvastatin, a member of the statin class of drugs, in vitro and in vivo models of FLT3/ITD AML, and to identify the underlying mechanisms of action. Taken together, these findings suggest that this novel therapy, which reasonably interferes with tumor metabolism, may be a promising option for treating FLT3/ITD AML.
2 Material and Methods
2.1 Reagents
Simvastatin was purchased from MedChemExpress (HY-17502; Shanghai, China). Simvastatin 8 mg (20 mM) was dissolved in 0.2 mL absolute ethyl alcohol and 0.25 mL 0.1N NaOH, and was subsequently incubated at 50°C for 2 h and neutralized with hydrochloric acid (HCl) to pH 7.2. Mevalonic acid powder was purchased from MedChemExpress (HY-113071) and was dissolved in dimethylsulfoxide (DMSO) [D12345; Invitrogen, Waltham, MA, USA]. Farnesyl pyrophosphate (FPP) solution was purchased from Sigma-Aldrich (F6892-1VL; St Louis, MO, USA) and geranylgeranyl pyrophosphate (GGPP) solution was purchased from GlpBio (G6025-1VL; Montclair, CA, USA).
2.2 Acute Myeloid Leukemia (AML) Cell Culture Conditions
Human FLT3/ITD AML cell lines MOLM-13 and MV4-11, and human stem cell AML cell lines KASUMI and KG-1α were purchased from ATCC (Rockefeller, MD, USA). All cell lines were incubated in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies, New York, NY, USA) and 1% penicillin/streptomycin. Cells were cultured in a humidified environment of 37 °C and 5% CO2. All cell lines were mycoplasm-free.
2.3 Primary Samples
This study was authorized by the Ethics Committee of the First Affiliated Hospital of Xiamen University and was implemented according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients. Bone marrow (BM) samples from 10 first-time-diagnosed AML patients, and peripheral blood specimens of 10 healthy hematopoietic stem cell transplantation donors, were collected. Mononuclear cells were isolated from the collected samples by density gradient centrifugation of lymphatic vessels (BD, NJ) and incubated in RPMI 1640 medium supplemented with 10% FBS for subsequent drug trials.
2.4 Cell Viability Assay
The cytotoxicity of simvastatin was determined by Cell Counting Kit-8 (CCK-8) [HY-K0301; MedChemExpress]. First, 2 × 104 cells/well of AML cells were seeded into 96-well plates with a total volume of 100 uL of growth medium and drug in a humidified environment containing 5% CO2 at 37°C. Second, AML cells were treated with 2.5, 5, 10, 20, or 40 μM simvastatin alone or in combination with 7.5, 15, 30, 60, or 120 μM mevalonic acid, 1.25, 2.5, 5, 10, or 20 μM FPP, or 0.625, 1,25, 2.5, 5, or 10 μM GGPP at different concentrations for 22, 46, or 70 h. Third, 10 μL of CCK-8 reagents were added to each well and incubated in the incubator for an extra 2 h. Finally, absorbance was measured at 490 nm using an enzyme-labeled instrument (ELx800; BioTek Instruments Inc., Winooski, VT, USA). Half of the maximal inhibitory concentration (IC50) was reckoned using Graphpad Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
2.5 Cell Apoptosis Assay
2 × 105 cells/well of AML cells were seeded into 12-well plates and treated with 2.5, 5, 10, 20, or 40 μM simvastatin for 24, 48 or 72 h. The cells were then harvested with the supernatant containing floating cells and washed twice with binding buffer. The cells were then incubated with 5 μL Annexin V-FITC for 15 min at 4 °C in the dark, and 5 μL propidium iodide (PI) was added for another 5 min. The excess dye was then washed with ice-cold phosphate-buffered saline (PBS) and followed by flow cytometry (BD Bioscience, Oxford, UK) and Flowjo software (BD Bioscience) analysis.
2.6 Cell Cycle Analysis
First, 2 × 105/well of AML cells were incubated in 12-well plates and exposed to 0, 2.5, 5, or 10 μM simvastatin. After drug exposure for 24 h, cells were harvested, fixed with 70% precooled ethanol, and incubated overnight on ice. The cells were then stained with freshly prepared PI solution for 30 min in the dark (on ice), which contained 200 μg/mL DNase-free RNase A, 20 μg/mL PI, and 0.1% Triton X-100. Finally, cells were subjected to flow cytometry (BD Bioscience) and the cellular DNA complements were analyzed using FACS C6 software.
2.7 Western Blot Analysis
AML cell lines were plated at 2 × 106 cells/well in six-well plates. Cells were treated with 2.5, 10, or 40 μM simvastatin alone or 40 μM simvastatin combined with 120 μM mevalonic acid, 10 μM GGPP, or 20 μM FPP for 48 h. Protein was extracted using the solution method and whole-cell lysates were extracted and quantified from each sample. Reagents used included Ripa Lysate (Beyotime Technology Co. Ltd, Shanghai, China; P0013C), PMSF solution (Beyotime Technology Co. Ltd; ST507-10ml), protease inhibitor (APExBIO Technology LLC, Houston, TX, USA; K1007), phosphatase inhibitor (APExBIO Technology LLC; K1015-A, K1015-B), and BCA protein assay kit (Beyotime Technology Co. Ltd; P0012). After electrophoresis and PVDF membrane transfer, the separated proteins were probed to the primary antibodies at 4℃ overnight, then cleaned three times with TBST, and incubated with secondary HRP-conjugated antibodies (1:10,000; Abcam, Cambridge, UK) at room temperature for 1.5 h. The primary antibodies included anti-phospho-ATM (Cell Signaling, #5883S), anti-phospho-ATR (Cell Signaling, #2853S), anti-phospho-CHK1 (Cell Signaling Technology, Inc., Danvers, MA, USA; #2348S), anti-phospho-CHK2 (Cell Signaling Technology, Inc.; #2197S), anti-P53 (Cell Signaling Technology, Inc.; #2527S), anti-p21 (Cell Signaling Technology, Inc.; #2947S), anti-BCL11A (Cell Signaling Technology, Inc.; #75432S), anti-RAS (Cell Signaling Technology, Inc.; #3339S), anti-RhoB (Cell Signaling Technology, Inc.; #63876S), anti-Rap1 (Cell Signaling Technology, Inc.; #2399S), anti-MDR1(Cell Signaling Technology, Inc.; #13342), and antiphospho-MDM2 (Cell Signaling Technology, Inc.; #86934S). The antibody anti-HMGCR (#174830) was purchased from Abcam (UK), and the antibody anti-TBX2 (#0507R) was purchased from Bioss Antibodies (Beijing, China). Blots were visualized using the ECL Western Blotting Detection Kit (GeneFlow, Lichfield, UK).
2.8 Animal Study
Four- to six-week-old BALB/C nude mice (Beijing HFK Bioscience Co. Ltd, Beijing, China) were inoculated subcutaneously with 2 × 106 MOLM-13 cells to construct an FLT3/ITD AML mouse model. Three days after inoculation, mice were randomly divided into two groups (five mice per group); the experimental group was intraperitoneally injected with simvastatin 20 mg/kg/day, and the other group was injected with PBS as the control group. The mice body weights were measured daily. After 10 days of abdominal administration, mice were sacrificed and the tumors were resected, weighted, and measured. Tumor volume was reckoned using the formula V = (L × W2)/2 (L indicates length; W indicates width). The tumor sections were stained with hematoxylin and eosin (H&E) and terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) to analyze the morphology and death of tumor cells.
2.9 Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) Staining
The fresh tumor tissue blocks of nude mice were fixed in 10% formalin for 24 h. The tissue slides were then prepared and cleaned with clean xylene. After washing with gradient ethanol (100%, 95%, 85%, 75%, 65%), the paraffin slide is then put into the prepared 4% formaldehyde, fixed for 15 min, and washed and digested (20 μg/mL protease K, 8–10 min). The glass slide was incubated in a wet box at 37 ℃ for 1 h in the dark, and the reaction was then terminated. After further washing, the film was sealed, and, finally, local green fluorescence of apoptotic tissue was detected using an EVOSTMM7000C imaging system fluorescence microscope.
2.10 Statistical Analysis
Mean ± standard error of the mean of at least three independent experiments was used to represent the results. Statistical analyses were carried out using GraphPad Prism 7 software. The two-tailed Student’s t test was used to compare the variables between two groups, and one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test were used to compare the variables among multiple groups. A p value <0.05 indicated statistical significance.
3 Results
3.1 Simvastatin Inhibited Cell Proliferation in FLT3/ITD AML Cell Lines
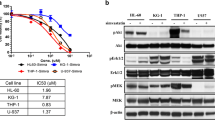
CCK8 was used to determine the inhibitory effect of simvastatin on MOLM-13, MV4-11, Kasumi, and KG-1a. The cell viability rates of each cell line at different concentrations of simvastatin for 24, 48 and 72 h are shown in Fig. 1. Exposure to simvastatin resulted in a significant inhibition of cell viability in the FLT3/ITD AML cells MOLM-13 and MV4-11 in a dose- and time-dependent manner. In contrast, the human non-FLT3/ITD AML cell lines KG1a and Kasumi-1 were much less sensitive to simvastatin in the same range of concentrations. The IC50 values of MOLM-13, MV4-11, Kasumi, and KG-1a treated with simvastatin for 24, 48 and 72 h were calculated and are summarized in Table 1. The IC50 values of KG1A and Kasumi treated with simvastatin for 24 and 48 h could not be calculated; however, their IC50 values at 72 h were 77.43 ± 7.42 μM and 58.63 ± 16.54 μM, respectively, while the IC50 values of MOLM-13 and MV4-11 at 72 h were only 5.12 ± 0.79 μM and 6.43 ± 2.80 μM, respectively. Overall, these findings indicate that simvastatin suppressed the proliferation of MOLM-13 and MV4-11 cells in a dose- and time-dependent manner, while Kasumi and KG1a cells were more resistant to simvastatin.
Effect of simvastatin on AML cell proliferation. MOLM-13, MV4-11, Kasumi, and KG1a were incubated with 0, 2.5, 5, 10, 20, or 40 μM simvastatin for a 24 h, b 48 h, and c 72 h. A CCK-8 kit and Graphpad Prism 7.0 were utilized to analyze cell viability and calculate the IC50 value. *p < 0.05, **p < 0.01, ***p < 0.001 versus untreated controls. AML acute myeloid leukemia, CCK-8 Cell Counting Kit-8, IC50 half maximal inhibitory concentration
3.2 Simvastatin Induced Apoptosis and Cell Cycle Arrest in FLT3/ITD AML Cell Lines
Since MOLM-13 and MV4-11 cells were sensitive to simvastatin treatment, flow cytometry was performed to detect the effects of simvastatin regarding cell cycle and apoptosis on these two AML cell lines to further verify the anti-leukemia efficacy of simvastatin. Generally, the apoptosis rate of MOLM-13 and MV4-11 increased correspondingly with the increase in simvastatin concentration and the extension of the incubation time (Fig. 2A–D), of which the tendency was consistent with the results of the cell survival rate. The apoptosis effects of simvastatin on Kasumi and KG-1a were simultaneously tested by using Annexin V/PI double staining. The results showed that there was a statistical difference in the killing effect after 72 h of high concentration (20 and 40 μM for Kasumi, 40 μM for KG1a), while there was almost no effect after 72 h of low concentration or 24 and 48 h of simvastatin treatment (Electronic supplementary Fig. S1a–d). As for the cell cycle shown in Fig. 2E–H, simvastatin induced a G0/G1-phase arrest at a concentration of 10 μM in both MOLM-13 and MV4-11 cells for 24 h, reflected by the increased number of cells in the G0/G1 phase, while cells in the S phase and G2/M were relatively decreased. These data indicated that in addition to inducing cell apoptosis, simvastatin could also play a role by blocking the cell cycle that contributed to its anti-leukemia effect.
Simvastatin induced apoptosis as well as cell cycle arrest in FLT3/ITD AML cell lines. a MOLM-13 and c MV4-11 were incubated with the indicated concentrations of simvastatin for 24, 48, and 72 h. Annexin V/PI double staining was then performed to determine the percentage of apoptotic cells. The corresponding numerical statistics of a and c are displayed in the histograms on the left as b and d. e MOLM-13 and f MV4-11 were exposed to 0, 2.5, 5, 10 μM simvastatin for 24 h. PI staining was then performed to analyze the cell cycle ratio of each phase. The corresponding numerical statistics of a and c are displayed in the histograms on the left as g and h. *p < 0.05, **p < 0.01, ***p < 0.001. AML acute myeloid leukemia, PI propidium iodide
3.3 Simvastatin Impaired the Mevalonate, MEK/ERK, and p38-MAPK Pathways in FLT3/ITD AML Cell Lines
Simvastatin can suppress tumor cell proliferation and induce cell apoptosis and cell cycle arrest through various mechanisms. Among these, the mevalonate pathway (Fig. 3a) was considered to be the primary mechanism that accounted for the anti-tumor effect of simvastatin. Mevalonate is the precursor of isoprenoids such as FPP and GGPP, and isoprenylation catalyzed by farnesyltransferase or geranylgeranyltransferase is required for small GTPases (e.g. Rho, RAS) to attach to the plasma membrane and subsequently participate in signal transduction pathways that regulate growth and survival [22]. Notably, GGPP produced by the mevalonate cascade also functions as an important substrate in the activation process of Rap1 [30]. Therefore, these effectors of HMGCR were assessed by Western blotting, where we found that the expressions of HMGCR in MOLM-13 and MV4-11 cells were decreased under the action of simvastatin, in concert with the downregulation of Rap1 and RhoB, while the expressions of RAS were upregulated (Fig. 3b). To get an exclusive deep dive, we centered our attention on Rap1-mediated pathways, where we found that the phosphorylation level of MEK, ERK, and p38 were significantly downregulated, which imparted us to speculate that the underlying mechanisms of action of simvastatin relied on the MEK/ERK and p38-MAPK signaling pathways (Fig. 3c, d).
Effect of simvastatin on the mevalonate pathway and the downstream targets. a Illustration of how simvastatin acts on the mevalonate pathway. b MOLM-13 and MV4-11 were exposed to 40 μM simvastatin for 48 h, and Western blotting analysis was then performed to determine the protein expression level of HMGCR, RAS, Rap1 and RhoB. c MOLM-13 and d MV4-11 cells were exposed to the designated concentrations of simvastatin for 48 h, and ERK, MEK and p38 and their phosphorylation levels were detected by Western blotting. HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase, FPP farnesyl pyrophosphate, GGPP geranylgeranyl pyrophosphate, SIM simvastatin, GAPDH glyceraldehyde-3-phosphate dehydrogenase
3.4 Mevalonate and Geranylgeranyl Pyrophosphate Can Alleviate the Cytotoxicity Induced by Simvastatin in FLT3/ITD AML Cell Lines
To determine which components of the mevalonate pathway are modulated upon simvastatin treatment, we combined simvastatin with three mitigating agents (mevalonate, FPP, GGPP) and tested the rescue effect of each component. The results showed that mevalonate and GGPP could relieve simvastatin from inhibiting AML cell proliferation, while FPP failed to show activity in remission (Fig. 4A, B). Similarly, when the combination of simvastatin plus mevalonate or GGPP was applied, the protein expression of HMGCR and Rap1 in MOLM-13 and MV4-11 cells did not differ significantly from the control group (Fig. 4c, d), which indicates that mevalonate and GGPP functioned as the activators of the mevalonate pathway in accordance with the results of the cell viability assay.
Mevalonate and GGPP counteracted the actions of simvastatin. a MOLM-13 and b MV4-11 cells were treated with the indicated concentrations of simvastatin alone or in combination with mevalonate, FPP, or GGPP for 48 h. CCK-8 was used to detect cell viability. The significant groups, with the presence of simvastatin (40 μM) alone or combined with mevalonate (120 μM), GGPP (10 μM) or FPP (20 μM), were then subjected to Western blot analysis to detect the expression level of interested proteins on c MOLM-13 and d MV4-11 cells. *p < 0.05, **p < 0.01, ***p < 0.001). FPP farnesyl pyrophosphate, GGPP geranylgeranyl pyrophosphate, CCK-8 Cell Counting Kit-8, SIM simvastatin, Meva mevalonate, Ctrl control, HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase, GAPDH glyceraldehyde-3-phosphate dehydrogenase
3.5 Simvastatin Reduced Tumor Burden in FLT3/ITD Xenograft Mice Models
On the basis of these in vitro results, we were encouraged to detect the anti-leukemia activity of simvastatin in vivo. MOLM-13 cells were used to establish mouse xenograft models by subcutaneous inoculation, the mice were randomly divided into the control and simvastatin treatment groups (Fig. 5a). We observed that although the mice in the simvastatin treatment group had a moderate and short-term weight loss compared with the control group on days 2–4 after drug treatment, they gradually recovered on day 5, and no significant drug toxicity was observed thereafter (Fig. 5b). After 10 days’ consecutive administration of simvastatin, the mice were sacrificed and the tumors were harvested and weighed. Compared with the mice in the control group, the tumor size (Fig. 5c) and tumor weight (Fig. 5d) of the mice in the simvastatin treatment group were significantly reduced, as well as tumor volume (Fig. 5e). Immunohistochemistry analysis was also performed and the results showed that the mice in the simvastatin treatment group had fewer infiltrating MOLM-13 cells and more apoptotic cells could be observed (Fig. 5f). In summary, these results indicated that simvastatin may have a certain inhibitory effect on the growth of AML tumors in vivo.
Simvastatin inhibited tumor growth in FLT3/ITD xenograft mice models. a Schematic diagram of the animal study procedures (n = 5). b Mice body weights were measured every day after tumor cell inoculation. c After 10 days of continuous drug treatment, the mice were sacrificed and the mice and tumors were then imaged for comparison. The corresponding numerical statistics of d tumor weight and e tumor volume were also determined. The tumor tissue sections were stained with H&E and TUNEL for immunohistochemistry analysis. f Cells with green fluorescence were positive for TUNEL staining, and apoptosis occurred. H&E staining: inflammatory cells infiltrated and vacuoles increased in the medication group. **p < 0.01. H&E hematoxylin and eosin, TUNEL terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling, Ctrl control, SIM simvastatin, DAPI 4′,6-diamidino-2-phenylindole
3.6 Simvastatin Attenuated Primary AML Blasts While Sparing Normal Peripheral Blood Mononuclear Cells
Having identified that simvastatin exerts cytotoxicity to AML cells both in vivo and in vitro, we further evaluated the effect of simvastatin on primary AML blasts and normal peripheral blood mononuclear cells (PBMCs). The clinical characteristics of AML patients (including age, sex, white blood cell count, chromosome and immunotype) are summarized in Table 2. On the one hand, we found that the apoptosis rate of primary cells with FLT3 mutation was higher than that of non-FLT3-mutant primary cells (Fig. 6a). On the other hand, simvastatin exhibited no obvious cytotoxicity to the normal PBMCs under the same condition (n = 10) [Fig. 6b]. These results suggested that FLT3-mutant AML blasts were more susceptible to simvastatin-induced growth inhibition than non-FLT3 AML blasts and normal PBMCs.
Effect of simvastatin on primary AML blasts and normal PBMCs. a Apoptosis ratios of primary AML bone marrow mononuclear cells and b healthy human PBMCs after treatment for 48 h by the indicated concentrations of simvastatin. *p < 0.05. AML acute myeloid leukemia, PBMCs peripheral blood mononuclear cells, Ctrl control
4 Discussion
FLT3/ITD mutation serves as an important indicator of AML regarding diagnosis, prognosis and relapse, which would help to guide the therapeutics decisions throughout a patient’s disease course [31]. Since 2006, targeting FLT3 in AML with TKIs had been granted marketing authorization and has achieved encouraging results. However, the defects of TKIs, like the elevation of FLT3 ligand levels in AML patients, which could compensate for the outcome, are severe and hard to tackle, thus calling for new remedies to overcome some of the challenges. Since metabolic reprogramming is a hallmark of malignancy, targeting metabolic reprogramming has emerged as an important strategy for anticancer therapy [32]. Like abnormal glucose and glutamine metabolism, abnormal cholesterol metabolism also plays a key role in tumors. Cholesterol metabolism is closely related to tumor genesis, development, and prognosis. Abnormal changes in genes related to cholesterol metabolism enzymes have been shown to affect tumor cell proliferation, invasion and migration in prostate, breast, colorectal and brain cancers [33]. As the rate-limiting enzyme for cholesterol biosynthesis, HMGCR has been identified as a potential target for the therapy of cancers. Currently, the repositioning of statins in the treatment of cancer has attracted a lot of attention. Statins have been developed and are widely used as a serum cholesterol-reducing drug that functions as an HMGCR inhibitor by preventing the conversion of HMG-CoA into mevalonate and inhibiting cholesterol synthesis [34, 35]. Since malignant cells are prone to elevating mevalonate synthesis, which promotes the growth of tumor cells [17, 18], HMGCR is thus equipped with antiproliferation activity, at least in part, by inhibiting the formation of mevalonate. Additionally, inhibition of HMG-CoA reductase can also reduce the production of other intermediate products of the mevalonate pathway, including isoprenylation, FPP and GGPP [36], which suggests that statins could have pleiotropic mevalonate-independent effects via these intermediate products. In this study, we confirmed that simvastatin inhibited the HMGCR on the mevalonate pathway in the FLT3/ITD AML cells (Fig. 3b). Moreover, the rescue experiments (Fig. 3c, d) further consolidated that intermediate products of the mevalonate pathway, mevalonate and GGPP, but not FPP, acted as the facilitators whereby simvastatin exerted its influence. This is because a second molecule, isopentenyl PPi, is required to convert FPP to GGPP. As isopentenyl PPi is also depleted by statin exposure, it will not be utilized by statin-treated cells [37]. A previous study showed that statins could inhibit the glycosylation of FLT3, which led to the loss of surface expression and induced cell death [26]. The difference is that our research starts with another lipid metabolic pathway, showing that statins can inhibit the synthesis of hydroxy-methylglutaryl-coenzyme A reductase (HMGR) through another lipid metabolic pathway, reduced synthesis of its downstream GGPP, which undoubtedly enriched the mechanistic network of statin therapy for FLT3/ITD + AML.
Notably, the GGPP produced by the mevalonate cascade also functions as an important substrate in the activation process of Rap1 [30], which is involved in many biological processes, including cell adhesion, cell growth, cell apoptosis, cytoskeleton remodeling, and intracellular vesicle transport [38, 39]. In adrenal tumors and ovarian tumors, Rap1 can participate in the proliferation and migration process by activating the ERK/MAPK, MER3/6/p38-MAPK, and PI3K/AKT pathways [40, 41]. Rap1 and RAS have high sequence similarity, and they have overlapping binding partners. Rap1 has also been shown to oppose and mimic RAS-driven cancer phenotypes. RAS and Rap1 cooperate to initiate and maintain ERK signal transduction, which is activated in many malignant tumors [42]. In our study, we found that expression of Rap1 was downregulated and expression of RAS was upregulated after simvastatin treatment. We hypothesized that this could be due to the fact that Rap1 and RAS have overlapping binding partners; simvastatin-induced downregulation of Rap1 resulted in relative upregulation of RAS, but this did not affect the inhibition of downstream ERK signaling (Fig. 3c, d). At the same time, although RAS has always been considered an oncogene that promotes cell proliferation, we clearly observed inhibition of AML cell proliferation after the use of simvastatin (Fig. 1). Intriguingly, FLT3 is the upstream receptor of the RAS/RAF/MER/ERK signaling cascade, which links it to one of the explanations for the underlying mechanism of simvastatin in the FLT3-mutant AML cells.
5 Conclusions
The present study demonstrates that simvastatin selectively and effectively eradicates FLT3/ITD AML cells both in vitro and in vivo. Mechanistically, the anti-leukemia activity of simvastatin is associated with the disruption of the HMG-CoA reductase pathway and downregulation of the MER/ERK and p38-MAPK signaling pathways. Together, this preclinical study creates such high expectations and merits future studies to optimize FLT3/ITD AML regimens by combining simvastatin with TKIs and other traditional chemotherapy drugs.
References
O’Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:926–57. https://doi.org/10.6004/jnccn.2017.0116.
Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593–606. https://doi.org/10.1016/S0140-6736(18)31041-9.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Volpe G, Clarke M, Garcia P, et al. Regulation of the Flt3 gene in haematopoietic stem and early progenitor cells. PLoS One. 2015;10: e0138257. https://doi.org/10.1371/journal.pone.0138257.
Cancer Genome Atlas Research N, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. https://doi.org/10.1056/NEJMoa1301689.
Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. https://doi.org/10.1056/NEJMoa1516192.
Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. https://doi.org/10.1056/NEJMoa1112304.
Rose D, Haferlach T, Schnittger S, et al. Subtype-specific patterns of molecular mutations in acute myeloid leukemia. Leukemia. 2017;31:11–7. https://doi.org/10.1038/leu.2016.163.
McMahon CM, Ferng T, Canaani J, et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019;9:1050–63. https://doi.org/10.1158/2159-8290.CD-18-1453.
Zhou F, Ge Z, Chen B. Quizartinib (AC220): a promising option for acute myeloid leukemia. Drug Des Devel Ther. 2019;13:1117–25. https://doi.org/10.2147/DDDT.S198950.
Angenendt L, Rollig C, Montesinos P, et al. Chromosomal abnormalities and prognosis in NPM1-mutated acute myeloid leukemia: a pooled analysis of individual patient data from nine international cohorts. J Clin Oncol. 2019;37:2632–42. https://doi.org/10.1200/JCO.19.00416.
Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. https://doi.org/10.1182/blood-2016-08-733196.
Dohner K, Thiede C, Jahn N, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135:371–80. https://doi.org/10.1182/blood.2019002697.
Imataki O, Ishida T, Kida JI, et al. Cost-effectiveness analysis of transplantation-ineligible elderly patients with acute leukemia harboring a molecular target: Ph-positive acute leukemia and FLT3-mutated acute myeloid leukemia. J Clin Med Res. 2022;14:432–5.
Engen CB, Wergeland L, Skavland J, Gjertsen BT. Targeted therapy of FLT3 in treatment of AML-current status and future directions. J Clin Med. 2014;3:1466–89. https://doi.org/10.3390/jcm3041466.
Gruenbacher G, Thurnher M. Mevalonate metabolism governs cancer immune surveillance. Oncoimmunology. 2017;6: e1342917. https://doi.org/10.1080/2162402X.2017.1342917.
Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene. 2012;31:4967–78. https://doi.org/10.1038/onc.2012.6.
Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–46. https://doi.org/10.1124/pr.111.004994.
Israelsen WJ, Vander Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51. https://doi.org/10.1016/j.semcdb.2015.08.004.
Kress S, Stein A, Maurer P, et al. Expression of hypoxia-inducible genes in tumor cells. J Cancer Res Clin Oncol. 1998;124:315–20. https://doi.org/10.1007/s004320050175.
van der Weide K, de Jonge-Peeters SD, Kuipers F, et al. Combining simvastatin with the farnesyltransferase inhibitor tipifarnib results in an enhanced cytotoxic effect in a subset of primary CD34+ acute myeloid leukemia samples. Clin Cancer Res. 2009;15:3076–83. https://doi.org/10.1158/1078-0432.CCR-08-3004.
van der Weide K, Korthuis PM, Kuipers F, et al. Heterogeneity in simvastatin-induced cytotoxicity in AML is caused by differences in Ras-isoprenylation. Leukemia. 2012;26:845–8. https://doi.org/10.1038/leu.2011.259.
Dimitroulakos J, Nohynek D, Backway KL, et al. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: a potential therapeutic approach. Blood. 1999;93:1308–18.
de Jonge-Peeters SD, van der Weide K, Kuipers F, et al. Variability in responsiveness to lovastatin of the primitive CD34+ AML subfraction compared to normal CD34+ cells. Ann Hematol. 2009;88:573–80. https://doi.org/10.1007/s00277-008-0633-2.
Nam DH, Lee H, Park JC, et al. Long-term statin therapy improves oncological outcome after radical gastrectomy for stage II and III gastric cancer. Anticancer Res. 2014;34:355–61.
Williams AB, Li L, Nguyen B, et al. Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia. Blood. 2012;120:3069–79. https://doi.org/10.1182/blood-2012-01-403493.
Ahern TP, Lash TL, Damkier P, et al. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15:e461-468. https://doi.org/10.1016/S1470-2045(14)70119-6.
Vallianou NG, Kostantinou A, Kougias M, Kazazis C. Statins and cancer. Anticancer Agents Med Chem. 2014;14:706–12. https://doi.org/10.2174/1871520613666131129105035.
Sehdev A, Shih YC, Huo D, et al. The role of statins for primary prevention in non-elderly colorectal cancer patients. Anticancer Res. 2014;34:5043–50.
Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–40. https://doi.org/10.1242/jcs.00238.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312. https://doi.org/10.1038/s41375-018-0357-9.
Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473. https://doi.org/10.1126/science.aaw5473.
Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–45. https://doi.org/10.1038/s41580-019-0190-7.
Fritz V, Benfodda Z, Henriquet C, et al. Metabolic intervention on lipid synthesis converging pathways abrogates prostate cancer growth. Oncogene. 2013;32:5101–10. https://doi.org/10.1038/onc.2012.523.
Niessner H, Beck D, Sinnberg T, et al. The farnesyl transferase inhibitor lonafarnib inhibits mTOR signaling and enforces sorafenib-induced apoptosis in melanoma cells. J Investig Dermatol. 2011;131:468–79. https://doi.org/10.1038/jid.2010.297.
Duncan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem. 2004;279:33079–84. https://doi.org/10.1074/jbc.M400732200.
Wong WW, Clendening JW, Martirosyan A, et al. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol Cancer Ther. 2007;6:1886–97. https://doi.org/10.1158/1535-7163.MCT-06-0745.
Mutvei AP, Nagiec MJ, Hamann JC, et al. Rap1-GTPases control mTORC1 activity by coordinating lysosome organization with amino acid availability. Nat Commun. 2020;11:1416. https://doi.org/10.1038/s41467-020-15156-5.
Fukuhra S, Sakurai A, Yamagishi A, et al. Vascular endothelial cadherin-mediated cell-cell adhesion regulated by a small GTPase, Rap1. J Biochem Mol Biol. 2006;39:132–9. https://doi.org/10.5483/bmbrep.2006.39.2.132.
Mochizuki N, Ohba Y, Kiyokawa E, et al. Activation of the ERK/MAPK pathway by an isoform of rap1GAP associated with G alpha(i). Nature. 1999;400:891–4. https://doi.org/10.1038/23738.
Sawada Y, Nakamura K, Doi K, et al. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–7. https://doi.org/10.1242/jcs.114.6.1221.
Shah S, Brock EJ, Ji K, Mattingly RR. Ras and Rap1: a tale of two GTPases. Semin Cancer Biol. 2019;54:29–39. https://doi.org/10.1016/j.semcancer.2018.03.005.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Genhong Li, Jingwei Yao2, Zhen Lu, Lian Yu, Qinwei Chen, Lihong Ding, Zhihong Fang, Yin Li, and Bing Xu declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
This human tissue samples experiment was approved by the First Affiliated Hospital of Xiamen University Ethics Review Board in accordance with the Declaration of Helsinki. Informed consent was obtained from each participant. All xenograft mouse experimental protocols were approved by the Animal Care and Use Committee of Xiamen University.
Availability of data and materials
The datasets and materials in the current study are available from the corresponding author upon reasonable request.
Funding
This work was financially supported by the National Natural Science Foundation of China (81770126, 81900160, and 81800163).
Authors' contributions
XB, YJW, LY and FZH designed the study, analyzed the data, wrote the paper and supported the experiments. LGH and LZ conceptualized and designed the study, performed the CCK8, flow cytometric analysis and Western blot experiments as well as analyzed the data. YL, CQW and DLH performed animal experiments. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, G., Yao, J., Lu, Z. et al. Simvastatin Preferentially Targets FLT3/ITD Acute Myeloid Leukemia by Inhibiting MEK/ERK and p38-MAPK Signaling Pathways. Drugs R D 23, 439–451 (2023). https://doi.org/10.1007/s40268-023-00442-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00442-6