Abstract
GSK961081 (batefenterol) is a novel bifunctional molecule composed of a muscarinic antagonist and a β2‐agonist. The aims of this substudy were (1) to characterize the population pharmacokinetics (PK) and pharmacodynamics (PD) of GSK961081 in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD); and (2) to investigate the relationship between systemic exposure to GSK961081 and key cardiac-related safety parameters. Three once-daily doses (100, 400, and 800 μg) and three twice-daily doses (100, 200, and 400 μg) of GSK961081 DISKUS were investigated. A two-compartment disposition PK model with first-order absorption adequately described the plasma GSK961081 concentration–time data. An empirical maximum-effects PD model adequately described the forced expiratory volume in 1 s (FEV1) response relationship with the covariate baseline FEV1 on day 1. No clear relationships between GSK961081 plasma drug levels and cardiac-related safety parameters were apparent. The PK and PD models will be used to guide the dose selection and development of GSK961081 in patients with COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A two-compartment model accurately described the distribution of the GSK961081 concentration–time data. |
The pharmacokinetic and pharmacodynamic models described the observed data well, with adequate goodness-of-fit diagnostic plots. |
A muscarinic antagonist and a β2‐agonist in one molecule may potentially lead to the development of another regimen to support the current strategy of combining β2‐agonists and anti‐muscarinics as inhaled bronchodilators for treatment of chronic obstructive pulmonary disease. |
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease of the respiratory system and is a major cause of morbidity and mortality worldwide [1]. The primary aims of the pharmacological treatment of chronic, stable COPD are to optimize lung function, reduce exacerbations, and improve exercise tolerance and quality of life [2, 3]. Current treatment strategies include the use of β2‐agonist (BA) therapy and anti‐muscarinic therapy delivered as inhaled bronchodilators [2].
GSK961081 (batefenterol) is a novel bifunctional molecule composed of both a muscarinic antagonist (MA) and a BA separated by an inert linker portion [4]. The combination of an MA with a BA results in greater bronchodilation in the airways than either component alone [5]. The bifunctional nature of GSK961081 has previously been demonstrated in vitro [6] and in vivo [7].
A phase IIb clinical trial of GSK961081 reported an improvement in the trough forced expiratory volume in 1 s (FEV1) of 155–277 mL after 28 days of treatment [8]. GSK961081 has a rapid onset of action and was well tolerated [8, 9]. The safety of adding short-acting bronchodilators (SABAs) to a GSK961081 treatment regimen was assessed and showed that SABAs may be used as a rescue therapy on top of muscarinic β2‐agonist (MABA) therapy [10].
A single compound with MABA activity, such as GSK961081, has advantages over the use of two separate compounds. As a single pharmacokinetic (PK) profile exists for both pharmacological activities, there is the potential to maximize the synergy between the two mechanisms. The technical and clinical development pathway is also simpler for a single compound than for a co‐formulation of two separate compounds [11, 12].
PK and pharmacodynamic (PD) modelling techniques are a recognized method used to determine the optimal dose and dose regimen of drugs under development [13, 14]. The aim of this substudy was to characterize the population PK and PD of GSK961081 in patients with moderate-to-severe COPD; the primary results from the study (GlaxoSmithKline study number MAB115032; http://www.clinicaltrials.gov registration number NCT01319019) have been published elsewhere [8]. The resulting data will be used to help define the most appropriate dose and dose interval in future clinical trials of GSK961081. Long-acting β2-agonists have been associated with cardiac-related effects, showing potential to increase the heart rate and cause corrected QT interval (QTc) prolongation and ventricular arrhythmias in patients with COPD [15, 16]. Therefore, we also investigated the relationship between systemic exposure to GSK961081 (containing a β2-agonist) and key cardiac-related safety parameters.
2 Methods
2.1 Study Design
This PK and PD assessment of GSK961081 was part of a 4‐week, multicenter, randomized, double-blind, double-dummy, placebo- and salmeterol-controlled parallel-group study, which has been published elsewhere [8] (GlaxoSmithKline study number MAB115032; http://www.clinicaltrials.gov registration number NCT01319019). In this substudy, we report the PK and PD analyses of the active treatment, GSK961081. Three once-daily doses (100, 400, and 800 μg) administered in the morning and three twice-daily doses (100, 200, and 400 μg) of GSK961081 DISKUS were investigated.
2.2 Study Patients
Male and female outpatients aged ≥40 years who were either current or former smokers with a smoking history of ≥10 pack‐years were recruited. The patients had a clinical diagnosis of moderate‐to‐severe stable COPD, based on spirometric normative values according to the National Health and Nutritional Examination Survey III [17] (post-bronchodilator FEV1/forced vital capacity <70 and FEV1 ≥30 and ≤70 % predicted). Patients receiving a stable dose of inhaled corticosteroids were eligible for enrollment. Patients diagnosed with asthma were excluded.
The study was approved by the medical ethics committees at the participating centers, and all participants gave their written informed consent for participation. The study was conducted according to the Declaration of Helsinki [18] and Good Clinical Practice guidelines [19]. The characteristics of the study population are shown in Table 1.
2.3 Study Assessments
Spirometry FEV1 was performed in all patients, using a Vitalograph (Biomedical Systems, Brussels, Belgium). The day 29 trough FEV1 was defined as the mean of the 11 and 12 h measurements after the evening dosing on day 28.
PK blood sampling was performed in a subset of patients at overnight sites. On day 28, following administration of both the morning and the evening doses, blood samples were collected at 1 h to 0 min before dosing and then at 0–30 min, 30 min–2 h, 2–6 h, and 6–11 h after dosing.
PK analysis of GSK961081 concentrations was performed using a validated high-performance liquid chromatography and mass spectrometry method with a lower limit of quantification (LLOQ) of 25 pg/mL. The detailed bioanalytical methodology has been reported previously [4]. PK samples obtained from patients in the placebo and salmeterol treatment groups were not analyzed.
2.4 Pharmacokinetic and Pharmacodynamic Analyses
Plasma concentrations of GSK961081, expressed as natural logarithms and trough FEV1 values, were modelled using NONMEM version 7 software (ICON Developments Solutions, Dublin, Ireland). The first-order conditional estimation method with interaction was used for the PD model, and all data were used in the model-building. The Monte Carlo importance sampling method was used for the PK model. The M3 methodology was used to maximize the likelihood for the plasma GSK961081 data, treating those data that were below the LLOQ as censored for the PK model [20]. The percentage of PK data below the LLOQ was >50 % for all GSK961081 treatments, except for 800 μg once daily on days 1 and 28, and 400 μg twice daily on day 28. Therefore, the PK model-building data set comprised data from the 800 μg once-daily and 400 μg twice-daily treatment groups on day 28 only, of which 30 and 27 % of the data, respectively, were below the LLOQ. The full PK data set comprised data from all GSK961081 treatment groups.
Model selection was based on the objective function values computed by NONMEM, goodness-of-fit graphical checking, and physiological plausibility of parameters. Potential covariates were investigated using a stepwise forward addition and backward elimination approach for the PD model only [21]. The covariates baseline FEV1 on day 1, age, weight, sex, height, smoking status, and inhaled corticosteroid use were investigated. A p value of <0.01 was used in the forward step and a p value of <0.001 was used in the backward step. Simulation-based diagnostics and re-estimation methods were used to evaluate the performance of the PK and PD final models [21].
Relationships between selected PD endpoints [including the glucose level, heart rate, QT interval corrected for the heart rate using Fridericia’s correction (QTcF), and potassium level] and systemic exposure to GSK961081 [the maximum plasma drug concentration (C max)] were explored graphically. Placebo data were plotted using a value of 0 pg/mL, and GSK961081 values below the LLOQ were plotted as 12.5 pg/mL (i.e., half the LLOQ of the GSK961081 assay).
3 Results
3.1 Pharmacokinetics
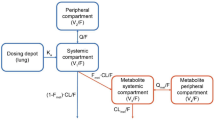
A total of 405 GSK961081 PK samples from 47 patients were included in the model-building PK data set. This comprised data from the 800 μg once-daily and 400 μg twice-daily treatment groups only (see Sect. 2). A two-compartment disposition model (ADVAN4 TRANS4) with first-order absorption was adequate to describe the plasma GSK961081 concentration–time data (Table 2). Interindividual variability could be estimated reliably for the apparent elimination clearance. Covariates were not included in the PK model, because of the limited data set.
The PK model described the observed data well, with adequate goodness-of-fit diagnostic plots (Fig. 1). A visual predictive check of the model showed no model misspecifications and good simulation properties for both the model-building data set and the full data set (Fig. 2). The full data set comprised 1112 GSK961081 PK samples from 128 patients (see Sect. 2). Bootstrapping confirmed the reliability of the PK model parameter estimates (data not shown).
Goodness-of-fit of the final pharmacokinetic model. a Logarithmic plasma GSK961081 concentrations on day 28 versus population predictions. b Logarithmic plasma GSK961081 concentrations on day 28 versus individual predictions. c Conditional weighted residuals versus population predictions. d Conditional weighted residuals versus time. The solid lines indicate the identity lines, and the dashed lines indicate the loess smooths
Visual predictive check of the final pharmacokinetic model, showing the logarithmic plasma GSK961081 concentrations versus time on day 28 for a 100 μg twice daily, b 200 μg twice daily, c 400 μg twice daily, d 100 μg once daily, e 400 μg once daily, and f 800 μg once daily. The horizontal lines indicate the lower limits of quantification for the pharmacokinetic assay, the solid lines indicate the population means, the dashed lines indicate the 95 % prediction intervals, the dotted lines indicate the observed means, and the dashed-dotted lines indicate the upper limits of the observed 95 % prediction intervals. Shaded areas indicate the 95 % confidence interval of the prediction interval. TRT treatment
3.2 Pharmacodynamics
A total of 347 trough FEV1 samples from 347 patients were included in the PD data set. A summary of patient demographics and clinical characteristics at screening was published in the primary paper (see the GSK961081 and placebo treatment groups only) [8]. An empirical maximum-effects (E max) model (Eq. 1) was adequate to describe the FEV1 response relationship (Table 3), with the baseline FEV1 on day 1 as a covariate (Eq. 2):
where E max is the trough FEV1 at the maximum effect, E 0 is the FEV1 at zero dose, ED50 is the dose producing 50 % of the maximum effect, and CON is the baseline FEV1 covariate effect.
The PD model described the observed data well, with adequate goodness-of-fit diagnostic plots (Fig. 3). A visual predictive check of the model showed no model misspecifications, good simulation properties (Fig. 4), and good agreement with the primary endpoint analysis [8]. Bootstrapping confirmed the reliability of the PD model parameter estimates (data not shown).
Goodness-of-fit of the final pharmacodynamic model. a Trough forced expiratory volume in 1 s (FEV1) values on day 29 versus population predictions. b Trough FEV1 values on day 29 versus individual predictions. c Conditional weighted residuals versus population predictions. d Conditional weighted residuals versus total daily doses. The solid lines indicate the identity lines, and the dashed lines indicate the loess smooths
Visual predictive check of the final pharmacodynamic model. a Day 29 trough forced expiratory volume in 1 s (FEV1) values versus total daily doses; the white circles indicate observed data, the solid line indicates the population means, and the dashed lines indicate the 95 % prediction intervals. b Day 29 placebo-corrected changes from baseline trough FEV1 values versus total daily doses; the black circles indicate once-daily dosing, the black triangles indicate the twice-daily dosing least squares means (with 95 % confidence intervals) from Wielders et al., the solid line indicates the pharmacodynamic model population medians, and the dashed lines indicate the pharmacodynamic model 95 % prediction intervals
3.3 Relationship Between Exposure and Safety Endpoints
The PK/PD analyses evaluated the relationship between key safety endpoints and systemic exposure to GSK961081. No clear relationships between maximum glucose, heart rate, QTcF and minimum potassium versus individual observed GSK961081 plasma drug levels (C max) were apparent (Fig. 5). It should be noted that these analyses were limited by high variability in the placebo response and a large number of patients with a C max below the LLOQ. Exploratory PK/PD analysis by way of generalized linear models did not show any significant relationships (data not shown). Similar evaluation of weighted mean changes in glucose levels, heart rate, QTcF, and potassium levels (0–4 h) from baseline versus individual observed GSK961081 C max values also showed no clear relationships with safety endpoints (data not shown).
Scatter plots of observed maximum plasma GSK961081 concentration (C max) values versus pharmacodynamic endpoints (regression plots) from 0 to 4 h on day 1 (left-hand panels) and day 28 (right-hand panels) for a maximum glucose level, b maximum heart rate, c maximum QT interval corrected for the heart rate using Fridericia’s correction (QTcF), and d maximum potassium level. The white circles indicate once-daily dosing, and the crosses indicate twice-daily dosing. For C max, placebo = 0 pg/mL, NQ = LLOQ/2, LLOQ = 25 pg/mL (indicated by the dashed vertical lines). bpm beats per minute, LLOQ lower limit of quantification, NQ not quantifiable
4 Discussion
This substudy is the first to report the population PK and PD of GSK961081 in patients with moderate-to-severe COPD. This information is important in the guidance of dose selection and subsequent clinical development of this novel compound. A two-compartment model accurately described the distribution of the GSK961081 concentration–time data. This substudy had a small number of patients because of limited PK sampling and a large number of non-quantifiable data. Due to the limited data available, covariates were not included in the PK model. We followed the recommendations of Ribbing and Jonsson [22], who suggested that stepwise selection of covariates or significance testing of covariates should not be used in data sets of less than 50–100 subjects.
An E max model described the trough FEV1 response on day 29. The primary study showed that there was only a small increase in the trough FEV1 from 400 to 800 μg, and concluded that a total daily dose of 400 μg may be the optimal dose [8]. These results are consistent with the final PD model reported here. As PK/PD models are an important tool in drug development [13, 14], our PK/PD modelling results with GSK961081 will aid dose selection for further studies of this novel compound in patients with COPD.
There were some limitations to this substudy. The number of different treatment doses investigated was small, and once-daily and twice-daily doses were combined for the PD analysis. However, there was no apparent influence of dosing regimen on the PD model, indicating that there was no apparent difference between once-daily and twice-daily dosing for the primary endpoint of the day 29 trough FEV1—a finding also reported in the primary publication [8].
Long-acting β2-agonists have been associated with cardiac-related effects [15, 16]. Over the doses of GSK961081 assessed in the current study, PK/PD analyses revealed no apparent relationship between individual C max values and any increase in the heart rate or QTcF. Furthermore, no clear relationship with blood potassium or glucose values was observed. The range of C max values in this substudy was limited, and we were not able to quantify this parameter in all patients. However, these results support the safety findings reported in the primary publication [8] that GSK961081 has no effect on glucose levels, potassium levels, heart rate, or blood pressure. In addition, although GSK961081 was associated with prolongation of various QTc intervals (by 3–5 ms, in comparison with placebo or salmeterol), there was no apparent dose response [8]. A previous study that investigated GSK961081 at a dose of 1200 µg once daily demonstrated that there was no prolongation of the QT interval [23]. Collectively, these data suggest that at the wide range of doses evaluated to date, GSK961081 may not be expected to induce cardiac-related effects; however, the current data are preliminary, and longer-term studies are required in patients with COPD to fully evaluate the tolerability profile of this compound.
COPD treatment guidelines recommend the use of combination bronchodilator therapy if patients remain symptomatic on a single bronchodilator [2]. Preclinical investigation of the effect of delivering two bronchodilators with different mechanisms of action has demonstrated a significant synergistic interaction in the bronchorelaxant response of human airways [24–27]. As a bifunctional molecule composed of both an MA and a BA, GSK961081 is postulated to have an advantage over combinations using two separate compounds, as the pharmacological activities are from a single molecule, thus ensuring co-localization at the site of action, potentially increasing the degree of bronchodilation with similar side effects.
5 Conclusion
The PK and PD models described in this substudy will be used as tools to guide the dose selection and clinical development of GSK961081 in patients with COPD. This novel compound combines two active moieties—an MA and a BA—in one molecule and may potentially lead to the development of another regimen to support the current strategy of combining β2‐agonists and anti‐muscarinics as inhaled bronchodilators for COPD treatment [2]. A further potential role of an MABA molecule may be as part of a triple COPD therapy delivered via a single inhaler [10].
References
World Health Organization. Health topics: chronic obstructive pulmonary disease. 2015. http://www.who.int/topics/chronic_obstructive_pulmonary_disease/en/. Accessed 19 Feb 2015.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. 2015. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed 19 Feb 2015.
Celli BR, MacNee W. Standards of the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46.
Norris V, Ambery C, Riley T. Pharmacokinetics and pharmacodynamics of GSK961081, a novel inhaled muscarinic antagonist b2-agonist, and fluticasone propionate administered alone, concurrently and as a combination blend formulation in healthy volunteers. Clin Pharmacol Drug Dev. 2014;3:305–13.
Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23:257–67.
Aiyar J, Steinfeld T, Pulido-Rios MT, Chin K, Lee TW, Jasper J, Thomas R, Hegde S, Mammen M. In vitro characterization of TD−5959: a novel bifunctional molecule with muscarinic antagonist and β2−adrenergic agonist activity. Am J Respir Crit Care Med. 2009;179:A4552.
Pulido-Rios MT, McNamara A, Kwan K, Martin W, Thomas R, Mammen M, Hegde S. TD−5959: a novel bifunctional muscarinic antagonist−β2-adrenergic agonist with potent and sustained in vivo bronchodilator activity in guinea pigs. Am J Respir Crit Care Med. 2009;179:A6195.
Wielders PL, Ludwig-Sengpiel A, Locantore N, Baggen S, Chan R, Riley JH. A new class of bronchodilator improves lung function in COPD: a trial with GSK961081. Eur Respir J. 2013;42:972–81.
Bateman ED, Kornmann O, Ambery C, Norris V. Pharmacodynamics of GSK961081, a bi-functional molecule, in patients with COPD. Pulm Pharm Ther. 2013;26:581–7.
Norris V, Ambery C. Bronchodilation and safety of supratherapeutic doses of salbutamol or ipratropium bromide added to single dose GSK961081 in patients with moderate to severe COPD. Pulm Pharm Ther. 2013;26:574–80.
Jacobsen JR, Aiyar J, Hegde S, Hughes AD, Mammen M. Dual‐pharmacology bronchodilators for the treatment of COPD. In: Hansel TT, Barnes PJ, editors. New drugs and targets for asthma and COPD. Progressive respiratory research, vol. 39. Basel: Karger; 2000. p. 39–45.
Cazzola M, Lopez-Campos JL, Puente-Maestu L. The MABA approach: a new option to improve bronchodilator therapy. Eur Respir J. 2013;42:885–7.
Gibbs JP. Prediction of exposure–response relationships to support first-in-human study design. AAPS J. 2010;12:750–8.
Bäckman P, Adelmann H, Petersson G, Jones CB. Advances in inhaled technologies: understanding the therapeutic challenge, predicting clinical performance, and designing the optimal inhaled product. Clin Pharmacol Ther. 2014;95:509–20.
Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–21.
Milic M, Bao X, Rizos D, Liu F, Ziegler MG. Literature review and pilot studies of the effect of QT correction formulas on reported beta2-agonist-induced QTc prolongation. Clin Ther. 2006;28:582–90.
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–87.
World Medical Association. World Medical Association Declaration of Helsinki—ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended [most recently] by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. 2015. http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed 19 Feb 2015.
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline: guidance for good clinical practice E6 (R1). 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 19 Feb 2015.
Ahn EA, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–21.
Mandema JW, Verotta JW, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–28.
Ribbing J, Jonsson EN. Power, selection bias and predictive performance of the population pharmacokinetic covariate model. J Pharmacokinet Pharmacodyn. 2004;31:109–34.
Norris V, Zhu C, Ambery C. The pharmacodynamics of GSK961081 in patients with COPD. Eur Respir J. 2011;38(Suppl. 55):823.
Calzetta L, Cazzola M, Page CP, Rogliani P, Facciolo F, Matera MG. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) 3/4 inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther. 2015;32:15–23.
Cazzola M, Calzetta L, Page C, Rogliani P, Facciolo F, Gavaldà A, Matera MG. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur J Pharmacol. 2014;745:135–43.
Cazzola M, Calzetta L, Segreti A, Facciolo F, Rogliani P, Matera MG. Translational study searching for synergy between glycopyrronium and indacaterol. COPD. 2015;12:175–81.
Calzetta L, Page P, Spina D, Cazzola M, Rogliani P, Facciolo F, Matera MG. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346:414–23.
Acknowledgments
We wish to acknowledge the investigators, staff, and patients at the study sites, without whom this research could not have been conducted. Editorial support (in the form of development of the manuscript first draft, in consultation with the authors) was provided by Ian Grieve, PhD, at Gardiner‐Caldwell Communications (Macclesfield, Cheshire, UK). Editorial support in the form of editorial suggestions on draft versions of this paper, assembling tables, collating author comments, copyediting, fact checking, and referencing were provided by Jackie Phillipson, PhD, at Gardiner-Caldwell Communications (Macclesfield, Cheshire, UK). Editorial support in the form of collation of author comments, redrawing of figures, and resubmission was provided by Joanne Ashworth of Fishawack Indicia Ltd. All editorial support was funded by GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of interest
CLA, RC, and JHR are employees of, and hold stock in, GlaxoSmithKline. PW has received reimbursement for attendance at symposia and speaker’s fees from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, GlaxoSmithKline, and Novartis Pharma. PW’s research institute has received grants from AstraZeneca and GlaxoSmithKline. ALS reports no conflict of interests.
Ethical standards
This study was approved by the medical ethics committees at the participating centers, and all participants gave their written informed consent for participation. The study was conducted according to the Declaration of Helsinki [18] and Good Clinical Practice guidelines [19].
Support statement
This study was funded by GlaxoSmithKline.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ambery, C.L., Wielders, P., Ludwig-Sengpiel, A. et al. Population Pharmacokinetics and Pharmacodynamics of GSK961081 (Batefenterol), a Muscarinic Antagonist and β2-Agonist, in Moderate-to-Severe COPD Patients: Substudy of a Randomized Trial. Drugs R D 15, 281–291 (2015). https://doi.org/10.1007/s40268-015-0104-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-015-0104-x