Abstract
Scleritis, an inflammatory disease of the eye affecting scleral tissue, presents unique challenges in the older adult population. Unlike their younger counterparts, older individuals manifest a distinct spectrum of the disease with different underlying etiologies, co-morbidities, altered immune function, and an increased risk of systemic side effects from medication choices. Addressing these complexities necessitates a comprehensive and multidisciplinary approach. Treatment of choice will depend on any underlying cause but generally involves non-steroidal anti-inflammatory drugs, systemic or local corticosteroids, and potentially disease-modifying anti-rheumatic drugs. Utilization of these therapeutic agents in older adults warrants careful consideration because of their potential side-effect profiles. This article critically examines the specific concerns for the use of these drugs in older patients and reviews the existing literature on their use in this specific cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The underlying causes of scleritis in older adults is often related to systemic disease and is more likely to require systemic medications. |
The choice of treatment can present challenges given pre-existing comorbidities in this population and the side-effect profiles of the medications used. |
With careful individualized drug selection and screening and monitoring, scleritis can be successfully and safely managed using systemic medications, including non-steroidal anti-inflammatory drugs, corticosteroids, and disease-modifying anti-rheumatic drugs. |
1 Introduction
The sclera is the outer layer of the eye, protecting the inner structures and giving form to the globe. It comprises connective tissue; collagen, elastin, and proteoglycans. It forms 80% of the globe, from the limbus to the insertion of the optic nerve and is avascular, receiving nutrients from the choroidal plexus and overlying episclera. Scleritis is a painful inflammation of the sclera that can involve any part of the globe and can lead to vision-threatening complications [1]. In 25% of patients, it presents as a bilateral disease and will eventually involve both eyes in 50% of patients. Etiology in older adults (generally defined as aged >60 or >65 years in the literature) often differs from that in younger individuals. The spectrum of autoimmune disorders varies, with an increased frequency of granulomatosis with polyangiitis (GPA) and rheumatoid arthritis (RA), and a higher risk of certain infectious etiologies including viral infection, bacterial infection, and tuberculosis (TB).
Additionally, older adults frequently have more co-morbidities, such as hypertension, diabetes mellitus, and cardiovascular disease, which can impact treatment choices. Medications to manage these conditions may affect scleritis treatments, and side-effect profiles of drugs used to manage scleritis may differ significantly in comparison to younger patients. Managing scleritis in older adults requires a delicate balance between addressing the underlying cause, controlling inflammation, and considering the individual’s overall health. Collaborative efforts between ophthalmologists, rheumatologists, and other specialists are essential to ensure the best outcomes in this population.
This review article offers a comprehensive exploration of scleritis in older adults, covering its classification, etiology, and treatment considerations. Special attention is given to the challenges of managing scleritis in older adults with co-morbidities, highlighting the need for careful treatment decisions.
Literature searches were carried out using PubMed and MEDLINE databases, in addition to Google Scholar. General search terms used included “scleritis,” “elderly,” and/or “older adults”. Search terms were then added more specifically for each of the treatment sections: “NSAIDs” or “non-steroidal anti-inflammatory drugs”; “systemic corticosteroids” and/or “prednisone” and/or “oral prednisone” and/or “prednisolone” and/or “oral prednisolone”; “intravenous corticosteroids” and/or “intravenous methylprednisolone” and/or “IV steroids” and/or “IV methylprednisolone” and; “disease modifying anti-rheumatic drugs” and/or “DMARDs”; “topical therapy” and/or “topical treatment” and/or “topical NSAIDs” and/or “topical steroids”; “subconjunctival steroid” and/or “sub-tenon steroid” and/or “periocular steroid”. Additionally, in the disease-modifying anti-rheumatic drug (DMARD) section, a separate search was carried out with the additional terms “biologics” and “anti-TNF”. For the section on the treatment of infectious scleritis, the specific infection and individual drug names were added to the general search terms. In searches where there were few or no relevant articles found, specifically in the sections on systemic corticosteroids, DMARDs, and treatment of infectious scleritis, the search term “scleritis” was removed, providing articles on other conditions where similar treatments are used. This is further discussed throughout the article.
2 Classifying Scleritis
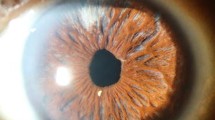
The insertions of the extraocular recti muscles divide the sclera into anterior and posterior sections and scleritis is classified according to the anatomic site of the inflammation: anterior, posterior or, rarely, both [2]. Anterior scleritis is more common, accounting for up to 80% of patients. It is categorized according to its clinical appearance as diffuse or nodular, and whether it is necrotizing. Patients with anterior scleritis complain of extreme boring ocular pain that may worsen with eye movement. Typically, the pain is continuous and may wake the patient from sleep. If there is corneal involvement, there may be reduced vision. In diffuse anterior scleritis, the inflammation can involve large parts of the sclera with redness and engorged, tortuous episcleral blood vessels (Fig. 1). In nodular scleritis, patients will present with a firm, immobile, painful red nodule with engorged episcleral blood vessels. Diagnosis is based on clinical findings and ocular erythema that persists after topical application of 10% phenylephrine. In contrast, the condition of episcleritis, a more benign inflammation of the conjunctival and superficial episcleral vasculature, classically blanches with this dose of topical phenylephrine and the pain described by patients is usually less intense. While there can also be nodules in this condition, they are confined to the more superficial episcleral [1]. It is important to differentiate between these two conditions as the treatment response will be different; episcleritis will be more likely to respond well to topical therapy whereas true scleritis usually requires systemic treatment. Additionally, unlike scleritis, episcleritis is not vision threatening. Causes of visual loss in scleritis include irregular astigmatism, corneal complications (including peripheral ulcerative keratitis), and glaucoma [2, 3].
Necrotizing scleritis is a more serious presentation than anterior scleritis and is imminently vision threatening with a higher risk of fast progression to scleral perforation [3]. Involved areas appear white early on with a distinct absence of blood vessels (Fig. 2a). As the disease progresses, ulceration and necrosis of the sclera appear (Fig. 2b), with possible involvement of the cornea, ciliary body, or trabecular meshwork. Following resolution of anterior scleritis, a bluish hue may remain that does not represent active inflammation, rather a re-arrangement of the scleral collagen. Scleromalacia perforans is a form of necrotizing anterior scleritis without inflammation. It is rare and typically involves both eyes, presenting without redness or pain. It is seen more often among elderly female patients and is associated with RA. In the sclera, there are areas of infarcted scleral vessels with scleral thinning and necrosis. Because of the lack of symptoms, patients often present late with extensive scleral thinning and secondary blurred vision due to astigmatism.
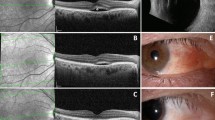
Posterior scleritis is defined as involvement of the sclera posterior to the muscle insertions. Diagnosis is challenging because clinical findings can be non-specific. Similar to anterior scleritis, patients complain of extreme ocular pain and headaches, but anterior ocular examination may be normal with no signs of ocular redness or vessel engorgement. A fundal examination may reveal optic nerve edema, macular edema, choroidal folds, retinal striae, or serous retinal detachments (Fig. 3). Diagnosis is confirmed based on B-scan ultrasonography demonstrating scleral thickening or fluid in the interface between the optic nerve and sclera (“T sign”).
3 Epidemiology and Etiology of Scleritis
Scleritis has an incidence rate between 1.0 and 5.2 cases per 100,000 persons/year and a prevalence that ranges from 1.7 to 5.2 per 100,000 persons [1, 4,5,6,7]. The average age of onset is between 30 and 50 years and women account for up to 60% of cases [8]. Though the incidence of scleritis is less common after the age of 65 years [7], older-onset disease is associated with a worse visual outcome [8]. Associations with systemic diseases are seen in up to half of cases and can be related to systemic autoimmune disease, infectious causes, recent ocular surgery, or various systemic drugs [8,9,10].
Scleritis is relatively uncommon in patients after the age of 60 years and they account for only 3% of all scleritis cases. In a recent study, the average age of these elderly patients was 67 years and two thirds were female [11]. Diffuse anterior scleritis was the most common subtype, followed by necrotizing scleritis. Systemic associations were found in 32% of patients. Interestingly, the most common systemic association was GPA. Because of the strong association with systemic disease, older patients presenting with scleritis should all have baseline screening investigations as well as a detailed systemic enquiry. These investigations are outlined in Table 1.
3.1 Autoimmune Disease-Associated Scleritis
Autoimmune diseases are associated with scleritis in approximately 40% of cases [12, 13]. Rheumatoid arthritis is the most common systemic disease in patients with scleritis, followed by GPA, Sjögren syndrome, and systemic lupus erythematosus (see Fig. 4) [14]. In 80% of patients, the underlying systemic disease is already known at the time of diagnosis of scleritis. For those without a known systemic disease, a new autoimmune disease is diagnosed during the follow-up at a rate of 4% per year.
3.2 Infectious Scleritis
Infectious causes are less common, though any breach of the conjunctivae or episclera can lead to micro-organisms invading the sclera. Systemic and local immunosuppression increase the risk of infectious scleritis, particularly the topical use of antimetabolites or radiotherapy. A recent claim-based study in the USA suggested an incidence of 1.6 per 100,000 people per year and a prevalence of 4.6 cases per 100,000 people [15]. Patients had a higher risk between the ages of 55–64 years and co-morbidity with autoimmune diseases increased the incidence by factor of 2.8 [15]. Bacteria remain an important cause of infectious scleritis and Pseudomonas aeruginosa accounts for as much as 80% of bacterial cases [14]. Tuberculosis remains a significant cause of morbidity, particularly in the developing world. Tuberculous scleritis is caused either by direct scleral inoculation by Mycobacterium tuberculosis or by an immune-mediated process. Additionally, the bacteria Nocardia (previously classified as a fungus) is also a recognized cause of severe scleritis and is more common in traumatic cases with exposure to soil or plant matter [14]. Among viruses, the herpes family including varicella-zoster virus and herpes simplex virus type-1 are the most frequent causes, and viral etiology, particularly zoster, may be more common in elderly individuals (see Fig. 5). Less commonly, scleritis can also be caused by a fungal pathogen. These are more common in developing countries and cause a rapidly progressive scleritis [14]. Where infectious scleritis is suspected, additional investigations, discussed further below, may be required.
3.3 Drug-Induced Scleritis
Several drugs are associated with the development of scleritis. Bisphosphonates, used primarily for the prevention of bone thinning in osteopenia and osteoporosis but also for Paget’s disease and certain bone cancers, were the first drug class shown to cause scleritis. It is considered the most sight-threatening side effect and can occur with either oral treatment or an intravenous infusion. It is an idiosyncratic response and, therefore, not dose related [16,17,18,19,20]. It is reported for different drugs in this group, occurring within 48 hours in over 80% of patients with pamidronate-associated scleritis [20, 21]. Other drugs related to scleritis include retinoids, topiramate, and vaccines, such as the influenza vaccine and the coronavirus disease 2019 mRNA vaccines [22, 23].
3.4 Surgically Induced Scleritis
Scleritis following ocular surgery is rare, accounting for 0.3% of cases in one multi-center study [9]. Single cases and small series reported scleritis occurring after pterygium surgery, strabismus surgery [24, 25], cataract surgery [26, 27], and glaucoma surgery [28]. Among patients with known scleritis, ocular surgery may trigger a recurrence of the disease [29].
4 Concerns Regarding Treating Scleritis in Older Adults
Treatment for scleritis aims to alleviate symptoms, particularly pain, and prevent vision-threatening complications. As an autoimmune disease, treatment is primarily based on systemic drugs to control the inflammation and prevent vision loss or recurrences. In a study examining 97 patients with scleritis across all age groups, 30% received non-steroidal anti-inflammatory drugs (NSAIDs), 31% needed the addition of systemic corticosteroids, and 26% required the addition of DMARDs [1]. Treatment tends to continue for months and may last years for patients with recalcitrant disease. Systemic side effects can occur with all these drug groups, limiting their use. Corticosteroids are particularly likely to cause short-term systemic side effects, including gastrointestinal (GI) complaints, behavioural changes, and weight gain. Long-term use raises concerns regarding accumulating side effects. Systemic hypertension and elevated blood glucose levels are concerns for many older patients and require continual monitoring. In patients with known diabetes, systemic corticosteroids can complicate the control of blood glucose levels. Close monitoring, adjustment of diabetic medication, and the use of short-acting insulin should be considered to control blood glucose levels. Long-term use of systemic corticosteroids can result in bone mass loss up to the point of osteoporosis and the need for treatment. For patients who do not tolerate systemic drugs, local treatment is a possible option. Reports of the use of local injections of corticosteroids suggest they are effective in controlling scleritis [30]. Treatments last for several months and can be repeated as needed if relapses occur. Concerns regarding scleral necrosis induced by corticosteroid injections limit their use to patients with non-necrotizing anterior scleritis [31,32,33,34].
5 Treatment of Scleritis
5.1 NSAIDs
Scleritis is an inflammatory condition and so it follows that one of the first-line treatment options for this condition is systemic NSAIDs [35]. Non-steroidal anti-inflammatory drugs are one of the most commonly prescribed medications in primary care worldwide [36,37,38]. They are a highly effective class of drugs for treating inflammation, including scleritis. Sainz de la Maza et al. [39], Bauer et al. [40], and Agrawal et al. [41] all demonstrated that in their cohorts, a high proportion of patients responded to treatment with oral NSAIDs alone. Bauer et al. and Agrawal et al. only included patients with non-necrotizing nodular or diffuse anterior scleritis and there was no analysis of different age groups. Sainz de la Maza et al. showed that in cases of anterior (nodular or diffuse) non-infectious non-necrotizing scleritis in their cohort, 36.7% responded to NSAIDs alone but, interestingly, the likelihood of this was significantly reduced in patients of an older age group. This could represent lower efficacy in this group or that older patients have a higher likelihood of systemic disease, such as RA, predisposing to more severe forms of scleritis that may necessitate more aggressive therapy.
Importantly, NSAIDs also have a well-known side-effect profile including GI upset and bleeding, cardiovascular complications, and nephrotoxicity. They are generally metabolized in the liver and excreted in the urine. The pharmacokinetics and dynamics of NSAIDs are likely altered in most aging bodies because of reduced muscle mass and water content, and thus elderly patients are potentially more susceptible to the side effects [42]. High-risk groups include patients over the age of 75 years and those taking concurrent corticosteroids or antiplatelet/anticoagulant therapy [43].
The American Geriatric Society recommend using NSAIDs with caution and limiting their use to the lowest effective dose and shortest duration. Long-term use should be avoided “unless other alternatives are not effective” [44]. Side effects should be monitored for, including renal function, and in any patients with an estimated glomerular filtration rate less than 30 mL/min, NSAIDs should ideally be avoided because of the risk of progression of chronic kidney disease [43, 45]. The American Geriatric Society also advocates the use of a gastroprotective medication alongside the use of NSAIDs, such as a proton pump inhibitor, owing to the incidence of GI bleeding and ulceration secondary to the use of NSAIDs increasing with age [46]. Additionally, NSAIDs have been shown to be associated with an increased risk of hospital admission because of congestive cardiac failure [47] and should be used with caution in patients already diagnosed with this condition and certainly avoided in those who are symptomatic [44].
Therefore, while NSAIDs can be an effective medication in scleritis treatment, they should be used cautiously and selectively in elderly patients, particularly those with co-morbidities. A summary of recommended doses, common side effects, and special considerations for use in older adults can be found in Table 2.
5.2 Systemic Corticosteroids
In a failure of NSAIDs to control symptoms, the next step would typically be oral corticosteroids in the form of prednisone. They may also be started as first line in patients with posterior or necrotizing scleritis, contraindications to NSAIDs, in those judged to have a higher degree of scleral inflammation, or those with associated systemic disease [1, 48]. In cases of severe inflammation, such as necrotizing scleritis with impending perforation, intravenous corticosteroids may be used and are discussed separately below.
Oral corticosteroids are typically started at high doses and slowly tapered over a number of weeks and months. The speed and length of taper will depend on the individual response and any undesired side effects. Studies have shown that up to 50% of patients with anterior nodular or diffuse scleritis respond to oral corticosteroids [1, 39]. In older patients, particularly those of smaller build, it may be worth considering lowering the initial corticosteroid dose owing to the diminution of muscle mass and plasma volume that accompanies aging in order to lessen or avoid the side effects discussed below (see Table 2) [49]. There is, however, no robust evidence to support this in the treatment of patients with scleritis.
The general side effects of oral corticosteroids are well known and have been mentioned above. While to the best of our knowledge there are no specific studies on prednisone use in elderly patients with scleritis, the use of high-dose oral corticosteroids in other inflammatory conditions such as giant cell arteritis and GPA has been linked with higher treatment-related damage in elderly patients [50, 51]. The course of treatment in these conditions is lengthy, with a range from 6 to 18 months, and it has been shown that side effects are related to the dose and duration of treatment, as well as protective medication and individual susceptibility [52,53,54,55]. Similarly, in patents with polymyalgia rheumatica, advancing age and cumulative corticosteroid dose have been identified as risk factors for corticosteroid-related adverse events, again suggesting that both dose and duration of treatment are important [56]. While the side effects of corticosteroids were significant in the older population, it is important to note that the burden of the disease was also higher, making it difficult to achieve the balance between risk and benefit.
While the course of corticosteroid treatment in scleritis is unlikely to be comparably as long as in these conditions, and therefore difficult to directly apply such results to, it still highlights the need for ensuring that corticosteroids are used with caution in elderly patients with co-morbidities. It is important to consider appropriate protective therapies and to monitor for any adverse reactions to treatment. A particularly important adjuvant to consider in elderly patients is bisphosphonates, with good evidence that their use reduces the risk of vertebral fractures and corticosteroid-induced bone loss in patients taking an average of 5 mg or more per day of oral corticosteroids [57]. It has been mentioned previously that bisphophonates can also cause scleritis; this remains a rare side effect and the authors suggest that the documented benefits of bisphophonates in this cohort of patients outweigh the risk. If, however, a patient develops scleritis secondary to bisphosphonate treatment, it is generally stopped, as the induced scleritis usually responds to discontinuation of the drug. It is also recommended to perform bone scanning in patients who have been receiving systemic corticosteroid therapy for 3 months or longer and to consider a baseline bone scan if it is anticipated that the patient will require a prolonged course of glucocorticoids [58]. Additionally, the use of gastroprotection is also recommended in patients taking oral corticosteroids, especially those who may also be taking NSAIDs for other reasons, owing to the increased risk of GI bleeding with this combination [44].
As mentioned above, there are some patients with scleritis, who will require a course of pulsed intravenous methylprednisolone (IVMP) to gain rapid disease control prior to commencing a tapering course of high-dose oral corticosteroids, often in addition to immune-modulating therapy [59]. Side effects that have been associated with high-dose intravenous therapy include cardiovascular complications, hepatic toxicity, avascular necrosis of the hip, and acute delirium.
Pulsed IVMP has been shown to cause tachycardia and in some patients can induce cardiac arrythmias; most commonly atrial fibrillation, though cases of ventricular tachycardia have been reported [60, 61]. Other cardiac complications include significant hypertension and potential subsequent myocardial infarction [62]. When treating elderly patients, it should be borne in mind that a significant number will already have cardiac disease and this risk will need to be considered. Furthermore, studies in patients receiving IVMP for thyroid orbitopathy have demonstrated a risk of significantly increased liver enzymes, with some patients progressing to acute liver failure [63]. These were patients with previously normal liver function, thus caution should be exercised in patients who may already have abnormal liver enzymes due to other diseases. Avascular necrosis carries a significant morbidity and is known to be a side effect of systemic corticosteroids. There is risk of it occurring both in oral and intravenous therapy but is more strongly associated with IVMP, presumably because the risk is directly rated to the dose and increases with higher individual doses; it is less closely linked with cumulative dosing [64]. Of note, it has been shown to occur more commonly in patients with systemic lupus erythematosus [65], one of the systemic conditions commonly linked with scleritis. With regard to age, perhaps counter-intuitively, corticosteroid-induced avascular necrosis tends to occur in young to middle-aged patients, with one study reporting 75% of cases occurring in patients aged between 30 and 60 years [66] and thus this is not a concern specific to elderly patients. Treatment for it, however, is less successful in older patients and it therefore carries a higher morbidity in this group [67].
A further consideration in the use of corticosteroids, both IVMP and oral prednisone, in the elderly patient is the potential risk of delirium. Fortunately, this side effect is less common although does appear to be more frequent at higher doses (>40 mg of prednisone daily) and is likely to present within the first 1–2 weeks of treatment [68, 69]. Older age (>60 years) has also been shown to be an independent risk factor for transition to delirium following treatment with systemic corticosteroids, though these were high-dose corticosteroids in patients who were already hospitalized [70]. In patients already displaying evidence of a cognitive decline, this should be closely monitored.
While systemic corticosteroids have their problems, particularly in an elderly cohort, they are still an important part of scleritis treatment. With close monitoring and a considered approach, they can be used relatively safely in elderly patients [49, 71].
5.3 DMARDs
Bearing in mind the desire to limit the dose and duration of oral corticosteroids, in patients requiring frequent or prolonged courses of prednisone, those showing progression despite corticosteroid therapy or in patients with severe disease, DMARDs may be required. These drugs are generally slower to act and patients will generally need to remain on a maintenance dose of corticosteroids until an effect is reached. There are various different “types” of DMARDs and these can be seen, divided by subgroups, in Table 3. In current practice, the main agents used as first-line DMARDs are antimetabolites; these could be considered as “conventional” DMARDs. Other successful therapies include alkylating agents such as cyclophosphamide [72] or calcineurin inhibitors. Following this, the group of anti-tumor necrosis factor (TNF) drugs may be considered. Other options include the CD20 antibody rituximab and the interleukin-6-receptor antibody tocilizumab [73]. The choice and timing of therapy will depend on the clinical presentation, progression, and multiple patient factors and a full discussion around this is beyond the scope of this article. The decision around when to change between these therapies must be assessed on a case-by-case basis depending on the ocular response to treatment, severity of disease (and, therefore, the need to gain control quickly), and the patient’s ability to tolerate the drug.
There are particular considerations to be borne in mind when using these drugs in older adults. In elderly patients, immunosenescence may predispose to the risk of over-immunosuppression [74], although there is no robust evidence to suggest this in patients with scleritis being treated with DMARDs. Furthermore, doses of these medications may need to be altered in older adults to reduce side effects and toxicity (see Table 2). All of these drugs have potentially toxic side effects on the kidneys, liver and/or hematopoiesis, and require blood monitoring in all patients, not just elderly patients. Baseline blood work should include a full blood count and renal and liver function. Additionally, the risk of reactivation of latent infection is discussed below and patients should also be screened for hepatitis and TB prior to commencing DMARDs.
There is evidence to suggest that the pharmacokinetic properties of some of the DMARD agents are altered in older age groups. The trough concentrations of calcineurin inhibitors, for example, were found to be 50% higher in patients aged over 65 years presumably owing to a reduction in cytochrome P450 3A4 metabolism with age [75]. Likewise, the metabolism of methotrexate is affected by age, and elderly patients seem to be more susceptible to toxicity with this medication and are likely to require a lower dose than younger patients to achieve the same efficacy [76, 77]. Conversely, elderly patients may require higher doses of mycophenolate than younger patients owing to a generally lower serum albumin level resulting in a higher clearance of unbound mycophenolic acid [78].
Infections are an important cause of morbidity and mortality in elderly patients, with the aging immune system itself being a risk factor for infection. It follows, therefore, that the addition of pharmacological immunosuppression serves to increase this risk. The majority of data regarding immunosuppression in the elderly patient come from the literature on RA. The conventional treatment for RA is methotrexate, which is reasonably well tolerated; one particular study with a 5-year follow-up showed discontinuation in only 25% of patients over 65 years with RA, mainly due to a GI and hepatic upset rather than infection, although rates of infection overall were still increased [79]. Conversely, there are several retrospective cohorts of patients with RA treated with anti-TNF agents suggesting that drug discontinuation in elderly patients was mainly related to adverse events (specifically infections) compared to drug ineffectiveness in the younger cohort [80,81,82]. Other large studies, however, have failed to demonstrate an overall elevated infection risk in patients with RA aged over 65 years treated with anti-TNF drugs [83, 84] and thus the overall evidence is hard to determine.
What is well known, however, is that treatment with anti-TNF agents can cause reactivation of latent TB and evidence suggests that older age (>60 years) is an independent risk factor for this [85, 86]. Screening for TB prior to commencing treatment is, therefore, vital, as is closely liaising with infectious disease specialists in cases of positive screening results. The risk of treatment for TB in an elderly patient, which may itself cause toxicity, must be weighed against any benefit of treatment. Another opportunistic infection to be mindful of is herpes zoster virus. The risk of reactivation of herpes zoster virus in a cohort of patients with RA was shown to be similar between anti-TNFs and conventional DMARDs, with older age being an independent risk factor [87]. Interestingly, the concomitant use of systemic corticosteroids doubled the risk [88].
It is important to consider that these data on infection rates may not be directly transferable to patients with scleritis given that RA is itself an independent risk factor for infection [89]. This is highlighted in a systemic review of patients with RA compared to other inflammatory rheumatological diseases being treated with methotrexate; the rate of overall infection was found to be increased in patients taking methotrexate with RA but not in those with other inflammatory rheumatological diseases [90]. There will of course be some overlap as RA is a potential cause of scleritis. A recent review of the use of biologic DMARDs in elderly patients with psoriatic arthritis suggested that rates of infections in this age group were not significantly higher than in younger patients; however, when they did occur, they tended to be more serious [91]. Another cohort of immunosuppressed patients are those who have had solid organ transplants. The level of immunosuppression in these patients is generally higher than those with rheumatological disease but data show that patients in this cohort over the age of 50 years have a two-fold increase in the risk of developing bacteremia and sepsis in comparison to their younger counterparts [92]. Similarly, there is also evidence of an increase in the rate of opportunistic infections in individuals aged over 50 years receiving immunosuppressive therapy for inflammatory bowel disease [93]. Some studies looking at the CD20 inhibitor rituximab have also shown an increased rate of infections occurring in older patients [94, 95]
Immunosenescence is also likely to be a contributing factor in the development of malignancy due to decreased immunosurveillance, and it is known that cancer incidence and mortality increase after the age of 65 years. Immunosuppressive therapy has been shown to increase the rates of malignancy in elderly patients, although, again, there are no specific data on those patients receiving immunosuppressive therapy for scleritis. The risk for malignancy in patients with solid organ transplants is two to three times higher than the general population and within this cohort, patients aged over 60 years have a five-fold increase in risk compared with those aged under 45 years [96, 97].
The literature on RA and rates of malignancy are inconsistent and difficult to interpret. There are studies that have suggested an increase in rates of malignancy in elderly patients receiving anti-TNF therapy; one describing an overall cancer risk increase [83] and one specifically for non-melanoma skin cancer [98]. An increased risk of lymphoma has been demonstrated in patients taking biologic DMARDs compared with the general population but not in comparison to patients with RA taking conventional DMARDs [99], and thus it is difficult to ascertain whether this increased risk was due to the treatment or to the underlying condition; patients with RA are known to have a globally increased of lymphoma at baseline [100]. A study from the Swedish Registry of Biologics reported an increased incidence of cervical cancer in women aged over 60 years receiving anti-TNF therapies [101]. Other large studies, however, have not found an increased overall cancer risk in elderly patients using anti-TNF drugs or other biologics [102,103,104,105]. Current knowledge advocates ongoing monitoring of the cancer risk in elderly patients and increased surveillance both prior to and during treatment with DMARDs.
5.4 Topical Treatment
Topical therapies that have been used for the treatment of scleritis include NSAID drops and corticosteroid drops, usually prednisone acetate 1%. Topical NSAIDs can be used as first-line treatment in patients with episcleritis, with some success, but no studies suggest this as an effective treatment for scleritis. Topical corticosteroids are a treatment option, with one trial showing resolution of scleritis symptoms in 43% of patients with non-necrotizing anterior scleritis with prednisone acetate 1% drops administered every 2 hours for 2 weeks [106]. In the broader literature, however, topical corticosteroid drops are not recommended as monotherapy but can be useful when used in conjunction with systemic treatment, such as oral NSAIDs [1, 41, 107].
An alternative treatment option is the injection of periocular corticosteroids, including subconjunctival and sub-tenon injections (see Fig. 6). As mentioned above, these are only recommended for use in patients with non-infectious, non-necrotizing scleritis but have shown good efficacy [108,109,110]. Sohn et al. [111] demonstrated a response rate of around 90% to subconjunctival triamcinolone, with two thirds of patients remaining symptom free at 24 months post-treatment. Albini et al. [33] showed similar response rates, though did not have the same length of follow-up. Neither study had any patients go on to develop scleral thinning or perforation.
The main advantage of local therapies is the avoidance of the major side effects that come with systemic treatment; an advantage that is particularly pertinent when treating older patients with multiple co-morbidities. In the above studies, many of the patients receiving treatment were those who either had contraindications to, or were unable to tolerate, systemic medications. It is important to remember, however, that there is still the potential for local side effects with both topical and periocular corticosteroids: namely cataract and raised intraocular pressure (IOP). The administration of subconjunctival triamcinolone was shown to cause a rise in IOP between 27% and 50% of patients [33, 108,109,110,111]. The majority of these patients were successfully managed with medical therapies (topical drops and oral acetazolamide), though in one study, 3% of patients went on to require glaucoma surgery [111]. Considering these results were from patients with no pre-existing glaucoma and previously normal IOP, this has to be a major consideration in a cohort of elderly patients—a group in which a higher number of patients will already have pre-existing glaucoma. Similarly, older patients are also more likely to have pre-existing cataracts that may be exacerbated by local corticosteroid treatment, necessitating the need for cataract surgery [33, 110, 111]. This is associated with a higher rate of post-operative complications in patients with scleritis, such as post-operative corneal edema, prolonged anterior chamber inflammation, and raised IOP, as well as the risk of recurrence of the disease [112, 113].
Local therapies are an important part of the armamentarium for treating scleritis, specifically non-necrotizing, non-infectious scleritis. They are a viable option for patients in whom systemic treatment is problematic and may, therefore, be particularly useful in an elderly cohort. The risk of raised IOP and subsequent glaucoma, however, is very real and should always be borne in mind when considering administration of these treatments.
6 Treatment of Infectious Scleritis
The treatment stepladder described above applies largely to non-infectious scleritis. As mentioned above, infectious scleritis is a less common but important entity and the mainstay of treatment in these cases will be treatment of the underlying infection as well as control of the inflammatory response to the inciting organism.
The majority of infectious scleritis is viral in origin, with herpes zoster virus being the most common [14]. This may be apparent as the cause of the scleritis in cases where patients present with the characteristic vesicular rash over the V1 distribution. It can, however, occur after the initial episode of zoster and hence a careful history is required. If there is clinical suspicion without the classic rash, obtaining an anterior chamber sample for herpes polymerase chain reaction can be useful [114]. Herpes zoster is generally treated with oral anti-viral drugs, commonly valaciclovir, alongside systemic corticosteroids when required. Valaciclovir is an oral prodrug of acyclovir and is 90% renally excreted [115], making patients with altered renal function more prone to toxic side effects. As with previously discussed systemic medications, this is of particular importance in elderly patients; renal function should be established prior to commencing treatment and the dose adjusted as necessary. There have been multiple reports of patients with acyclovir-associated encephalopathy in the literature, most of whom had pre-existing renal disease [116, 117]. Acyvlovir can also precipitate renal dysfunction in and of itself, and monitoring of creatinine levels and glomerular function during treatment is advised. It is, however, a generally well-tolerated therapy and has been used for many years.
Decreased renal function is also a major factor in the treatment of toxoplasma-induced infectious scleritis. This is most commonly treated with co-trimoxazole [118], which is nephrotoxic and again requires careful renal monitoring in elderly patients as it has the ability to induce an acute kidney injury [119]. The alternative treatment of triple therapy (pyrimethamine, sulfadiazine, and folinic acid) has a myriad of side effects including leukopenia and thrombocytopenia and requires weekly blood tests for monitoring, as well as potentially causing a GI and dermatological upset, which all need to be taken into account when prescribing for older patients [120, 121]. Fortunately, scleritis is a rare presentation of toxoplasma and patients are likely to have presented concurrent retinochoroiditis in the same eye, alerting the clinician to the likely diagnosis [122]. If there is doubt about the diagnosis, again, an anterior chamber sample can be obtained and sent for toxoplasma polymerase chain reaction.
Bacterial scleritis is usually secondary to a keratitis, following ocular surgery or subsequent to trauma and is treated as per the causative organism. In such cases, the inciting cause should be apparent from the history and ocular examination. It is important to differentiate surgically induced necrotizing scleritis from infectious scleritis; surgically induced necrotizing scleritis would be expected to have a longer latent period. In suspected bacterial scleritis, swabs or tissue samples for culture are crucial in determining the correct antibiotic treatment. Until the organism is known, broad-spectrum systemic and topical antibiotics should be used. The most accessible location for tissue sample will be scrapings from a keratitis or an ulcerated scleral nodule. Scleral or corneo-scleral biopsy would be advised if: initial scrapings are negative and there is a lack of clinical response to treatment; initial scrapings are positive but there is a poor response to recommended treatment; if there is a large area of abscess requiring drainage; and if surgical intervention is otherwise required, for example, in the case of a corneal or scleral perforation [14].
Tuberculosis should also be considered as an underlying cause of scleritis and all patients presenting with scleritis should be screened for this with Quantiferon Gold testing and a plain chest X-ray, as clinically it is not always easy to distinguish from non-infectious scleritis. A thorough clinical and systemic history is also crucial. It usually runs a chronic course and does not respond as well to anti-inflammatory treatment [122, 123]. Treatment for TB is prolonged, requiring at least 6 months of therapy with multiple agents and should be undertaken under the supervision of an experienced infectious diseases physician. Older patients are at greater risk of hepatic toxicity with these drugs and very regular blood monitoring is necessary [124]. Patients taking ethambutol require a regular evaluation of visual acuity, color vision, and the visual field because of the risk of optic neuropathy. Streptomycin, used for drug-resistant TB, should not be used in elderly patients because of the higher risk of irreversible auditory and vestibular damage [124].
A small percentage of infectious scleritis will be secondary to fungal infection and the main systemic treatments for this would be the “azole” group of anti-fungal agents [14]. Reduced hepatic function as a result of aging and the effect of polypharmacy in elderly patients already taking multiple medications competing for the same metabolic pathways can result in an increased half-life of this group of drugs and subsequent toxicity [125]. Liver function should be monitored in patients being commenced on treatment with voriconazole or similar agents.
7 Conclusions and Future Directions
Treatment of scleritis in older adults can be challenging, and achieving the balance between gaining adequate disease control without treatment having a negative impact on the rest of the individual patient’s health and well-being is the desired goal. Specific challenges include side effects of the mainstream systemic drugs used to treat scleritis, namely NSAIDs and corticosteroids, and the associated pre-existing comorbidities in more elderly patients that are likely to be exacerbated. The overall effect of aging on the human body must also be considered, particularly the general reduction in renal function and, to a lesser degree in most cases, hepatic function. With careful monitoring and selective use, however, these drugs can still be used effectively and safely in the older population.
Evidence from the current body of literature suggests that DMARDs are a valid option in elderly patients. While they certainly can have side effects, including infections and toxicity, the majority of studies, particularly the more recent studies on anti-TNF therapy, do not generally suggest that the rates of adverse events are increased in older patients in comparison to younger individuals on these drugs. In fact, the long-term effects of corticosteroid use may cause more problems than DMARDs. Discussions with individual patients and, where appropriate, family members, including informed consent of potential side effects, alongside careful monitoring is required. It is also important to remember the role of topical and local therapies in the treatment of certain patients, particularly in the short-term management of patients unable to tolerate first-line systemic therapies. This review is limited in its specific applicability to patients with scleritis. The data presented are largely extrapolated from studies on other systemic conditions because of a lack of evidence in the literature on the treatment of scleritis in older populations. There is a need for further studies in this area to provide some robust evidence for this specific cohort, both on the efficacy of the different therapeutic options as well as side effects.
Other more long-term future directions could include the implementation of more targeted treatment decisions with the use of large study data on the effect of individual characteristics such as age, sex, ethnicity, renal function, hepatic function, and bone mass on the efficacy of treatments and the risk of side effects. There could be the potential for the use of artificial intelligence alongside such “big data” to be able to provide more individualized treatment plans for each patient.
References
Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130(4):469–76.
Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007;33(4):835–54.
Tuft SJ, Watson PG. Progression of scleral disease. Ophthalmology. 1991;98(4):467–71.
Braithwaite T, Adderley NJ, Subramanian A, Galloway J, Kempen JH, Gokhale K, et al. Epidemiology of scleritis in the United Kingdom from 1997 to 2018: population-based analysis of 11 million patients and association between scleritis and infectious and immune-mediated inflammatory disease. Arthritis Rheumatol. 2021;73(7):1267–76.
Thong LP, Rogers SL, Hart CT, Hall AJ, Lim LL. Epidemiology of episcleritis and scleritis in urban Australia. Clin Exp Ophthalmol. 2020;48(6):757–66.
Homayounfar G, Nardone N, Borkar DS, Tham VM, Porco TC, Enanoria WT, et al. Incidence of scleritis and episcleritis: results from the Pacific Ocular Inflammation Study. Am J Ophthalmol. 2013;156(4):752–8.
Honik G, Wong IG, Gritz DC. Incidence and prevalence of episcleritis and scleritis in Northern California. Cornea. 2013;32(12):1562–6.
Armbrust KR, Kopplin LJ. Characteristics and outcomes of patients with scleritis in the IRIS® Registry (Intelligent Research in Sight) Database. Ophthalmol Sci. 2022;2(3): 100178.
de-la-Torre A, Cabrera-Pérez M, Durán C, García S, Cuevas M, Carreño N, et al. Clinical patterns and risk factors in scleritis: a multicentric study in Colombia. Graefes Arch Clin Exp Ophthalmol. 2022;260(12):3957–67.
Xu TT, Reynolds MM, Hodge DO, Smith WM. Epidemiology and clinical characteristics of episcleritis and scleritis in Olmsted County, Minnesota. Am J Ophthalmol. 2020;217:317–24.
Magesan K, Surya J, Sridharan S, Nair V, Agarwal M, Agarwal AE, et al. Clinical profile of scleritis presenting for the first time in the elderly. Ocul Immunol Inflamm. 2023;31(4):696–700.
Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111(3):501–6.
Lavric A, Gonzalez-Lopez JJ, Majumder PD, Bansal N, Biswas J, Pavesio C, et al. Posterior scleritis: analysis of epidemiology, clinical factors, and risk of recurrence in a cohort of 114 patients. Ocul Immunol Inflamm. 2016;24(1):6–15.
Murthy SI, Sabhapandit S, Balamurugan S, Subramaniam P, Sainz-de-la-Maza M, Agarwal M, et al. Scleritis: differentiating infectious from non-infectious entities. Indian J Ophthalmol. 2020;68(9):1818–28.
Zhang Y, Amin S, Lung KI, Seabury S, Rao N, Toy BC. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: a claims-based analysis. PLoS ONE. 2020;15(8): e0237995.
Fraunfelder FW, Fraunfelder FT. Adverse ocular drug reactions recently identified by the National Registry of drug-induced ocular side effects. Ophthalmology. 2004;111(7):1275–9.
Hemmati I, Wade J, Kelsall J. Risedronate-associated scleritis: a case report and review of the literature. Clin Rheumatol. 2012;31(9):1403–5.
Provost C, Sené T, Lecler A. Bisphosphonate-induced posterior scleritis. Ophthalmology. 2021;128(3):371.
Tabbara KF. Nodular scleritis following alendronate therapy. Ocul Immunol Inflamm. 2008;16(3):99–101.
Chartrand NA, Lau CK, Parsons MT, Handlon JJ, Ronquillo YC, Hoopes PC, et al. Ocular Side effects of bisphosphonates: a review of literature. J Ocul Pharmacol Ther. 2023;39(1):3–16.
Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol. 2003;135(2):219–22.
Hashimoto Y, Yamana H, Iwagami M, Ono S, Takeuchi Y, Michihata N, et al. Ocular adverse events after coronavirus disease 2019 mRNA vaccination: matched cohort and self-controlled case series studies using a large database. Ophthalmology. 2023;130(3):256–64.
Sanjay S, Handa A, Kawali A, Shetty R, Bhakti Mishra S, Mahendradas P. Scleritis and episcleritis following coronavirus disease (COVID-19) vaccination. Ocul Immunol Inflamm. 2023;31(6):1184–90.
Akbari MR, Mohebbi M, Johari M, Mirmohammadsadeghi A, Mahmoudi A. Multifocal surgically induced necrotizing scleritis following strabismus surgery: a case report. Strabismus. 2016;24(3):101–5.
Pujari A, Chaniyara MH, Sharma P, Sharma N. Necrotizing scleritis following uncomplicated strabismus surgery. Indian J Ophthalmol. 2020;68(11):2555–7.
Hong CM, Shin MH, Kim SJ, Seo SW, Chung I, Yoo WS. Bilateral posterior scleritis after sequential bilateral cataract surgery: a case report. BMC Ophthalmol. 2022;22(1):321.
Matsuura K, Uotani R, Inoue Y. Bilateral diffuse anterior scleritis after unilateral cataract surgery: a case report. J Pak Med Assoc. 2021;71(3):1014–6.
Aktas Z, Bektas C, Hasanreisoglu M. Panscleritis as an unusual complication of gonioscopy-assisted transluminal trabeculotomy. J Glaucoma. 2019;28(2):e21–3.
Wong JC, Savsani E, Mahmoudzadeh R, Salabati M, Razeghinejad R, Lee D, et al. Glaucoma surgical outcomes in patients with a history of scleritis. Ocul Immunol Inflamm. 2023;21:1–7.
Roufas A, Jalaludin B, Gaskin C, McCluskey P. Subconjunctival triamcinolone treatment for non-necrotising anterior scleritis. Br J Ophthalmol. 2010;94(6):743–7.
Fraunfelder FT, Watson PG. Evaluation of eyes enucleated for scleritis. Br J Ophthalmol. 1976;60(3):227–30.
Tu EY, Culbertson WW, Pflugfelder SC, Huang A, Chodosh JC. Therapy of nonnecrotizing anterior scleritis with subconjunctival corticosteroid injection. Ophthalmology. 1995;102(5):718–24.
Albini TA, Zamir E, Read RW, Smith RE, See RF, Rao NA. Evaluation of subconjunctival triamcinolone for nonnecrotizing anterior scleritis. Ophthalmology. 2005;112(10):1814–20.
Zamir E, Read RW, Smith RE, Wang RC, Rao NA. A prospective evaluation of subconjunctival injection of triamcinolone acetonide for resistant anterior scleritis. Ophthalmology. 2002;109(4):798–805 (discussion 807).
Foster CS, Kothari S, Anesi SD, Vitale AT, Chu D, Metzinger JL, et al. The ocular immunology and uveitis foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61(1):1–17.
Davis A, Robson R. The dangers of NSAIDs: look both ways. Br J Gen Pract. 2016;66(645):172–3.
Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl. 1):i1–57.
Cameron C. Non-steroidal anti-inflammatory drugs: making better treatment choices. Best Pract J. 2013;55:9–18.
Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, Doctor PP, Tauber J, Foster CS. Scleritis therapy. Ophthalmology. 2012;119(1):51–8.
Bauer AM, Fiehn C, Becker MD. Celecoxib, a selective inhibitor of cyclooxygenase 2 for therapy of diffuse anterior scleritis. Am J Ophthalmol. 2005;139(6):1086–9.
Agrawal R, Lee CS, Gonzalez-Lopez JJ, Khan S, Rodrigues V, Pavesio C. Flurbiprofen: a nonselective cyclooxygenase (COX) inhibitor for treatment of noninfectious, non-necrotizing anterior scleritis. Ocul Immunol Inflamm. 2016;24(1):35–42.
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–50.
Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–91.
American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052–81.
Stillman MJ, Stillman MT. Choosing nonselective NSAIDs and selective COX-2 inhibitors in the elderly: a clinical use pathway. Geriatrics. 2007;62(2):26–34.
Sabzwari SR, Qidwai W, Bhanji S. Polypharmacy in elderly: a cautious trail to tread. J Pak Med Assoc. 2013;63(5):624–7.
Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160(6):777–84.
Abdel-Aty A, Gupta A, Del Priore L, Kombo N. Management of noninfectious scleritis. Ther Adv Ophthalmol. 2022;14:25158414211070880.
Kaiser FE, Doe RP. Steroid use in the elderly: guidelines for avoiding adverse effects. Postgrad Med. 1984;76(1):65–8, 72–4.
Kamali S, Erer B, Artim-Esen B, Gul A, Ocal L, Konice M, et al. Predictors of damage and survival in patients with Wegener’s granulomatosis: analysis of 50 patients. J Rheumatol. 2010;37(2):374–8.
Kermani TA, Warrington KJ, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et al. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol. 2015;42(7):1213–7.
Koldingsnes W, Nossent H. Predictors of survival and organ damage in Wegener’s granulomatosis. Rheumatology (Oxford). 2002;41(5):572–81.
Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum. 2005;52(7):2168–78.
Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49(5):703–8.
Chandran A, Udayakumar PD, Kermani TA, Warrington KJ, Crowson CS, Matteson EL. Glucocorticoid usage in giant cell arteritis over six decades (1950 to 2009). Clin Exp Rheumatol. 2015;33(2 Suppl. 89):S98–102.
Gabriel SE, Sunku J, Salvarani C, O’Fallon WM, Hunder GG. Adverse outcomes of antiinflammatory therapy among patients with polymyalgia rheumatica. Arthritis Rheum. 1997;40(10):1873–8.
Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10(10): CD001347.
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–37.
McCluskey P, Wakefield D. Intravenous pulse methylprednisolone in scleritis. Arch Ophthalmol. 1987;105(6):793–7.
Vasheghani-Farahani A, Sahraian MA, Darabi L, Aghsaie A, Minagar A. Incidence of various cardiac arrhythmias and conduction disturbances due to high dose intravenous methylprednisolone in patients with multiple sclerosis. J Neurol Sci. 2011;309(1–2):75–8.
van der Hooft CS, Heeringa J, Brusselle GG, Hofman A, Witteman JC, Kingma JH, et al. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166(9):1016–20.
Walasik-Szemplińska D, Kamiński G, Sudoł-Szopińska I. Life-threatening complications of high doses of intravenous methylprednisolone for treatment of Graves’ orbitopathy. Thyroid Res. 2019;12:13.
Marinó M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid. 2004;14(5):403–6.
Powell C, Chang C, Naguwa SM, Cheema G, Gershwin ME. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9(11):721–43.
Kalla AA, Learmonth ID, Klemp P. Early treatment of avascular necrosis in systemic lupus erythematosus. Ann Rheum Dis. 1986;45(8):649–52.
D’Aubigné RM, Frain PG. [Theory of osteotomies]. Rev Chir Orthop Reparatrice Appar Mot. 1972;58(3):159–67.
Serong S, Haversath M, Tassemeier T, Dittrich F, Landgraeber S. Results of advanced core decompression in patients with osteonecrosis of the femoral head depending on age and sex-a prospective cohort study. J Orthop Surg Res. 2020;15(1):124.
Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549–60.
Ikeda N, Yamada S, Yamamoto M, Tanaka K, Fujii T, Tsuji T, et al. Two cases of steroid dementia showing partial recovery during 2-year follow-up. Psychiatry Clin Neurosci Rep. 2022;1: e49.
Schreiber MP, Colantuoni E, Bienvenu OJ, Neufeld KJ, Chen KF, Shanholtz C, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480–6.
Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood. 2010;116(13):2215–23.
Pujari SS, Kempen JH, Newcomb CW, Gangaputra S, Daniel E, Suhler EB, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117(2):356–65.
de Fidelix TS, Vieira LA, de Freitas D, Trevisani VF. Biologic therapy for refractory scleritis: a new treatment perspective. Int Ophthalmol. 2015;35(6):903–12.
Ivulich S, Snell G. Long-term management of elderly patients taking immunosuppressive medications. Aust J Gen Pract. 2020;49(3):100–6.
Jacobson PA, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12(12):3326–36.
Drosos A. Methotrexate intolerance in elderly patients with rheumatoid arthritis: what are the alternatives? Drugs Aging. 2003;20(10):723–36.
Bologna C, Viu P, Jorgensen C, Sany J. Effect of age on the efficacy and tolerance of methotrexate in rheumatoid arthritis. Br J Rheumatol. 1996;35(5):453–7.
Romano P, Agena F, De Almeida Rezende Ebner P, MassakazuSumita N, KamadaTriboni AH, Ramos F, et al. Longitudinal pharmacokinetics of mycophenolic acid in elderly renal transplant recipients compared to a younger control group: data from the nEverOld Trial. Eur J Drug Metab Pharmacokinet. 2019;44(2):189–99.
Gardner G, Furst DE. Disease-modifying antirheumatic drugs. Potential effects in older patients. Drugs Aging. 1995;7(6):420–37.
Cho SK, Sung YK, Kim D, Won S, Choi CB, Kim TH, et al. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2016;17:333.
Murota A, Kaneko Y, Yamaoka K, Takeuchi T. Safety of biologic agents in elderly patients with rheumatoid arthritis. J Rheumatol. 2016;43(11):1984–8.
Busquets N, Tomero E, Descalzo M, Ponce A, Ortiz-Santamaría V, Surís X, et al. Age at treatment predicts reason for discontinuation of TNF antagonists: data from the BIOBADASER 2.0 registry. Rheumatology (Oxford). 2011;50(11):1999–2004.
Genevay S, Finckh A, Ciurea A, Chamot AM, Kyburz D, Gabay C. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;57(4):679–85.
Radovits BJ, Kievit W, Fransen J, van de Laar MA, Jansen TL, van Riel PL, et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(9):1470–3.
Kim HW, Park JK, Yang JA, Yoon YI, Lee EY, Song YW, et al. Comparison of tuberculosis incidence in ankylosing spondylitis and rheumatoid arthritis during tumor necrosis factor inhibitor treatment in an intermediate burden area. Clin Rheumatol. 2014;33(9):1307–12.
Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford). 2005;44(6):714–20.
McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48(10):1364–71.
Winthrop KL, Baddley JW, Chen L, Liu L, Grijalva CG, Delzell E, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309(9):887–95.
Crowson CS, Hoganson DD, Fitz-Gibbon PD, Matteson EL. Development and validation of a risk score for serious infection in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(9):2847–55.
Ibrahim A, Ahmed M, Conway R, Carey JJ. Risk of infection with methotrexate therapy in inflammatory diseases: a systematic review and meta-analysis. J Clin Med. 2018;8(1):15.
Sandhu VK, Ighani A, Fleming P, Lynde CW. Biologic treatment in elderly patients with psoriasis: a systematic review. J Cutan Med Surg. 2020;24(2):174–86.
Candel FJ, Grima E, Matesanz M, Cervera C, Soto G, Almela M, et al. Bacteremia and septic shock after solid-organ transplantation. Transplant Proc. 2005;37(9):4097–9.
Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48(5):595–600.
Payet S, Soubrier M, Perrodeau E, Bardin T, Cantagrel A, Combe B, et al. Efficacy and safety of rituximab in elderly patients with rheumatoid arthritis enrolled in a French Society of Rheumatology registry. Arthritis Care Res (Hoboken). 2014;66(9):1289–95.
Mielnik P, Sexton J, Lie E, Bakland G, Loli LP, Kristianslund EK, et al. Does older age have an impact on rituximab efficacy and safety? Results from the NOR-DMARD Register. Drugs Aging. 2020;37(8):617–26.
Nair N, Gongora E, Mehra MR. Long-term immunosuppression and malignancy in thoracic transplantation: where is the balance? J Heart Lung Transplant. 2014;33(5):461–7.
Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation. 1999;68(12):1859–64.
Amari W, Zeringue AL, McDonald JR, Caplan L, Eisen SA, Ranganathan P. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford). 2011;50(8):1431–9.
Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1101–36.
Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–95.
Kim SC, Glynn RJ, Giovannucci E, Hernández-Díaz S, Liu J, Feldman S, et al. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis. 2015;74(7):1360–7.
Askling J, van Vollenhoven RF, Granath F, Raaschou P, Fored CM, Baecklund E, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum. 2009;60(11):3180–9.
Dreyer L, Mellemkjær L, Andersen AR, Bennett P, Poulsen UE, JuulsgaardEllingsen T, et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides: a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79–82.
Haynes K, Beukelman T, Curtis JR, Newcomb C, Herrinton LJ, Graham DJ, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65(1):48–58.
Setoguchi S, Solomon DH, Weinblatt ME, Katz JN, Avorn J, Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2757–64.
McMullen M, Kovarik G, Hodge WG. Use of topical steroid therapy in the management of nonnecrotizing anterior scleritis. Can J Ophthalmol. 1999;34(4):217–21.
Tappeiner C, Walscheid K, Heiligenhaus A. Diagnosis and treatment of episcleritis and scleritis. Ophthalmologe. 2016;113(9):797–810.
Croasdale CR, Brightbill FS. Subconjunctival corticosteroid injections for nonnecrotizing anterior scleritis. Arch Ophthalmol. 1999;117(7):966–8.
Sen HN, Ursea R, Nussenblatt RB, Buggage RR. Subconjunctival corticosteroid injection for the treatment of non-necrotising anterior scleritis. Br J Ophthalmol. 2005;89(7):917–8.
Shaw SJ, Giegengack M, Kurup S. Sub-tenon triamcinolone injection for non-ecrotizing anterior scleritis: reducing treatment burden of systemic medication. Investig Ophthalmol Vis Sci. 2012;53(14):5505.
Sohn EH, Wang R, Read R, Roufas A, Teo L, Moorthy R, et al. Long-term, multicenter evaluation of subconjunctival injection of triamcinolone for non-necrotizing, noninfectious anterior scleritis. Ophthalmology. 2011;118(10):1932–7.
Vergouwen D, Berge JT, Rothova A. Recurrences of scleritis after ocular surgery. Ocular Immunol Inflamm. 2022;30(4):1029–30.
Palkar A, Sudharshan S, George AE, Ganesh SK, Biswas J, Dutta MP. Cataract surgery in the setting of scleritis. Ocul Immunol Inflamm. 2021;29(7–8):1540–3.
Loureiro M, Rothwell R, Fonseca S. Nodular scleritis associated with herpes zoster virus: an infectious and immune-mediated process. Case Rep Ophthalmol Med. 2016;2016:8519394.
Huguenel C, Felton D, Bruccoleri R, Salhanick S. Case files of the Harvard medical toxicology fellowship: valacyclovir neurotoxicity and unintentional overdose. J Med Toxicol. 2015;11(1):132–6.
Prasad B, McIsaac M, Toppings J. Valacyclovir-associated neurotoxicity treated with intensification of peritoneal dialysis. BMJ Case Rep. 2017;2017: bcr2017220678.
Kenzaka T, Sugimoto K, Goda K, Akita H. Acute kidney injury and acyclovir-associated encephalopathy after administration of valacyclovir in an elderly person with normal renal function: a case report and literature review. Medicine (Baltimore). 2021;100(21): e26147.
Kamath YS, Rathinam SR, Kawali A. Ocular toxoplasmosis associated with scleritis. Indian J Ophthalmol. 2013;61(6):295–7.
Fraser TN, Avellaneda AA, Graviss EA, Musher DM. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother. 2012;67(5):1271–7.
Rothova A, Meenken C, Buitenhuis HJ, Brinkman CJ, Baarsma GS, Boen-Tan TN, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115(4):517–23.
Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 2002;134(1):102–14.
Lhaj HA, Benjelloun A, Bouia Y, Bennouk Y, Mouzari Y, Kamouni YE, et al. Latent tuberculosis-related scleritis: a case report. BMC Res Notes. 2016;9(1):446.
Nanda M, Pflugfelder SC, Holland S. Mycobacterium tuberculosis scleritis. Am J Ophthalmol. 1989;108(6):736–7.
Rajagopalan S. Tuberculosis in older adults. Clin Geriatr Med. 2016;32(3):479–91.
Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging. 2018;13:657–67.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
Laura Butler, Oren Tomkins-Netzer, Or Reiser, and Rachael L. Niederer have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Author Contributions
LB, OR, and OTN wrote the manuscript, RL provided images for the figures, and all authors reviewed the manuscript.
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Butler, L., Tomkins-Netzer, O., Reiser, O. et al. Management of Scleritis in Older Adults. Drugs Aging 41, 287–302 (2024). https://doi.org/10.1007/s40266-024-01105-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-024-01105-0