Abstract
Background and Objective
The dual orexin receptor antagonist daridorexant, studied in two phase III trials, dose-dependently improved objective and subjective sleep variables and daytime functioning in adults with insomnia. Because treatment of insomnia in older adults is challenging and has limited options, the purpose of the current analysis was to further analyse the phase III trial studying the higher doses of daridorexant, those that showed efficacy (daridorexant 50 mg, daridorexant 25 mg and placebo, nightly for 3 months), and compare the safety and efficacy of daridorexant in patients aged ≥ 65 (‘older adults’) to those aged < 65 years (‘younger adults’).
Methods
Analyses by age (≥ 65 years, n = 364; < 65 years, n = 566) were performed on data from the randomised, double-blind, placebo-controlled Trial 1 in adult patients with insomnia (NCT03545191). Efficacy endpoints included a change from baseline at month 1 and month 3 in polysomnography-measured wake after sleep onset (WASO) and latency to persistent sleep (LPS), self-reported total sleep time (sTST) and daytime functioning assessed using the validated Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ). Safety endpoints included adverse events and the Visual Analog Scale for morning sleepiness.
Results
At baseline, mean [standard deviation] WASO was numerically greater (110 [39] vs 92 [38] min) in older than younger adults, while LPS was comparable (~ 65 min). Mean baseline IDSIQ total and all domain scores were numerically lower (i.e. better) in older adults. Daridorexant caused similar reductions in WASO and LPS, and similar increases in sTST, from baseline, in both age groups; improvements were numerically greater with daridorexant 50 mg than 25 mg. At month 3, daridorexant 50 mg, compared with placebo, decreased WASO by a least-squares mean of 19.6 (95% confidence interval 9.7, 29.5) in older patients versus 17.4 min (10.7, 24.0) in younger patients and decreased LPS by a least-squares mean of 14.9 (7.5, 22.3) in older patients versus 9.7 min (3.7, 15.7) in younger patients. Daridorexant 50 mg increased sTST from baseline to month 3 by a least-squares mean of 59.9 (49.6, 70.3) in older patients versus 57.1 min (48.9, 65.3) in younger patients. Daridorexant 50 mg progressively improved IDSIQ total and domain scores from week 1 onwards similarly in both groups; daridorexant 25 mg improved IDSIQ scores, but only in younger adults. In both age groups, in comparison with placebo, the overall incidence of adverse events was comparable, and there were fewer falls on daridorexant. Daridorexant improved Visual Analog Scale morning sleepiness in both groups; daridorexant 50 mg increased the mean (standard deviation) Visual Analog Scale morning sleepiness score by 15.9 (20.7) in older adults and by 14.9 (18.7) in younger adults from baseline to month 3. In older adults, there was one case of sleep paralysis, and no cases of narcolepsy, cataplexy, or complex sleep behaviour.
Conclusions
In older patients with insomnia, as in younger patients, the efficacy of daridorexant is maximal on night-time and daytime variables at the higher dose of 50 mg. Older patients particularly require this dose to improve daytime functioning. Older patients are not at an increased risk of adverse events or residual effects the next morning after night-time administration of daridorexant, even at 50 mg. The dose of daridorexant does not need to be decreased for older patients.
Clinical Trial Registration
ClinicalTrials.gov (NCT03545191) [first posted: 4 June, 4 2018], https://clinicaltrials.gov/ct2/show/NCT03545191.
Plain Language Summary
The burden of chronic insomnia (difficulty in falling/staying asleep or not getting enough sleep) increases with age yet treatment options in older patients are limited. In older patients, because of a risk of side effects, guidelines suggest caution when prescribing sleep medications and, for some drugs, recommend starting at a lower dose. Daridorexant was approved in 2022 for the treatment of insomnia in adults following positive results in two trials that showed daridorexant significantly improved night-time sleep and daytime functioning over 3 months of treatment in adults with insomnia. Approximately 40% of patients taking part in these trials were aged 65 years or older. This current analysis compared the safety and benefits of daridorexant in older adults (aged at least 65 years) and younger adults (aged less than 65 years) in the trial that administered the highest two doses of daridorexant, 25 and 50 mg. The results showed that the benefits of daridorexant were comparable in both age groups over 3 months; compared with placebo, daridorexant improved night-time sleep (reduced time awake during the night, reduced time to fall asleep and increased total sleep time) and daytime functioning—patients had less daytime sleepiness and a better mood and feeling of alertness. In older patients, the benefits, particularly for daytime functioning, were greatest at the higher 50-mg dose, without any increase in side effects. Both doses of daridorexant were equally well tolerated in the two age groups, indicating that treatment with daridorexant at 50 mg can be safely started in older patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In older adults with chronic insomnia, daridorexant improves sleep onset, sleep maintenance, objective and subjective total sleep time, and daytime functioning in the domains of sleepiness, alert/cognition and mood. |
In older adults, the highest approved dose of 50 mg is needed to improve both night-time sleep and daytime functioning. |
Daridorexant at 50 mg given nightly for 3 months is at least as safe and efficacious in older as in younger adults. This effect is without any corresponding increase in adverse events and, in particular, no increased risk of residual effects the morning following night-time administration of daridorexant. |
1 Introduction
The world’s older population continues to grow; globally, more than 700 million people are currently aged 65 years and over, and by 2050, this is projected to double to 1.5 billion [1]. Insomnia disorder is one of the most common sleep disturbances in this older population and is characterised by a long-standing difficulty in initiating and/or maintaining sleep with a substantial impairment of daytime functioning [2, 3]. The prevalence of insomnia symptoms increases with advancing age, reaching up to ~ 50% in adults aged over 65 years [4,5,6]. The impact of insomnia in older adults is substantial, associated with daytime distress, reduced cognitive and physical function, and an increased risk of falls and even mortality, compared with counterparts without insomnia [7,8,9,10].
Treating insomnia in older adults should be a priority, yet it often presents a challenge to physicians because of a lack of evidence-based clinical guidelines as well as the multiple interacting comorbidities and accompanying polypharmacy that limit therapeutic options for this patient population [11,12,13,14]. While cognitive behavioural therapy for insomnia is recommended as a first-line therapy [15,16,17], it may not be available or sufficient for all [18] and its efficacy in older adults has not been adequately demonstrated [19]. Despite a number of approved pharmacotherapies targeting several pathways [20], there is a lack of agents that address all aspects of the disorder; many of these medications, for example, do not address sleep maintenance, an aspect that is particularly problematic in older patients [14, 21], nor daytime functioning impairment [22]. In addition, older adults may be more vulnerable than younger adults to the adverse events associated with commonly prescribed sedative hypnotics [23], such as residual morning somnolence, daytime cognitive and psychomotor impairment, falls and fractures [24,25,26,27]. Consequently, some guidelines recommend caution and advise use of the lowest effective dose when prescribing some types of sleep medications to older adults [16, 28, 29].
Dual orexin receptor antagonists have evolved as a new pharmaceutical class for the treatment of insomnia. They inhibit a major wake-promoting pathway responsible for the overactive wake signalling (hyperarousal) characteristic of insomnia by blocking the binding of the orexin A and B neuropeptides to the two orexin receptors [30]. Daridorexant is a new potent, selective, dual orexin receptor antagonist approved for the treatment of insomnia in adults aged ≥ 18 years [31]. It has a favourable pharmacokinetic/pharmacodynamic profile with a rapid absorption enabling a fast sleep onset, and a rapid elimination, enabling sleep maintenance without next-morning sleepiness and no accumulation upon repeated nightly dosing [32,33,34].
The daridorexant phase III development programme in adult patients (aged ≥ 18 years) with insomnia disorder included two 12-week studies—one of which evaluated both approved doses of daridorexant, 50 and 25 mg, together with placebo (ClinicalTrials.gov identifier NCT03545191), while the other evaluated lower doses of daridorexant (ClinicalTrials.gov identifier NCT03575104)—plus a 40-week long-term extension study. In the 12-week study of daridorexant 50 mg and 25 mg (Trial 1), daridorexant improved sleep onset, sleep maintenance and the patient’ subjective assessment of sleep quantity, and, at the highest dose of 50 mg, also improved various aspects of daytime functioning (sleepiness, mood, alert/cognition) [35], measured by a newly developed and validated patient-reported outcome instrument, the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) [36]. The safety profile of the higher 50-mg dose of daridorexant was comparable to the 25-mg dose, with no excess of next-morning sleepiness, somnolence, falls, physical dependence or withdrawal at drug cessation. In this study, approximately 40% of randomised patients were aged ≥ 65 years and a pre-specified subgroup analysis indicated that both efficacy and safety findings of daridorexant were consistent in both the older subgroup (aged ≥ 65 years) and the younger subgroup (aged < 65 years) [35], supporting earlier phase II results [37]. This article reports the results of a secondary analysis of this phase III trial, exploring further the similarities or differences in the efficacy and safety of daridorexant 50 and 25 mg between older and younger adults with insomnia disorder.
2 Methods
2.1 Study Design
The study design has been previously described in detail [35]. Briefly, this randomised, phase III, double-blind, placebo-controlled, parallel-group clinical trial (ClinicalTrials.gov identifier NCT03545191) included a screening period (7–18 days) followed by a single-blind placebo run-in period (13–24 days), after which patients were randomised (1:1:1) to receive, in a double-blinded manner, oral daridorexant 50 mg, daridorexant 25 mg or placebo every evening for 12 weeks. Study treatment dose adjustments were not permitted. The treatment period was followed by a 7-day, single-blind, placebo run-out period and then either a 23-day safety follow-up or enrolment into a 40-week placebo-controlled extension study (NCT03679884; results reported elsewhere [38]). The study was conducted in ten countries (Australia, Canada, Denmark, Germany, Italy, Poland, Serbia, Spain, Switzerland and the USA) at 75 sites between May 2018 and May 2020, in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guideline for Good Clinical Practice and local regulations. The protocol was approved by institutional review boards or independent ethics committees and all patients provided written informed consent. Safety and efficacy were monitored by an independent data monitoring committee and an independent safety monitoring board adjudicated blinded adverse events.
2.2 Study Participants
The key eligibility criteria were age 18 years or older, a diagnosis of insomnia disorder (according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) [2], an Insomnia Severity Index (ISI) score ≥ 15 and a self-reported history of disturbed sleep (≥ 30 min to fall asleep, ≥ 30 min awake during sleep time, self-reported total sleep time [sTST] of ≤ 6.5 h) for more than 3 nights per week for at least 3 months prior to screening. During the placebo run-in period, the following polysomnography (PSG) criteria were required to be met: latency to persistent sleep (LPS) ≥ 20 min; wake after sleep onset (WASO) ≥ 30 min; and mean total sleep time (TST) < 7 h recorded during two consecutive nights. Patients with a history of sleep-related breathing disorders, any sleep disorder other than insomnia, or suicide ideation/attempt, self-reported daytime napping (≥ 1 h/day ≥ 3 days/week), acute/unstable psychiatric conditions, or alcohol or drug abuse were excluded. Patients with a periodic limb movements arousal index ≥ 15/h, an apnea hypopnea index ≥ 15/h or restless legs syndrome during the PSG visit in the screening period were also excluded [35].
2.3 Efficacy Endpoints
This analysis was based on the two primary objective endpoints, change in WASO and LPS at month (M) 1 and M3 measured by PSG, and the two key secondary subjective endpoints, change in sTST at M1 and M3 as recorded in a sleep diary, and a weekly change in self-reported daytime functioning, assessed using the IDSIQ. The IDSIQ is a new validated instrument developed in accordance with US Food and Drug Administration guidance for determining patient-reported outcomes [36]. The IDSIQ contains 14 different questions assessing daytime functioning in subjects with insomnia disorder with a recall period of today; the questions are grouped into three domains each representing the main daytime symptoms and impacts of insomnia on sleepiness (four questions), alert/cognition (six questions) and mood (four questions) [Fig. S1 of the Electronic Supplementary Material (ESM)]. Each question is scored on an 11-point numerical scale (from 0 to 10) with lower scores denoting better daytime functioning. The IDSIQ was completed every evening using an electronic hand-held device and the scores were the weekly means. In Trial 1, the change from baseline to M1 and M3 sleepiness domains of the IDSIQ was a key secondary endpoint [35]; in this secondary analysis, weekly changes in all components of the IDSIQ were examined for differences between older and younger adults. Weekly changes from baseline in the morning Visual Analog Scale (VAS) for depth and quality of sleep, in the evening VAS for ability to function and daytime alertness, and in ISI scores at M1 and M3 were also evaluated.
2.4 Safety Endpoints
Safety endpoints included treatment-emergent adverse events (TEAEs), discontinuation of double-blind treatment because of TEAEs, adjudicated adverse events of special interest (AESIs) and next-morning residual effects (change from baseline in VAS-assessed morning sleepiness). Adjudicated adverse events of special interest were defined as narcolepsy-like symptoms related to excessive daytime sleepiness, complex sleep behaviour events including hallucinations, sleep paralysis and cataplexy, and suicide/self-injury.
2.5 Statistical Analysis
In Trial 1, randomisation was stratified by age group (< 65 years; ≥ 65 years) ensuring a similar proportion of patients per age group in all treatment groups. In this secondary analysis, the efficacy and safety of daridorexant 50 mg and 25 mg were evaluated in patients aged ≥ 65 years (termed ‘older adults’) and in adult patients aged < 65 years (termed ‘younger adults’). Efficacy endpoints were analysed in the full analysis set defined as all randomised participants. Safety endpoints were analysed in the safety set defined as all participants who received at least one dose of the study drug.
Descriptive statistics are presented for all endpoints. The change from baseline in WASO, LPS, sTST and IDSIQ scores in the older and younger adult subgroups was analysed using a linear mixed-effects model for repeated measures. The model was adjusted for the baseline value of each endpoint respectively and included factors for treatment (daridorexant 50 mg and daridorexant 25 mg; placebo), visit (M1; M3), interaction of treatment by visit and baseline by visit. Missing values were assumed missing-at-random. The WASO and LPS values are the mean of polysomnography recordings obtained over two consecutive nights during the 3-month double-blind treatment period. Data for sTST are based on the mean of daily entries in the 7 days before polysomnography nights. Baseline sleep parameters are presented as mean (standard deviation [SD]). Night-time efficacy results are reported as least-squares mean (LSM) with standard error of the mean and 95% CI for a change from baseline and for a difference to placebo at M1 and M3. Daytime efficacy data are presented as the mean change from baseline (VAS, IDSIQ) and 95% CI (IDSIQ). Insomnia Severity Index score data are presented as mean (SD). Data for ISI score ≥ 22 and patients with a ≥ 6-point decrease in ISI score from baseline are presented as n (%). Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC. USA).
All results presented are intentionally descriptive and avoid p values, as these secondary analyses were not type I error rate controlled or specifically powered. For the same reasons, tests for interactions between the treatment groups and age were not conducted.
3 Results
3.1 Study Population
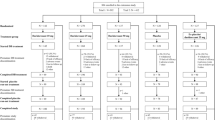
Of the 930 patients who were randomised, 364 (39%) were older adults aged ≥ 65 years and 566 (61%) were younger adults aged < 65 years (Fig. S2 of the ESM). Completion of double-blind treatment was similar across the older and younger age groups; 93% (339/364) and 91% (514/566), respectively.
In both age groups, the majority of patients were female (66% for older adults, 68% for younger adults) and Caucasian (95% for older adults, 87% for younger adults) [Table 1]. The mean (SD) age was 70 years (4) in the older group and 46 years (12) in the younger group. As expected, comorbidities were more prevalent in the older group (81% had at least one comorbidity, vs 62% in the younger group). Hypertension was the most common comorbidity, affecting 40% of older adults (vs 11% in younger adults). The older adult group was also more likely to be receiving concomitant medications (71 vs 48%), corresponding to the increased prevalence of reported comorbidities. Most common medications were statins, antihypertensives and antiplatelet agents (Table 1).
At baseline, mean (SD) WASO was numerically greater (worse) (110 [38.5] vs 92 [38.1] min) in older adults compared with younger adults and LPS and sTST were similar in both age groups. Conversely, ISI scores were slightly higher in the younger group (mean [SD] 19.5 [4.2] and 18.5 [3.9] in younger and older adults, respectively). Insomnia Daytime Symptoms and Impacts Questionnaire total score and all domain scores (for sleepiness, mood, alert/cognition) were numerically lower [i.e. better] (mean [SD] 69.9 [24.8], 21.3 [7.0], 18.2 [8.5], 30.4 [10.4] in older adults and 76.2 [24.4], 22.9 [6.9], 20.1 [8.7], 33.1 [10.1] in younger adults for the IDSIQ total score and sleepiness domain, mood domain and alert/cognition domain scores, respectively) in older compared with younger adults. Visual Analog Scale scores (for sleep quality and depth, daytime alertness, ability to function and morning sleepiness) were numerically higher (i.e. better) in older adults compared with younger adults (mean [SD] 38.6 [17.4], 38.8 [17.8], 43.4 [20.0], 43.3 [19.5], and 41.4 [19.1] in older adults and 34.0 [17.4], 34.3 [17.4], 37.5 [19.8], 38.1 [19.3], and 35.1 [18.3] in younger adults for VAS scores of quality of sleep, depth of sleep, daytime alertness, ability to function and morning sleepiness, respectively). Time since insomnia diagnosis was longer in the older group (mean [SD] 13.3 [12.0] years vs 8.9 [8.8] years for the younger group) [Table 1]. Baseline demographic and insomnia characteristics were generally similar across treatment groups within each age group (Table 1 of the ESM).
3.2 Night-Time Efficacy Endpoints
Daridorexant caused similar reductions from baseline in WASO and LPS, and similar increases in sTST in both age groups at M1 and M3; improvements were numerically greater with daridorexant 50 mg than 25 mg in both groups (Fig. 1, Table 2 of the ESM). At M3, daridorexant 50 mg, compared with placebo, decreased WASO by 19.6 (95% CI 9.7, 29.5) min in the older group versus 17.4 min (95% CI 10.7, 24.0) in the younger group and decreased LPS by 14.9 min (95% CI 7.5, 22.3) in the older group vs 9.7 min (95% CI 3.7, 15.7) in the younger group (Table 2 of the ESM). The change in sTST with daridorexant 50 mg compared with placebo was numerically greater in the older group compared with the younger group; at M3, the placebo-corrected change in sTST was 30.6 min (95% CI 16.1, 45.2) in the older group compared with 12.4 min (95% CI 0.7, 24.0) in the younger group. However, the change from baseline in sTST was similar in each age group (Table 2 of the ESM). The placebo response, in particular for WASO and sTST, was noticeably smaller in the older group than in the younger group.
Effect of daridorexant on objective and subjective sleep parameters by age group. Mean change from baseline in a wake after sleep onset (WASO), b latency to persistent sleep (LPS) and c self-reported total sleep time (sTST) in younger adults aged < 65 years and older adults aged ≥ 65 years administered daridorexant 25 mg, daridorexant 50 mg and placebo. The WASO and LPS values are the mean of polysomnography recordings obtained over 2 consecutive nights during the 3-month double-blind treatment period. Data for sTST are based on the mean of daily entries in the 7 days before polysomnography nights. Error bars show standard errors. SEM standard error of the mean
3.3 Daytime Efficacy Endpoints
In patients treated with daridorexant 50 mg, the IDSIQ total score and the three domain scores (sleepiness, alert/cognition, mood) decreased notably (i.e. improved) from baseline from week 1 onwards, similarly in both age groups, and the difference to placebo increased over time (Fig. 2). At M3, daridorexant 50 mg decreased the IDSIQ total score by − 21.0 (95% CI − 24.7, − 17.3) and by − 18.3
Effect of daridorexant on Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) total scores and domain scores by age group. Weekly mean change from baseline in a IDSIQ total score, b IDSIQ sleepiness domain score, c IDSIQ mood domain score and d IDSIQ alert/cognition domain score in younger adults aged < 65 years and older adults aged ≥ 65 years administered daridorexant 25 mg, daridorexant 50 mg and placebo. Lower IDSIQ scores indicate better patient-perceived daytime functioning
(95% CI − 21.3, − 15.2) in the older and younger groups, respectively. For the domain scores, in older and younger groups respectively, daridorexant 50 mg decreased the IDSIQ sleepiness score by − 6.2 (95% CI − 7.3, − 5.1) and − 5.4 (95% CI − 6.3, − 4.5), decreased the mood domain score by − 5.7 (95% CI − 6.9, − 4.6) and − 5.2 (95% CI − 6.2, − 4.2), and decreased the alert/cognition score by − 9.0 (95% CI − 10.6, − 7.4) and − 7.7 (95% CI − 9.0, − 6.4). Daridorexant 25 mg numerically reduced, but to a lesser extent than 50 mg, the IDSIQ sleepiness domain, the other two domains and the total score only in the younger group. In the older adult group, the effect of daridorexant 25 mg was similar to placebo. The placebo response on daytime functioning endpoints was comparable in the two age groups and increased over time.
3.4 VAS
In both age groups, improvements in all mean VAS scores were numerically higher with daridorexant 50 mg versus placebo as compared with 25 mg (Fig. 3). At M3, daridorexant 50 mg increased VAS depth of sleep by 19.3 (SD 23.6) in older adults and 19.6 (SD 21.4) in younger adults, increased the quality of sleep by 19.7 (SD: 23.3) in older adults and 21.0 (SD 21.4) in younger adults, increased daytime alertness by 16.9 (SD 23.0) in older adults and 15.6 (SD 19.3) in younger adults, and increased ability to function by 17.7 (SD 23.5) in older adults and 17.2 (SD 21.0) in younger adults.
Effect of daridorexant on Visual Analog Scale (VAS) depth and quality of sleep, daytime alertness and ability to function over time by age group. Weekly mean change from baseline in VAS a depth of sleep, b quality of sleep, c daytime alertness and d ability to function in younger adults aged < 65 years and older adults aged ≥ 65 years administered daridorexant 25 mg, daridorexant 50 mg and placebo. Higher VAS scores indicate better scores
In younger adults, all four VAS scores also increased (improved) with daridorexant 25 mg compared with placebo but to a lower extent than 50 mg. A similar dose response increase was seen in older adults for VAS depth and quality of sleep; for VAS daytime alertness and ability to function, daridorexant 25 mg had little noticeable effect compared with placebo in older adults. The placebo response for all VAS scores (depth and quality of sleep, daytime alertness and ability to function) was smaller in the older group than in the younger group at all assessed timepoints.
3.5 ISI
In both age groups, daridorexant 50 mg decreased (i.e. improved) the mean ISI scores from the baseline values, as well as decreased the proportion of patients with an ISI score ≥ 22 (i.e. severe insomnia), with the effect increasing over time from M1 to M3 (Table 2). In older adults, daridorexant 50 mg decreased the mean ISI score from 18.8 (SD 3.9) at baseline to 11.4 (SD 6.1) at M3 and, in younger adults, decreased the mean ISI score from 19.6 (SD 4.1) at baseline to 12.3 (SD 6.4) at M3. Compared with placebo, daridorexant 25 mg did not appear effective in improving ISI scores in the younger group at either timepoint and was numerically less efficacious than the 50-mg dose in the older group at both timepoints.
After 3 months treatment, the proportion of patients with remaining severe insomnia (ISI score ≥ 22) was particularly low in the older population: 3.6 and 4.5% at the 50-mg and 25-mg dose, respectively, compared with 25.2 and 16.5% at baseline. Compared with placebo, daridorexant 50 mg also numerically increased the proportion of patients with a ≥ 6 point decrease in total ISI scores (representing a clinically meaningful improvement [39]) from baseline at M1 and M3, similarly in older and younger adults. At M3, 58.0% of older adults and 55.6% of younger adults in the 50-mg group had a decrease of ≥ 6 points in their ISI score from baseline.
3.6 Safety
The overall safety profile of daridorexant in the older adults was comparable to that in the younger adults, with most values of TEAE incidence (based on the percentage of patients in the safety set) being nominally lower in the older group as compared with that of the younger group for daridorexant 50 mg (Table 3). Consistent with the overall study population [35], the most frequently reported TEAEs during 12-week double-blind treatment in both age groups and in all treatment groups were nasopharyngitis and headache (Table 3).
Of note, in older patients, somnolence, dizziness, and falls, which are of particular interest in this population, were reported at a similar low frequency in the daridorexant 50 mg (n = 1, 1, 1, respectively) and placebo (n = 1, 1, 4, respectively) groups. There were no noticeable differences between older and younger patients in the frequency of any individual AEs. The incidence of serious adverse events was low in both age groups treated with daridorexant (< 2%). One death was reported in the study, a serious adverse event of cardiac arrest in a 78-year-old patient receiving daridorexant 25 mg; this patient had a medical history of multiple cerebrovascular and cardiovascular conditions, including stroke and hypertension, and the event was assessed by the investigator as not related to treatment.
Independent safety board-adjudicated AESIs were infrequent in both age groups (Table 3). In older adults, two AESIs of mild severity were reported: excessive daytime sleepiness in one patient, a 69-year-old man receiving daridorexant 25 mg, and sleep paralysis in one patient, a 76-year-old woman receiving daridorexant 50 mg. The patient who reported sleep paralysis did not report a history of such events. In younger adults, five AESIs were reported: excessive daytime sleepiness was reported in three patients, one in each of the two daridorexant treatment groups and one in the placebo group; sleep paralysis in one patient, a 26-year-old male patient receiving daridorexant 25 mg; and hallucinations in a 25-year-old female patient receiving daridorexant 25 mg. No AESIs related to suicidal ideation/self-injury or cataplexy were reported in either age group.
For VAS morning sleepiness, as seen with the other VAS parameters, the placebo response was smaller in the older group than in the younger group at all timepoints. In both age groups, daridorexant did not worsen VAS morning sleepiness; on the contrary, daridorexant numerically increased (i.e. improved) the VAS morning sleepiness score (Fig. 4), and as compared with placebo, the improvement in morning sleepiness was greater in the older than the younger group. In older adults, the benefit was more pronounced for daridorexant 50 mg than for the 25-mg dose, whereas in younger adults, the treatment effect was similar between daridorexant doses. At M3, daridorexant 50 mg increased the VAS morning sleepiness score by a mean of 15.9 (SD 20.7) in older adults and 14.9 (SD 18.7) in younger adults from baseline.
Effect of daridorexant on the Visual Analog Scale (VAS) morning sleepiness score over time by age group. Safety analysis set. Mean weekly change from baseline in VAS morning sleepiness score (mm) in younger adults aged < 65 years and older adults aged ≥ 65 years administered daridorexant 25 mg, daridorexant 50 mg and placebo. The VAS score ranges from 0 to 100; from 0 ‘very sleepy’ to 100 ‘not sleepy at all’. A higher score indicates less morning sleepiness
4 Discussion
The burden of chronic insomnia increases with age [40]. Insomnia in older adults is often multifactorial, sometimes associated with chronic medical or psychiatric conditions, medication use, and changes in lifestyle and the environment that commonly accompany ageing. Ageing, per se, is associated with overactivity of the orexin/hypocretin system [41], and this could be the mechanism underlying the impaired sleep consolidation and increased sleep fragmentation often seen in older adults. An increased intrinsic excitability of orexin neurons, together with a loss of potassium channels (KCNQ2/3), was observed in aged mice, despite an age-related reduction in the total number of orexin neurons [41]. Aged mice had more fragmented sleep and, upon optogenetic stimulation of orexin neurons, longer wake bouts as compared with younger mice. If the orexin system in humans is modified by ageing and associated with a sleep of lower quality or quantity, it is of interest to ask if the effect of the novel dual orexin receptor antagonist (DORA) daridorexant differs between older and younger adults with insomnia disorder. The secondary analysis of the 3-month phase III study of daridorexant 50 and 25 mg versus placebo in insomnia allowed the comparison of older to younger adults. At baseline, WASO was nominally longer and TST shorter in the older adults compared with younger adults, while LPS was similar between the two age groups. These observations are not unexpected, as sleep maintenance difficulties and early morning awakenings are more common in older patients with insomnia than are sleep-onset difficulties [42,43,44]. Nevertheless, the older patients perceived at baseline their sTST, sleep quality and depth of sleep as similar or better compared with the younger patients, as also their estimation of daytime functioning.
Despite the baseline characteristics that suggested more severe deficits in objective sleep parameters and less severe deficits in subjective assessments and daytime functioning for the older patients compared with the younger patients, the improvements from baseline in night-time and daytime variables by daridorexant were very comparable between the two age groups. However, because the placebo responses were smaller in the older patients, several variables, in particular sTST, had larger placebo-corrected responses to daridorexant in older patients compared with younger patients. There was, however, one exception, in that in older patients, the lower dose of 25 mg did not produce any greater effect than placebo on daytime functioning, whether measured by the IDSIQ (on any domains) or by the VAS scores of daytime alertness and ability to function. One could speculate that the need for the 50-mg dose to improve all aspects of insomnia in older patients may, at least partly, be linked to the increased excitability of the orexin system in this age group, as reported in aged mice [41]. The increased comorbidities and the lesser perception of the contribution of insomnia in their daytime functioning impairment observed in the older patients may also have further reduced the response to the lower dose of 25 mg on daytime variables.
Concerning safety, in neither age group did daridorexant cause residual sleepiness the next morning and, to the contrary, there was a numerical improvement in morning sleepiness. The higher dose of 50 mg was most effective on reducing morning sleepiness in older patients, suggesting a direct result of a better quantity and quality of sleep, without the penalty of an excessive duration of action. Pharmacokinetic analysis of daridorexant was studied in older healthy adults [45]. In line with the well-documented physiological decrease in intrinsic metabolic drug clearance that occurs with advancing age [46, 47], the data suggest a slightly lower elimination of daridorexant. Next-morning plasma concentrations were also higher in older than younger patients with insomnia (unpublished data, Idorsia Pharmaceuticals). However, there was no correlation between daridorexant plasma concentration and a change from baseline in safety endpoints (coding sub-test total score and VAS morning sleepiness), neither in older nor in younger adults [35, 48, 49]. Thus, the slightly prolonged elimination did not translate into next-day residual effects [45]. These data suggest that the slightly higher exposure in older adults at the end of the night is not reflected in a difference in safety parameters.
All other safety endpoints were comparable in both age groups, despite the comorbidities and polypharmacy that were observed in the older patient group. Of note, in older adults, there was only one isolated case of sleep paralysis and no narcolepsy-like adverse events, including cataplexy, abnormal sleep behaviours or hallucinations. Falls, a common concern in the older population, which may be precipitated by some hypnotic drugs prescribed for insomnia [25, 50, 51], were infrequent. Specifically, the number of falls was numerically lower in patients receiving daridorexant (either dose) than in those receiving placebo, as suggested with other DORAs [52]. Daridorexant, like other approved DORAs, has been designated as a schedule IV-controlled substance by the US Drug Enforcement Agency. Such classification is not applicable to daridorexant in European Union member states. It should also be noted that in non-clinical studies, daridorexant has been shown to not induce dependence or a risk of abuse [53]. Accordingly, to date, there have been no reports of misuse, abuse or physical dependence with daridorexant and no withdrawal syndrome at drug cessation in older or younger adults [35, 48, 49, 54].
Many hypnotic drugs require caution when prescribed to older patients, with the lowest available starting dose often recommended for older patients [55]. The data with daridorexant, however, support using the same and highest effective dose of 50 mg, in older and younger patients, with no increased safety concern.
A strength of this analysis is that the older age group is representative of the older population with respect to comorbid conditions and concomitant medications, except for neuropsychiatric disorders, which were excluded as per the eligibility criteria. The eligibility criteria for the study did not specify an upper age limit for inclusion and thus the study population represents a broad group of older patients, including 15% (of the overall population) aged over 75 years. Limitations of the analysis include its post hoc nature (effect of age was pre-planned but the detailed comparisons were not type I error rate controlled or specifically powered) and the quite small sample size of very old patients aged over 75–80 years. The results presented are intentionally descriptive and avoid p values, owing to the possible, and undetectable, occurrence of false-positive or false-negative results. As such, the interpretation of the treatment effect relies more on the judgement of clinical relevance rather than on statistical significance. Based on the stringent inclusion criteria comparing objective measures of poor sleep, the patients had probably more severe insomnia than generally seen in practice where PSG is rarely performed. Additionally, the limited ethnic and racial diversity of the study population may only partially reflect such diversity beyond the clinical trial. Older patients may be taking a range of medications for a variety of conditions, and consideration should be given to pharmacokinetic and pharmacodynamic drug–drug interactions with daridorexant. As daridorexant is primarily metabolised by cytochrome P450 3A4 [56], concomitant use with cytochrome P450 3A4 inhibitors will increase exposure to daridorexant, which may increase the risk of adverse reactions [57], and concomitant use with cytochrome P450 3A4 inducers may decrease exposure to daridorexant, which may reduce efficacy [58]. Further, daridorexant is contra-indicated in patients with narcolepsy [35]. In addition, the current study did not evaluate whether cognitive disorders and depression, frequently seen in older patients with insomnia, influenced the effect of daridorexant, in particular on IDSIQ measures. Further studies in older adults with depression and/or cognitive impairment may be of interest and provide important information about daridorexant treatment in this population of patients. Last, there are no head-to-head trials comparing daridorexant to other DORAs, thus inferences about comparative efficacy or safety are limited.
5 Conclusions
The results from this analysis suggest that in older patients with insomnia, as in younger patients, the efficacy of daridorexant is dose dependent. In the older population, the higher daridorexant dose of 50 mg is necessary to improve daytime functioning and optimise the improvements in sleep onset and maintenance. This is without any increased risk of adverse events and, in particular, no increased risk of carry-over effects to the next morning after night-time administration of daridorexant. Older patients do not need lower doses of daridorexant than younger patients and the data from the trial show the clinically greater benefit of the 50-mg dose in comparison with the 25-mg dose for patients ≥ 65 years of age.
References
United Nations Department of Economics and Social Affairs Population Division. World population ageing 2019. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 30 Aug 2022.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
Ito E, Inoue Y. The international classification of sleep disorders, third edition. Am Acad Sleep Med. 2015;73(6):916–23.
Bjorøy I, Jørgensen VA, Pallesen S, Bjorvatn B. The prevalence of insomnia subtypes in relation to demographic characteristics, anxiety, depression, alcohol consumption and use of hypnotics. Front Psychol. 2020;11:527. https://doi.org/10.3389/fpsyg.2020.00527.
Pallesen S, Sivertsen B, Nordhus IH, Bjorvatn B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014;15(2):173–9. https://doi.org/10.1016/j.sleep.2013.10.009.
Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56(9):540–8. https://doi.org/10.1177/070674371105600905.
Hayley AC, Williams LJ, Kennedy GA, Holloway KL, Berk M, Brennan-Olsen SL, et al. Excessive daytime sleepiness and falls among older men and women: cross-sectional examination of a population-based sample. BMC Geriatr. 2015;15:74. https://doi.org/10.1186/s12877-015-0068-2.
Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201–7. https://doi.org/10.5665/sleep.2070.
Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56(9):1665–73. https://doi.org/10.1111/j.1532-5415.2008.01846.x.
Kirshner D, Kizony R, Gil E, Asraf K, Krasovsky T, Haimov I, et al. Why do they fall? The impact of insomnia on gait of older adults: a case-control study. Nat Sci Sleep. 2021;13:329–38. https://doi.org/10.2147/nss.S299833.
Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl. 2):S366–72.
Gulia KK, Kumar VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18(3):155–65. https://doi.org/10.1111/psyg.12319.
Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population: influence of previous complaints of insomnia. Arch Intern Med. 1992;152(8):1634–7.
Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017–24. https://doi.org/10.5664/jcsm.7172.
Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–62. https://doi.org/10.5664/jcsm.8986.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33. https://doi.org/10.7326/m15-2175.
Flaxer JM, Heyer A, Francois D. Evidenced-based review and evaluation of clinical significance: nonpharmacological and pharmacological treatment of insomnia in the elderly. Am J Geriatr Psychiatry. 2021;29(6):585–603. https://doi.org/10.1016/j.jagp.2020.10.011.
Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. 2018;33(6):955–62. https://doi.org/10.1007/s11606-018-4390-1.
Rios P, Cardoso R, Morra D, Nincic V, Goodarzi Z, Farah B, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281. https://doi.org/10.1186/s13643-019-1163-9.
Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29–35.
Tanielian M, Antoun J, Sidani M, Halabi A, Hoballah M, Hawatian K, et al. Sleep pattern and predictors of daily versus as-needed hypnotics use in middle-aged and older adults with insomnia. BMC Prim Care. 2022;23(1):98. https://doi.org/10.1186/s12875-022-01707-w.
Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. https://doi.org/10.1177/1179573518770672.
Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011;12(7):601–10. https://doi.org/10.2174/138920011796504527.
Nguyen KL, Watanabe JH. Association between sleep medications and falls and fall-related worries in community-dwelling older adults in the United States. J Contemp Pharm Pract. 2020;66(3):23–32. https://doi.org/10.37901/jcphp18-00022.
Kang D-Y, Park S, Rhee C-W, Kim Y-J, Choi N-K, Lee J, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health. 2012;45(4):219. https://doi.org/10.3961/jpmph.2012.45.4.219.
Gallacher J, Elwood P, Pickering J, Bayer A, Fish M, Ben-Shlomo Y. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). J Epidemiol Community Health. 2012;66(10):869–73. https://doi.org/10.1136/jech-2011-200314.
Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34(2):125–56. https://doi.org/10.2165/11539050-000000000-00000.
American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94. https://doi.org/10.1111/jgs.15767.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–49. https://doi.org/10.5664/jcsm.6470.
Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–81. https://doi.org/10.1038/nrn2092.
Markham A. Daridorexant: first approval. Drugs. 2022;82(5):601–7. https://doi.org/10.1007/s40265-022-01699-y.
Treiber A, de Kanter R, Roch C, Gatfield J, Boss C, von Raumer M, et al. The use of physiology-based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT-541468. J Pharmacol Exp Ther. 2017;362(3):489–503. https://doi.org/10.1124/jpet.117.241596.
Muehlan C, Brooks S, Zuiker R, van Gerven J, Dingemanse J. Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29(7):847–57. https://doi.org/10.1016/j.euroneuro.2019.05.009.
Muehlan C, Heuberger J, Juif PE, Croft M, van Gerven J, Dingemanse J. Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther. 2018;104(5):1022–9. https://doi.org/10.1002/cpt.1046.
Mignot E, Mayleben D, Fietze I, Leger D, Zammit G, Bassetti CLA, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–39. https://doi.org/10.1016/S1474-4422(21)00436-1.
Hudgens S, Phillips-Beyer A, Newton L, Seboek Kinter D, Benes H. Development and validation of the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ). Patient. 2020;14:249–68. https://doi.org/10.1007/s40271-020-00474-z.
Zammit G, Dauvilliers Y, Pain S, Sebok Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–32. https://doi.org/10.1212/WNL.0000000000009475.
Kunz D, Benes H, Garcia-Borreguero D, Dauvilliers Y, Plazzi G, Sassi-Sayadi M, et al. Long-term safety and efficacy of daridorexant in patients with insomnia disorder. In: Presented at World Sleep; 11-16 March, 2022: abstract 2145.
Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–94. https://doi.org/10.1185/03007990903167415.
McCall WV. Sleep in the elderly: burden, diagnosis, and treatment. Prim Care Companion J Clin Psychiatry. 2004;6(1):9–20. https://doi.org/10.4088/pcc.v06n0104.
Li SB, Damonte VM, Chen C, Wang GX, Kebschull JM, Yamaguchi H, et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science. 2022;375(6583): eabh3021. https://doi.org/10.1126/science.abh3021.
Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. https://doi.org/10.1016/j.jsmc.2017.09.001.
Carskadon MA, Brown ED, Dement WC. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;3(4):321–7. https://doi.org/10.1016/0197-4580(82)90020-3.
Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. https://doi.org/10.1016/j.neuron.2017.02.004.
Muehlan C, Boehler M, Brooks S, Zuiker R, van Gerven J, Dingemanse J. Clinical pharmacology of the dual orexin receptor antagonist ACT-541468 in elderly subjects: exploration of pharmacokinetics, pharmacodynamics and tolerability following single-dose morning and repeated-dose evening administration. J Psychopharmacol. 2020;34(3):326–35. https://doi.org/10.1177/0269881119882854.
Butler JM, Begg EJ. Free drug metabolic clearance in elderly people. Clin Pharmacokinet. 2008;47(5):297–321. https://doi.org/10.2165/00003088-200847050-00002.
Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44(1):33–60. https://doi.org/10.2165/00003088-200544010-00002.
Zammit G, Dauvilliers Y, Pain S, Sebok Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94:e2222–32. https://doi.org/10.1212/wnl.0000000000009475.
Dauvilliers Y, Zammit G, Fietze I, Mayleben D, Seboek Kinter D, Pain S, et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol. 2020;87(3):347–56. https://doi.org/10.1002/ana.25680.
Chen T-Y, Lee S, Buxton OM. A greater extent of insomnia symptoms and physician-recommended sleep medication use predict fall risk in community-dwelling older adults. Sleep. 2017. https://doi.org/10.1093/sleep/zsx142.
de Jong MR, Van der Elst M, Hartholt KA. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther Adv Drug Saf. 2013;4(4):147–54. https://doi.org/10.1177/2042098613486829.
Sogawa R, Emoto A, Monji A, Miyamoto Y, Yukawa M, Murakawa-Hirachi T, et al. Association of orexin receptor antagonists with falls during hospitalization. J Clin Pharm Ther. 2022;47(6):809–13. https://doi.org/10.1111/jcpt.13619.
Ufer M, Steiner MA, Post A, Dingemanse J, Toeroek M, Giusepponi M, et al. W150. Assessment of the abuse potential of daridorexant, a new dual orexin receptor antagonist for the treatment of insomnia disorder: data from preclinical and clinical studies. Neuropsychopharmacology. 2020;45(1):354. https://doi.org/10.1038/s41386-020-00892-5.
Ufer M, Kelsh D, Schoedel KA, Dingemanse J. Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem. Sleep. 2022;45(3): zsab224. https://doi.org/10.1093/sleep/zsab224.
Abad VC, Guilleminault C. Insomnia in elderly patients: recommendations for pharmacological management. Drugs Aging. 2018;35(9):791–817. https://doi.org/10.1007/s40266-018-0569-8.
Muehlan C, Fischer H, Zimmer D, Aissaoui H, Grimont J, Boss C, et al. Metabolism of the dual orexin receptor antagonist ACT-541468, based on microtracer/ accelerator mass spectrometry. Curr Drug Metab. 2019;20(4):254–65. https://doi.org/10.2174/1389200220666190206141814.
Boof ML, Alatrach A, Ufer M, Dingemanse J. Interaction potential of the dual orexin receptor antagonist ACT-541468 with CYP3A4 and food: results from two interaction studies. Eur J Clin Pharmacol. 2019;75(2):195–205. https://doi.org/10.1007/s00228-018-2559-5.
Gehin M, Wierdak J, Sabattini G, Sidharta PN, Dingemanse J. Effect of gastric pH and of a moderate CYP3A4 inducer on the pharmacokinetics of daridorexant, a dual orexin receptor antagonist. Br J Clin Pharmacol. 2022;88(2):810–9. https://doi.org/10.1111/bcp.15029.
Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. https://doi.org/10.1093/sleep/34.5.601.
Acknowledgements
The authors thank all the study investigators, IDMC and Independent Safety Board members and participants, study staff and nursing teams for their participation. The authors thank Martine Clozel and Pedro Pina (Idorsia Pharmaceuticals) for provision of pertinent comments and critical review of the manuscript. Medical writing support (writing the draft, revising the manuscript and journal styling) was provided by Jessica Beake (Beake Medicom Ltd), funded by Idorsia Pharmaceuticals Ltd, and figures were created by Nicolas Weber (Idorsia Pharmaceuticals).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Idorsia Pharmaceuticals Ltd, Allschwil, Switzerland. Idorsia funded the medical writing support (manuscript preparation).
Conflict of interest
IF reports consulting fees from Bayer, Bioproject, Hennig, Idorsia Pharmaceuticals, Jazz Pharmaceuticals, and STADA and speaker activity/reimbursement from Hennig, Idorsia Pharmaceuticals and Medice. CLAB reports consultancy fees from Bioproject, Takeda and Jazz Pharmaceuticals during the conduct of the trials. DM has been a clinical investigator for Apnimed, Eisai, Idorsia Pharmaceuticals, Imbrium, Janssen, Jazz Pharmaceuticals, Merck, Sage, Takeda and Vanda. WVM reports scientific advisor fees from Idorsia Pharmaceuticals and Janssen, and royalties from Wolter Kluwer. DSK and SP are employees of Idorsia Pharmaceuticals. IDSIQ was developed by Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A Hall M, Moul DE, Nofzinger EA and Kupfer DJ of the University of Pittsburgh and as amended by Idorsia Pharmaceuticals Ltd. IDSIQ© 2020, University of Pittsburg. All rights reserved. IDSIQ-14 derivative created 2020 by Idorsia Pharmaceuticals Ltd under license and distributed by Idorsia Pharmaceuticals Ltd under license.
Ethics approval
The study protocol was approved by the appropriate institutional review boards or independent ethics committees and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
All patients provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
The study sponsor will receive requests for individual participant data that underlie the results reported in this article, after deidentification, from researchers who provide a methodologically sound proposal. Please direct any requests to medicalinformationus@idorsia.com.
Code availability
Not applicable.
Author contributions
All authors were involved in the study conceptualisation. DSK and SP were involved in data curation. SP was involved in the formal analysis. SP, DSK and IF were involved in the methodology. DSK was involved in supervision. SP was involved in validation and visualisation. IF, DWM and CLAB were trial investigators or participated in the conduct of the study. SP and DSK verified the data. All authors had full access to the data, were involved with review and editing of the manuscript, approved the final version of the manuscript and had final responsibility for the decision to submit for publication and agreed to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fietze, I., Bassetti, C.L.A., Mayleben, D.W. et al. Efficacy and Safety of Daridorexant in Older and Younger Adults with Insomnia Disorder: A Secondary Analysis of a Randomised Placebo-Controlled Trial. Drugs Aging 39, 795–810 (2022). https://doi.org/10.1007/s40266-022-00977-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00977-4