Abstract
Background
Due to population aging, an increasing number of elderly patients with diabetes use insulin. It is therefore important to investigate the characteristics of new insulins in this population. Faster-acting insulin aspart (faster aspart) is insulin aspart (IAsp) in a new formulation with faster absorption. This study investigated the pharmacological properties of faster aspart in elderly subjects with type 1 diabetes mellitus (T1DM).
Methods
In a randomised, double-blind, two-period crossover trial, 30 elderly (≥65 years) and 37 younger adults (18–35 years) with T1DM received single subcutaneous faster aspart or IAsp dosing (0.2 U/kg) and underwent an euglycaemic clamp (target 5.5 mmol/L) for up to 12 h.
Results
The pharmacokinetic and pharmacodynamic time profiles were left-shifted for faster aspart versus IAsp. In each age group, onset of appearance occurred approximately twice as fast (~3 min earlier) and early exposure (area under the concentration–time curve [AUC] for serum IAsp from time zero to 30 min [AUCIAsp,0-30 min]) was greater (by 86% in elderly and 67% in younger adults) for faster aspart than for IAsp. Likewise, onset of action occurred 10 min faster in the elderly and 9 min faster in younger adults, and early glucose-lowering effect (AUC for the glucose infusion rate [GIR] from time zero to 30 min [AUCGIR,0-30 min]) was greater (by 109%) for faster aspart than for IAsp in both age groups. Total exposure (AUCIAsp,0-t) and the maximum concentration (C max) for faster aspart were greater (by 30 and 28%, respectively) in elderly than in younger adults. No age group differences were seen for the total (AUCGIR,0-t) or maximum (GIRmax) glucose-lowering effect.
Conclusion
This study demonstrated that the ultra-fast pharmacological properties of faster aspart are similar in elderly subjects and younger adults with T1DM.
ClinicalTrials.gov Identifier: NCT02003677.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In elderly subjects with type 1 diabetes mellitus, onset of appearance was twice as fast and early insulin exposure and early glucose-lowering effect were up to twofold greater for faster-acting insulin aspart (faster aspart) than for insulin aspart. |
While the total exposure and maximum concentration of faster aspart were ~30% greater in elderly than in younger adults, there were no age group differences in the total or maximum glucose-lowering effect of faster aspart. |
The ultra-fast pharmacokinetic and pharmacodynamic properties of faster aspart observed in younger adults are preserved in the elderly, suggesting that faster aspart also has the potential to improve postprandial glucose control over current rapid-acting insulin analogues in elderly patients with diabetes. |
1 Introduction
Due to a continuously growing elderly population, the number of elderly patients with diabetes who are being treated with insulin is also increasing [1]. One implication of this is that it has become even more important to characterise the pharmacokinetic and pharmacodynamic properties of any new insulin product specifically in the elderly population.
Faster-acting insulin aspart (faster aspart) is insulin aspart in a new formulation with two added excipients (l-arginine and niacinamide), which are both listed in the US Food and Drug Administration (FDA) inactive ingredient database as products approved for injection [2]. L-arginine acts as a stabilising agent, while niacinamide results in faster initial absorption after subcutaneous administration. The aim of this formulation is to improve postprandial glucose control over current rapid-acting insulins. Clinical pharmacology studies have shown that faster aspart has an onset of appearance that is twice as fast and a twofold higher early insulin exposure than insulin aspart, which leads to more than 50% greater early glucose-lowering effect [3–5]. In phase III trials conducted in subjects with type 1 (T1DM) and type 2 (T2DM) diabetes mellitus, postprandial glucose increments in standardised meal-tests were reduced with faster aspart versus insulin aspart [6, 7]. As the majority of subjects in these previous studies with faster aspart were below 65 years of age, there is a need for specific knowledge about the pharmacological properties of faster aspart in the elderly population.
The objectives of the current study were to investigate the overall pharmacokinetic and pharmacodynamic characteristics of faster aspart in elderly subjects compared with those in younger adults, and to compare the early pharmacokinetic and pharmacodynamic properties between faster aspart and insulin aspart in elderly subjects using the same comparison in younger adults as a reference. The study was conducted in subjects with T1DM in order to be able to assess the glucose-lowering effect of the investigational insulins in a glucose clamp setting, without interference from endogenous insulin secretion.
2 Methods
2.1 Trial Design
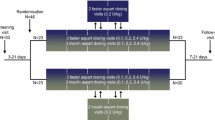
This was a randomised, single-centre (Profil, Neuss, Germany), double-blind, two-period, crossover trial in elderly and younger adults with T1DM. The trial protocol was reviewed and approved by the local health authority (Bundesinstitut für Arzneimittel und Medizinprodukte) and by an independent ethics committee (Ärztekammer Nordrhein). The trial was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice and relevant regulatory guidance on clinical trials in the elderly [8–10]. Written informed consent was obtained before initiation of any trial-related activities. The trial was registered at ClinicalTrials.gov (trial identifier: NCT02003677).
2.2 Participants
Eligible subjects were elderly (≥65 years) or younger adult (18–35 years) men and women diagnosed with T1DM ≥12 months before being included in the trial who were treated with multiple daily insulin injections or continuous subcutaneous insulin infusion for ≥12 months (total daily insulin dose <1.2 (I)U/kg/day and total daily bolus insulin dose <0.7 (I)U/kg/day), with glycosylated haemoglobin (HbA1c) ≤9.5% (80 mmol/mol), body mass index (BMI) 18.5–28.0 kg/m2 and fasting C-peptide ≤0.3 nmol/L. Subjects were excluded if they had clinically significant concomitant diseases or clinically significant abnormal values in clinical laboratory screening tests, were smokers or were treated with any drugs that might interfere with glucose metabolism.
2.3 Procedures
The trial consisted of a screening visit (3–21 days before the first dosing visit), two dosing visits separated by 3–12 days of washout and a follow-up visit (7–21 days after the second dosing visit). At the dosing visits, subjects received single 0.2 U/kg dosing of faster aspart (100 U/mL; Novo Nordisk, Bagsværd, Denmark) or insulin aspart (NovoRapid®; 100 U/mL; Novo Nordisk) in a randomised sequence. Both trial products were provided in a blinded PDS290 pen-injector prefilled pen (Novo Nordisk) and were administered by subcutaneous injection into a lifted skin fold of the lower abdominal wall above the inguinal area.
At each dosing visit, subjects attended the clinic in the morning after an overnight fast. They were advised to avoid any physical exercise during the last 2 days before each dosing visit and to come to the clinic by car, taxi or public transport. Subjects received a single dose of trial product in a euglycaemic glucose clamp setting (ClampArt®; Profil, Neuss, Germany) as previously described [4]. The blood glucose (BG) clamp target level was 5.5 mmol/L (100 mg/dL) and the clamp lasted up to 12 h after dosing. The quality of the conducted clamps [11] for each treatment and age group is presented in the Electronic Supplementary Material (Online Resource Table S1).
Blood samples for pharmacokinetic assessment were drawn within 2 min before dosing, then every 2 min from dosing until 20 min after dosing, every 5 min from 20 to 80 min, every 10 min from 80 min to 2 h, every 15 min from 2 to 3 h, and then at 3.5, 4, 5, 6, 7, 8, 10 and 12 h after dosing.
2.4 Assessments
Free serum insulin aspart concentrations (polyethylene glycol-precipitated) were measured by a validated insulin aspart-specific enzyme-linked immunosorbent assay. The glucose infusion rate (GIR) needed to keep the BG concentration at the clamp target level was recorded automatically every minute during the glucose clamp. Safety assessments included adverse events, local tolerability at the injection site, hypoglycaemic episodes, laboratory safety parameters, physical examination, vital signs and electrocardiogram. Hypoglycaemic episodes were defined as ‘confirmed’ when they were either ‘severe’ according to American Diabetes Association criteria, i.e. requiring third-party assistance [12], or verified by a plasma glucose level of <3.1 mmol/L (56 mg/dL).
2.5 Endpoints
Endpoints evaluating onset of exposure and onset of glucose-lowering effect included the pharmacokinetic endpoints, onset of appearance (time from trial product administration until the first time serum insulin aspart concentration was greater than or equal to the lower limit of quantification [LLOQ; 10 pmol/L]), time to early 50% of maximum insulin aspart concentration (t Early 50% Cmax) and time to maximum insulin aspart concentration (t max), and the pharmacodynamic endpoints, onset of action (time from trial product administration until the BG concentration had decreased by at least 0.3 mmol/L [5 mg/dL] from baseline, where baseline was defined as the mean BG concentration from −5 to −1 min), time to early 50% of maximum GIR (t Early 50% GIRmax) and time to maximum GIR (tGIRmax). Early exposure and early glucose-lowering effect were evaluated by deriving the early partial area under the concentration–time curve (AUC) values for serum insulin aspart (AUC for insulin aspart from time zero to 15 min [AUCIAsp,0-15 min], 30 min [AUCIAsp,0-30 min], 1 h [AUCIAsp,0-1 h], 1.5 h [AUCIAsp,0-1.5 h], and 2 h [AUCIAsp,0-2 h]) to assess pharmacokinetics and the AUCs for GIR (AUC for GIR from time zero to 30 min [AUCGIR,0-30 min], 1 h [AUCGIR,0-1 h], 1.5 h [AUCGIR,0-1.5 h], and 2 h [AUCGIR,0-2 h]) to assess pharmacodynamics. Overall exposure and glucose-lowering effect were evaluated by deriving the pharmacokinetic endpoints, total insulin aspart exposure (AUCIAsp,0-t) and maximum insulin aspart concentration (C max), and the pharmacodynamic endpoints, total glucose-lowering effect (AUCGIR,0-t; primary endpoint) and maximum GIR (GIRmax).
In order to calculate onset of appearance and AUCIAsp,0-15 min, the insulin aspart concentration was imputed during the period from dosing of trial product until the time of the first insulin aspart concentration above LLOQ using compartmental modelling. This approach was also used for the initial part of the AUC in calculating all other AUCIAsp endpoints for consistency. AUCIAsp,0-t was derived by calculating the AUC until the time of last quantifiable insulin aspart concentration and then extrapolating until 12 h (the last pharmacokinetic sampling timepoint) based on the terminal slope. AUCGIR,0-t was calculated until time of last GIR observation >0. In order to ensure robust calculation of t Early 50% GIRmax, GIRmax and tGIRmax, these endpoints were derived from LOESS smoothed GIR profiles (using a smoothing factor of 0.1). All other endpoints were derived from the raw profiles.
2.6 Statistical Analyses
No formal sample size calculation was performed. Before initiation of the trial, the number of subjects required to complete the trial was set to 40 (20 in each age group). With this sample size, assuming a total variance of log(AUCGIR,0-t) of 0.17 [3], the 95% confidence interval (CI) for the ratio of elderly/younger adults with respect to AUCGIR,0-t would be expected to range from 0.77 to 1.30 times the estimated age group ratio. This was considered a sufficient precision for the current study. To account for potential dropouts, it was pre-planned to randomise 44 subjects (22 subjects per age group).
All statistical analyses were performed at a 5% significance level using all randomised subjects who received at least one dose of trial product. SAS® version 9.3 (SAS Institute, Cary, NC, USA) was used for all analyses.
Pharmacokinetic and pharmacodynamic endpoints were log-transformed (except for onset of appearance, t Early 50% Cmax, t max, onset of action, t Early 50% GIRmax, tGIRmax and AUCGIR,0-30 min) and analysed in a linear mixed model with age group, treatment, interaction between age group and treatment, and period as fixed effects and subject as a random effect. The variance of the random subject effect and the residual variance depended on the age group. The pharmacokinetic and pharmacodynamic properties were compared between faster aspart and insulin aspart for elderly and younger adults using treatment ratios and 95% CIs derived by back-transforming the model-based treatment differences and corresponding CIs. Treatment ratios and 95% CIs for endpoints analysed without log-transformation were calculated by Fieller’s method [13]. To compare overall insulin exposure and overall glucose-lowering effect between elderly and younger adults for both faster aspart and insulin aspart, age group ratios and 95% CIs were calculated for AUCIAsp,0-t, C max, AUCGIR,0-t (statistical analysis of primary endpoint) and GIRmax from the same model as described above. For all analyses, a p value for test of no interaction between age group and treatment was derived.
Safety endpoints were summarised by descriptive statistics for subjects receiving at least one dose of trial product.
3 Results
3.1 Subject Disposition and Baseline Characteristics
A total of 85 subjects were screened and 67 subjects (30 elderly and 37 younger adults) were randomised and treated with trial product (Online Resource Fig. S1). Two subjects (one elderly and one younger adult) withdrew consent after the first dosing visit (insulin aspart and faster aspart, respectively). Thus, 29 elderly subjects and 36 younger adults completed the trial. All 67 randomised subjects were included in the pharmacokinetic and safety analyses. A total of 23 randomised subjects (8 elderly and 15 younger adults) were excluded from the pharmacodynamic analysis based on a blinded review conducted after completion of the first 44 subjects. During this blinded review, it was discovered that the Biostator algorithm [14], used at that time by ClampArt®, to determine the GIR based on BG concentrations did not consistently react fast enough to the rapid changes in metabolic activity induced by the study insulins. This led to high oscillations in BG and GIR, particularly shortly after dosing, in some clamp experiments depending on the exact glucodynamics. The technical issue affected at least one of the two glucose clamps in each of the 23 subjects, implying that clamp quality was insufficient for the valid calculation and interpretation of pharmacodynamic endpoints, especially in the early post-dosing period. The algorithm was therefore optimised to react faster to changes in BG and to reduce the high oscillations in BG and GIR observed with the Biostator algorithm. Both the Biostator algorithm and the optimised algorithm are proportional–integral–derivative (PID) controllers with comparable design, but with different aggressiveness. In order to achieve the planned number of completers for pharmacodynamic evaluation, 23 additional subjects were subsequently enrolled and assigned to the same treatment sequence as the subject being replaced. Thus, 44 subjects (22 in each age group) were included in the pharmacodynamic analysis. As the pharmacokinetic data were not affected by the technical clamp issues, the pharmacokinetic data obtained in the replaced subjects were included in the analysis (Online Resource Fig. S1).
Subject characteristics were comparable between age groups, except for the pre-defined difference in age between the two groups and the consequently longer duration of diabetes in the elderly group (Table 1).
3.2 Treatment Differences in Onset of Exposure and Glucose-Lowering Effect
In elderly subjects, both the mean serum insulin aspart concentration–time profile (Fig. 1) and the glucose-lowering effect–time profile (Fig. 2) were left-shifted for faster aspart compared with insulin aspart. Similar left-shifts of the pharmacokinetic and pharmacodynamic profiles were observed in younger adults (Online Resource Figs. S2 and S3).
Onset of appearance of faster aspart in the elderly subjects occurred twice as fast (~3 min earlier; p < 0.001), and t Early 50% Cmax was shorter by ~10 min (p < 0.001), as compared with insulin aspart (Table 2). In the elderly subjects, t max occurred 7 min earlier for faster aspart than for insulin aspart; however, the difference was not statistically significant (Table 2). Similar results were obtained with respect to onset of exposure in younger adults (Online Resource Table S2). Accordingly, the effect of treatment on onset of exposure did not differ statistically significantly between elderly and younger adults. p values for treatment by age group interaction were 0.904, 0.304 and 0.760 for onset of appearance, t Early 50% Cmax and t max, respectively.
Onset of action and t Early 50% GIRmax occurred ~10 min faster (p < 0.001) and ~6 min earlier (p = 0.003), respectively, with faster aspart compared with insulin aspart in the elderly subjects, while tGIRmax did not differ statistically significantly between treatments (Table 2). A similarly accelerated onset of glucose-lowering effect was obtained in the group of younger adults (Online Resource Table S2). No statistically significant differences between elderly and younger adults were observed with respect to treatment effect for onset of glucose-lowering effect. p values for the treatment by age group interaction were 0.707, 0.121 and 0.948 for onset of action, t Early 50% GIRmax and tGIRmax, respectively.
3.3 Treatment Differences in Early Exposure and Glucose-Lowering Effect
In the elderly subjects, early insulin exposure up to 2 h after dosing was statistically significantly greater for faster aspart than for insulin aspart (Fig. 3a). Insulin exposure was ~3-fold greater during the first 15 min after dosing (AUCIAsp,0-15 min; p < 0.001) and ~2-fold greater within the first 30 min after dosing (AUCIAsp,0-30 min; p < 0.001) for faster aspart than for insulin aspart. Similar results were obtained in younger adults, although the treatment difference for AUCIAsp,0-1.5 h and AUCIAsp,0-2 h did not reach statistical significance in this age group (Online Resource Fig. S4). The treatment effect on early exposure was not statistically significantly different between age groups. p values for treatment by age group interaction were 0.283, 0.449, 0.583, 0.622 and 0.663 for AUCIAsp,0-15 min, AUCIAsp,0-30 min, AUCIAsp,0-1 h, AUCIAsp,0-1.5 h and AUCIAsp,0-2 h, respectively.
Early exposure (a) and glucose-lowering effect (b) after administration of faster aspart versus insulin aspart (0.2 U/kg) in elderly subjects with type 1 diabetes mellitus. AUC area under the curve, CI confidence interval, GIR glucose infusion rate, IAsp insulin aspart, LS Mean least square mean, P-value treatment comparison of faster aspart versus insulin aspart
Early glucose-lowering effect was statistically significantly greater for faster aspart than for insulin aspart in the elderly subjects up to 2 h after dosing (Fig. 3b). An ~2-fold greater glucose-lowering effect was observed with faster aspart than with insulin aspart within the first 30 min after dosing (AUCGIR,0-30 min; p = 0.001). Similar treatment differences were observed in the group of younger adults, although the treatment difference for AUCGIR,0-2 h did not reach statistical significance in this age group (Online Resource Fig. S4). There was no statistically significant impact of age group on the treatment differences in early glucose-lowering effect. p values for treatment by age group interaction were 0.914, 0.605, 0.869 and 0.959 for AUCGIR,0-30 min, AUCGIR,0-1 h, AUCGIR,0-1.5 h and AUCGIR,0-2 h, respectively.
3.4 Treatment Differences in Overall Exposure and Glucose-Lowering Effect
AUCIAsp,0-t, C max, AUCGIR,0-t and GIRmax were all comparable for faster aspart and insulin aspart, irrespective of age group (Table 3 and Online Resource Table S3).
3.5 Age Group Differences in Overall Exposure and Glucose-Lowering Effect
AUCIAsp,0-t and C max for faster aspart were greater in elderly than in younger adults (estimated ratio of elderly/younger adults [95% CI] 1.30 [1.07–1.57], p = 0.008, and 1.28 [1.02–1.61], p = 0.031) (Table 4). For insulin aspart, the point estimates for the age group ratios were comparable with those for faster aspart, although no statistically significant difference was seen for C max. The effect of age on overall exposure did not differ statistically significantly between faster aspart and insulin aspart. p values for treatment by age group interaction were 0.867 and 0.431 for AUCIAsp,0-t and C max, respectively.
There were no statistically significant differences in AUCGIR,0-t and GIRmax between elderly and younger adults for faster aspart or for insulin aspart (estimated ratio of elderly/younger adults [95% CI] 0.93 [0.73–1.17], p = 0.517, and 0.85 [0.66–1.10], p = 0.209) (Table 4). p values for treatment by age group interaction were 0.403 and 0.430 for AUCGIR,0-t and GIRmax, respectively.
Least square means for AUCIAsp,0-t, C max, AUCGIR,0-t and GIRmax for both faster aspart and insulin aspart are presented for elderly subjects in Table 3 and for younger adults in Online Resource Table S3.
3.6 Safety
Faster aspart and insulin aspart were well-tolerated in both elderly and younger adults, and no safety issues were identified during the trial. There were no serious adverse events and all adverse events were either moderate (six events for faster aspart and eight events for insulin aspart) or mild (nine for faster aspart and two for insulin aspart) in intensity. No confirmed hypoglycaemic episodes and no injection-site reactions occurred during the trial.
4 Discussion
The main findings of the present study were the faster onset and greater early exposure and glucose-lowering effect observed with faster aspart versus insulin aspart in elderly subjects. The left shift of the pharmacokinetic and pharmacodynamic profiles of faster aspart versus insulin aspart in the elderly mirrors that seen in younger adults in the current study as well as in previous studies [3–5], indicating that the ultra-fast pharmacological properties of faster aspart are preserved in elderly patients with diabetes.
The faster onset and greater early glucose-lowering effect of faster aspart than with insulin aspart indicate that faster aspart better mimics the insulin action seen in the healthy state in response to a meal. Thus, faster aspart may provide better mealtime coverage than current rapid-acting insulins. Indeed, in subjects with T1DM, superiority of faster aspart over insulin aspart in 2-h postprandial glucose excursion and a statistically significant difference in favour of faster aspart in 1-h postprandial glucose excursion were demonstrated in a meal-test [6]. Likewise, in subjects with T2DM, the 1-h postprandial glucose excursion was statistically significantly reduced for faster aspart versus insulin aspart, while the treatment difference in 2-h postprandial glucose excursion in favour of faster aspart did not reach statistical significance [7]. These two latter trials were conducted mainly in younger adults [6, 7]. On the basis of the results of the current study we would hypothesise that similar improvements in postprandial glucose excursions would be achieved in elderly patients with diabetes. However, the potential for faster aspart to improve postprandial glucose control in elderly patients with diabetes still remains to be confirmed in further investigations.
The faster pharmacokinetic and pharmacodynamic properties of faster aspart may provide the option of post-meal administration, when necessary, thereby allowing the insulin dose to be more accurately adjusted to the actual food intake. This could be particularly useful in those elderly patients with cognitive impairment and/or irregular eating patterns. Potential benefits include improved control of postprandial glucose and, not least, a reduced risk of hypoglycaemia, which is considered a particularly serious problem and a barrier to optimal glycaemic control in elderly patients with diabetes [15, 16]. In a standardised meal-test in elderly subjects with T2DM, insulin aspart administered at meal ingestion and regular human insulin administered 30 min before meal ingestion resulted in similar insulin and postprandial glucose profiles [17]. Post-meal insulin lispro provided greater control of mean daily BG and a reduced incidence of hypoglycaemia and hyperglycaemia compared with regular human insulin administered 30 min before each meal in elderly subjects with T2DM living in nursing homes [18]. Thus, it appears that current rapid-acting insulins allow for post-meal dosing, while still providing at least as good postprandial glucose control as regular human insulin administered pre-meal. In a similar manner, faster aspart administered 20 min post-meal has been compared with insulin aspart administered at mealtime in adult subjects with T1DM [6]. The 2-h postprandial glucose increment was not statistically significantly different between post-meal faster aspart and mealtime insulin aspart, while the 1-h postprandial glucose increment was statistically significantly in favour of mealtime insulin aspart. The HbA1c reduction seen during the 26-week treatment period was non-inferior, and overall rates of severe and confirmed hypoglycaemia were comparable, for post-meal faster aspart versus mealtime insulin aspart [6]. Clinical evidence is needed to confirm the option of post-meal dosing of faster aspart in elderly patients with diabetes and the optimal time window for such administration.
We found a higher total exposure and C max of faster aspart in elderly than in younger adults. The same was seen for insulin aspart, although it was only statistically significant for total exposure. A similar trend has been observed in previous studies for the insulin degludec/insulin aspart combination, insulin degludec alone and for insulin detemir [19–21], and would be in accordance with a reduced insulin clearance with increasing age [22]. Importantly, we found comparable AUCGIR,0-t and GIRmax in elderly and younger adults, as previously shown [19]. The similar glucose-lowering effect in elderly and younger adults despite higher exposure in the elderly is in line with the well-known decrease in insulin sensitivity with aging [23, 24]. In this context, it is also important to emphasise that the insulin dose should always be adjusted on an individual basis, depending on each patient’s needs. It follows that faster aspart should be considered safe also in elderly diabetes patients in terms of possible hypoglycaemia, although clinical data are needed to confirm this.
The clamp quality in the current study was high both in terms of precision and control deviation (Online Resource Table S1). Thus, the technical clamp issues experienced during the initial part of the study seem to have had no adverse impact on the quality of the results, as would also be expected since the affected data were excluded during a blinded review (see Sect. 3.1). In fact, the control deviation (i.e. the mean difference between the measured and the target BG concentration) was lower than in a previous series of experiments using the Biostator algorithm [11]. Thus, optimising the algorithm as done during the present study may have contributed to reducing the control deviation.
Several review articles have emphasised the generally limited number of clinical trials investigating diabetes interventions in the elderly [25–27]. Accordingly, to our knowledge, glucose clamp studies comparing the pharmacokinetic and pharmacodynamic properties of an insulin between elderly and younger adults and/or between two insulins in elderly subjects have only been conducted for a few insulin products [19, 20, 28]. Therefore, a strength of the current study was the use of the euglycaemic glucose clamp technique to assess the pharmacodynamic properties. It is, however, also important to emphasise that an inherent limitation of the glucose clamp method is the experimental and standardised setting, which imposes some challenges in directly translating to clinical practice. Another limitation of the present study was the relatively few subjects per age group. Still, the number of subjects was higher than in similar previous studies in elderly subjects [19, 20], and was sufficient to demonstrate statistically significant differences in pharmacokinetic and pharmacodynamic properties between faster aspart and insulin aspart in both age groups.
5 Conclusion
The present study demonstrated that faster aspart has a faster onset and a greater early exposure and glucose-lowering effect than insulin aspart in elderly subjects with T1DM. The results observed in the elderly subjects are in line with those seen in the younger adults, suggesting that faster aspart also has the potential to improve postprandial glucose control compared with current rapid-acting insulins in elderly patients with diabetes.
References
Heller S, McCance DR, Moghissi E, Nazeri A, Kordonouri O. Diversity in diabetes: the role of insulin aspart. Diabetes Metab Res Rev. 2012;28:50–61.
US Food and Drug Administration. Inactive ingredient search for approved drug products. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed 7 Oct 2016.
Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17:682–8.
Heise T, Stender-Petersen K, Hövelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clinical Pharmacokinet. doi:10.1007/s40262-016-0473-5.
Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. Faster onset and greater early exposure and glucose-lowering effect with faster-acting insulin aspart vs. insulin aspart: a pooled analysis in subjects with type 1 diabetes [abstract]. Diabetes. 2016;65(Suppl. 1):A239.
Russell-Jones D, Bode B, de Block C, et al. Double-blind mealtime faster-acting insulin aspart vs insulin aspart in basal–bolus improves glycemic control in T1D: the onset® 1 trial [abstract]. Diabetes. 2016;65(Suppl. 1):A77.
Bowering K, Case C, Harvey J, et al. Faster-acting insulin aspart vs insulin aspart as part of basal-bolus therapy improves postprandial glycemic control in uncontrolled T2D in the Double-Blinded onset® 2 trial [abstract]. Diabetes. 2016;65(Suppl. 1):A63.
ICH Harmonised Tripartite Guideline E7. Studies in support of special populations: geriatrics. 24 June 1993. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E7/Step4/E7_Guideline.pdf. Accessed 7 Oct 2016.
ICH. E7 studies in support of special populations: geriatrics. Questions & answers. 6 July 2010. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E7/Q_As/E7_Q_As_step4.pdf. Accessed 7 Oct 2016.
European Medicines Agency. Committee for Medicinal Products for Human Use. Adequacy of Guidance on the Elderly Regarding Medicinal Products for Human Use. 14 December 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500049541.pdf. Accessed 7 Oct 2016.
Benesch C, Heise T, Klein O, Heinemann L, Arnolds S. How to assess the quality of glucose clamps? Evaluation of clamps performed with ClampArt, a novel automated clamp device. J Diabetes Sci Technol. 2015;9:792–800.
American Diabetes Association. Defining and reporting hypoglycaemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycaemia. Diabetes Care. 2005;28:1245–9.
Fieller EC. Some problems in interval estimation. J R Stat Soc Ser B Stat Methodol. 1954;16:175–85.
Clemens AH, Hough DL, D’Orazio PA. Development of the Biostator glucose clamping algorithm. Clin Chem. 1982;28:1899–904.
Thompson AM, Linnebur SA, Vande Griend JP, Saseen JJ. Glycemic targets and medication limitations for type 2 diabetes mellitus in the older adult. Consult Pharm. 2014;29:110–23.
Ligthelm RJ, Kaiser M, Vora J, Yale JF. Insulin use in elderly adults: risk of hypoglycemia and strategies for care. J Am Geriatr Soc. 2012;60:1564–70.
Meneilly GS. A comparison of insulin aspart and regular insulin in elderly patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:754–5.
Velussi M. Lispro insulin treatment in comparison with regular human insulin in type 2 diabetic patients living in nursing homes. Diabetes Nutr Metab. 2002;15:96–100.
Brunner M, Pieber T, Korsatko S, Kojzar H, Svendsen AL, Haahr H. The distinct prandial and basal pharmacodynamics of IDegAsp observed in younger adults are preserved in elderly subjects with type 1 diabetes. Drugs Aging. 2015;32:583–90.
Korsatko S, Deller S, Mader JK, et al. Ultra-long pharmacokinetic properties of insulin degludec are comparable in elderly subjects and younger adults with type 1 diabetes mellitus. Drugs Aging. 2014;31:47–53.
FDA. Levemir® [prescribing information]. February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021536s031s051lbl.pdf. Accessed 7 Oct 2016.
Minaker KL, Rowe JW, Tonino R, Pallotta JA. Influence of age on clearance of insulin in man. Diabetes. 1982;31:851–5.
Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin N Am. 2013;42:333–47.
Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–101.
Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–64.
Tanwani LK. Insulin therapy in the elderly patient with diabetes. Am J Geriatr Pharmacother. 2011;9:24–36.
Ober SK, Watts S, Lawrence RH. Insulin use in elderly diabetic patients. Clin Interv Aging. 2006;1:107–13.
Krones R, Schütte C, Heise T. The rapid-acting properties of insulin aspart are preserved in elderly people with type 2 diabetes. Diabetes Obes Metab. 2009;11:41–4.
Acknowledgements
The authors would like to thank Theis Gondolf, MD, Novo Nordisk, for his review of and input into the manuscript and Carsten Roepstorff, PhD, CR Pharma Consult, Copenhagen, Denmark for providing medical writing support, which was funded by Novo Nordisk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Novo Nordisk.
Conflict of interest
Tim Heise is a shareholder of Profil, which has received research funds from Adocia, AstraZeneca, Becton Dickinson, Biocon, Boehringer Ingelheim, Dance Biopharm, Eli Lilly, Grünenthal, Gulf Pharmaceutical Industries, Johnson & Johnson, Marvel, MedImmune, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma. In addition, Tim Heise is a member of advisory panels for Novo Nordisk and received speaker honoraria and travel grants from Eli Lilly, Mylan and Novo Nordisk. Kirstine Stender-Petersen, Jacob Bonde Jacobsen and Hanne Haahr are employees and shareholders of Novo Nordisk. Eric Zijlstra received travel grants from Dance Biopharm and Novo Nordisk and speaker honoraria from Novo Nordisk and Roche Diabetes Care. Ulrike Hövelmann declares no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Heise, T., Hövelmann, U., Zijlstra, E. et al. A Comparison of Pharmacokinetic and Pharmacodynamic Properties Between Faster-Acting Insulin Aspart and Insulin Aspart in Elderly Subjects with Type 1 Diabetes Mellitus. Drugs Aging 34, 29–38 (2017). https://doi.org/10.1007/s40266-016-0418-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-016-0418-6