Abstract
Rheumatoid arthritis (RA) is a chronic immune-mediated inflammatory disease which can induce progressive disability if not properly treated early. Over the last 20 years, the improvement of knowledge on the pathogenesis of the disease has made available several drugs targeting key elements of the pathogenetic process, which now represent the preferred treatment option after the failure of first-line therapy with conventional drugs such as methotrexate (MTX). To this category of targeted drugs belong anti-cytokine or cell-targeted biological agents and more recently also Janus kinase inhibitors (JAKis). In the absence to date of specific biomarkers to guide the therapeutic choice in the context of true precision medicine, the choice of the first targeted drug after MTX failure is guided by treatment cost (especially after the marketing of biosimilar products) and by the clinical characteristics of the patient (age, sex, comorbidities and compliance) and the disease (presence or absence of autoantibodies and systemic or extra-articular manifestations), which may influence the efficacy and safety profile of the available products. This viewpoint focuses on the decision-making process underlying the personalized approach to RA therapy and will analyse the evidence in the literature supporting the choice of individual products and in particular the differential choice between biological drugs and JAKis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rheumatoid arthritis is a chronic auto-immune inflammatory disease that requires early diagnosis and treatment to prevent the progression of joint damage. |
Several therapeutic options are now available after first-line treatment with conventional drugs, such as methotrexate, has failed. |
In the absence of predictive biomarkers, the choice of the first targeted drug after MTX failure is guided by clinical and pharmacoeconomic factors through a patient-centred decision-making process. |
1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease that primarily affects the joints and is characterized by progressive cartilage and bone damage, resulting in a high rate of disability. In addition, the possible involvement of other organs and systems (especially the respiratory and cardiovascular systems) and the development of disease- and therapy-related co-morbidities contribute to the deterioration of quality of life and life expectancy of these patients.
In the last 3 decades there has undoubtedly been immense progress in the field of rheumatology and rheumatoid arthritis in particular. Knowledge of the disease pathogenetic mechanisms has allowed the development of drugs that can radically alter the disease course and greatly improve the quality of life and survival of RA patients. This has been complemented by the use of therapeutic strategies aimed at intercepting the disease at its earliest stages and achieving a therapeutic goal, such as disease remission (treat-to-target strategy) [1].
However, many challenges remain. The aetiology of RA is still largely unknown, and there is still a lack of biomarkers for the prediction of response to individual drugs [2]. Furthermore, in recent years it has become increasingly clear that RA is a highly heterogeneous disease and that there are likely to be distinct patient subgroups and disease sub-phenotypes [3, 4]. In this scenario, the choice of the drugs to be used in the individual patient is still mainly left to the expertise of the rheumatologist and to a trial-and-error approach [5].
To date, there are two main categories of ‘advanced’ drugs available to the rheumatologist: biologics [biologic disease-modifying antirheumatic drugs (bDMARDs)], i.e., monoclonal antibodies or fusion molecules with anti-cytokine or anti-cellular targeting and, more recently, targeted synthetic agents (tsDMARDs), i.e., Janus kinase inhibitors (JAKis) with intracellular and pleiotropic action.
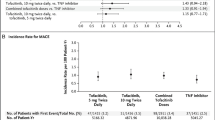
According to the 2019 recommendations of the European Alliance of Associations for Rheumatology (EULAR), the first-line treatment of patients with RA involves the use of methotrexate (MTX) or another conventional DMARD (csDMARDs), often combined with a low dose of bridging steroid. If first-line therapy fails, it is recommended to add a bDMARD or a tsDMARD to the ongoing treatment [6]. No preference is given between the two drug categories, as they are comparable in their efficacy profile. In the updated version of the 2022 EULAR recommendations for the management of RA, this suggestion remains essentially unchanged, confirming the possibility of using a bDMARD or tsDMARD as second-line therapy, but adding a caveat of requiring a careful assessment of the patient’s risk profile before starting any Janus kinase (JAK) inhibitor therapy [7]. This cautionary note is mainly based on the results of two studies. ORAL Surveillance, a phase IV randomized controlled post-marketing study, showed an apparently higher incidence of major cardiovascular events (MACE) and cancer in patients treated with tofacitinib than in those treated with tumour necrosis factor inhibitors (TNFis) in a study population selected for age (> 50 years) and the presence of at least one traditional cardiovascular risk factor [8]. The second study, B023, a multi-register observational study, showed higher rates of thromboembolic events in patients treated with the JAKi baricitinib compared with TNFis [9].
In light of this evidence, the US Food and Drug Administration (FDA) has decided to restrict the use of JAKis to patients who have failed (or are intolerant to) at least one TNFi [10]. Instead, the European Medicines Agency (EMA) recommends that the use of JAKis in patients aged 65 years and older, current or former smokers and patients with cardiovascular, neoplastic and thromboembolic risk factors should be considered only in the absence of valid therapeutic alternatives [11]. Similarly, as mentioned above, the latest version of the EULAR recommendations stated that risk factors for cardiovascular events and malignancies, including age over 65 years, current or former smoking, other cardiovascular risk factors, other risk factors for malignancy and risk factors for thromboembolic disease, should be considered when considering prescribing a JAKi [7]. In line with the principle of shared decision-making, JAKis therefore remain a viable second-line treatment option after careful assessment of the risk profile and appropriate communication with the patient.
So the question remains: In a patient with RA who does not respond to csDMARDs, which drug (or which class of drugs) should be used?
In this narrative review, we analyse the main factors to consider when choosing a treatment for this RA patient category, including the pharmacodynamic properties of the drugs, the demographic and clinical characteristics of the patients and disease-related factors.
The searches were conducted between December 2022 and January 2023 in the databases MEDLINE, Scopus, and Web of Science. The search terms included: rheumatoid arthritis, treatment strategy, treatment recommendations, biologics, Janus kinase inhibitors and targeted therapy. Every individual search term was supplemented with relevant free text terms. The search result was limited to articles that were written in English as well as articles published during the last 10 years. The evidence presented in the manuscript is illustrated and summarized in the Table 1.
2 Overview of Targeted Therapies
2.1 bDMARDs
The bDMARDs approved to date for the therapy of RA include five TNFis (infliximab, adalimumab, golimumab, certolizumab pegol and etanercept), two IL6 inhibitors (tocilizumab and sarilumab), a monoclonal anti-B-cell CD20 antibody (rituximab) and a T-lymphocyte co-stimulation inhibitor (abatacept). They are all monoclonal antibodies, with the exception of abatacept and etanercept, which are fusion proteins. Their key feature is their therapeutic target selectivity. Compared with conventional immunosuppressive drugs, bDMARDs are highly specific for their target molecule or cell, ensuring selective target suppression while maintaining adequate function of the various (cellular and non-cellular) components of the immune system.
The first bDMARDs approved in 1999 were two TNFis, infliximab and etanercept. Since then, the family has grown to include five drugs that differ in chemical structure, route of administration and therapeutic interval, but have similar efficacy and safety profiles. Use of these drugs has been shown to dramatically reduce disease activity and, in responding patients, slow or halt clinical and radiographic progression and improve patient-reported outcomes. Their success has paved the way for the development of other molecules with different mechanisms of action. Abatacept, a fusion protein whose mechanism of action is to inhibit lymphocyte co-stimulation by antigen-presenting cells, and rituximab, a monoclonal antibody that can induce depletion of mature pre-B and B lymphocytes by binding to the CD20 receptor, were approved in 2005 and 2006. The last class of approved bDMARDs are inhibitors of the receptor (soluble and membrane-bound) of IL6, a pro-inflammatory cytokine whose role in the pathogenesis of RA is known and extensively described [12].
2.2 JAK Inhibitors
JAKis are a class of drugs that work by inhibiting the activity of one or more members of a family of enzymes known as Janus kinases. These enzymes are intracellular non-receptor tyrosine kinases that act by transducing signals mediated by various cytokines through the JAK–signal transducer and activator of transcription (STAT) pathway, which consists of four JAKs (JAK1, JAK2, JAK2 and TYK2) and seven STATs (STAT1-4, STAT5A and 5B, STAT6). A plethora of inflammatory mediators (IL-2, IL-4, IL-6, IL-7, IL-9, IL-11, IL-12-23, IL-15, and type I–II interferons) and haematopoietic growth factors [such as IL-3, IL-5, granulocyte colony-stimulating growth factor (GM-CSF), erythropoietin and thrombopoietin] carry out their intracellular signalling via this pathway [13]. As many of these molecules play a central role in the RA network, the use of agents targeting JAK inhibition allows modulation of multiple pathways simultaneously and potentially a broader effect on disease pathogenesis.
Tofacitinib, the first JAKi approved (in 2012 by the FDA and in 2017 by the EMA), is a selective inhibitor of JAK1,3 with minor activity against JAK2 and TYK2, whereas baricitinib is a selective inhibitor of JAK1,2 with moderate activity against TYK2 and minimal activity against JAK3. In later years, drugs with more selective activity against JAK1 were investigated, mainly to minimize the negative effects of JAK2 inhibition on the haemopoietic system, leading to the approval of the “second-generation JAKis” or “selective JAKis”, upadacitinib and filgotinib [14]. However, the selectivity of JAKis has been evaluated in vitro, while it is still unclear whether this really affects their use in vivo [15]. Unfortunately, there are no head-to-head comparisons between pan-JAK inhibitors and selective JAK inhibitors to better define the impact of theoretical selectivity on their safety and efficacy profile.
3 Drug-Related Factors
3.1 Immunogenicity
All biologics are potentially immunogenic, meaning that they can induce an immune response against themselves, which is expressed in particular by the formation of anti-drug antibodies (ADAs) [16]. The main clinical consequences of immunogenicity are a reduction in clinical efficacy and the development of administration reactions, ranging from mild and localized to severe acute reactions such as anaphylaxis and serum sickness [17]. A clear correlation between the development of infusion reactions or injection-site symptoms and the presence of circulating ADAs has been shown [18]. Similarly, the presence of ADAs is associated with a reduced clinical response and a higher rate of treatment discontinuation [19]. The administration of JAKis is not able to induce immunogenicity, and therefore, no hypersensitivity reactions or reduction in clinical response due to the presence of ADAs has been observed.
3.2 Costs
As clearly stated by the EULAR Task Force experts, the pharmaco-economic issue is one of the aspects to be considered in the overall management of RA patients [7]. The costs of advanced therapies with both bDMARDs and tsDMARDs are significantly higher than those associated with conventional therapies, and this has led to limitations in their use and disparities in treatment between countries worldwide [20]. Biosimilars (currently available for infliximab, etanercept, adalimumab, rituximab and recently for tocilizumab) have been shown to be as clinically effective as their reference biologics [21]. Their use in clinical practice has been found to be cost-effective and represents a valid and recommended strategy to reduce healthcare expenditure [22,23,24].
3.3 Efficacy in Disease Activity
During their development programs, all JAK inhibitors have been tested in head-to-head comparisons with TNFis. In patients with an inadequate response to methotrexate (MTX-IR), tofacitinib 5 mg was compared with adalimumab in the ORAL Strategy study, achieving the non-inferiority endpoint of an American College of Rheumatology (ACR) response of at least 50% (ACR50); 46% versus 44%) [25]. Similarly, filgotinib demonstrated non-inferiority to adalimumab in achieving a response of Disease Activity Score for 28 joints based on the C-reactive protein level (DAS28-CRP) ≤ 3.2 at week 12 (FINCH1 study) [26]. In the RA-BEAM and SELECT-COMPARE studies, both baricitinib (74% versus 66%, p < 0.05) and upadacitinib (71% versus 63%, p < 0.01) demonstrated statistically significant superiority over adalimumab in achieving the primary endpoint of ACR20 [27, 28]. A better performance of baricitinib and upadacitinib also occurred in more stringent secondary outcomes, such as ACR70 in the RA-BEAM (45% versus 35%, p < 0.01 for baricitinib versus adalimumab), and DAS28-CRP ≤ 2.6 response in the SELECT-COMPARE trial (29% versus 18%, p < 0.01 for upadacitinib versus adalimumab) [27, 28]. Upadacitinib was also studied in patients who had an inadequate response to at least one biologic (bDMARD-IR) compared with abatacept, both in combination with a csDMARD. At the end of the study (SELECT-CHOICE), upadacitinib was superior to abatacept in terms of DAS28-CRP response at week 12 [29].
In the real world, recent studies have not shown any clinically significant differences between the use of available classes of drugs in patients with MTX-IR [30, 31]. A meta-analysis found a lower efficacy of TNFis compared with non-TNFis [relative risk (RR) 0.88; 95% confidence interval (CI) 0.81–0.95; p < 0.01] and of bDMARDs compared with JAKis (RR 0.86; 95% CI 0.79–0.94; p < 0.01) [32]. However, as these results come from observational studies that were neither randomized nor double-blind, they should be interpreted with caution.
3.4 Efficacy on Patient-Reported Outcomes (PROs)
It is well recognized that pain and fatigue are among the most common symptoms reported by RA patients. These symptoms, which have a significant impact on patients’ health-related quality of life (HRQOL), are poorly captured by current disease activity scores and often persist in the absence of clinically detectable signs of active disease. Many different patient-reported outcomes (PROs) have been validated in RA, providing a more comprehensive assessment of the disease and its impact on patients’ HRQOL.
The results of PROs observed in randomized controlled trials (RCTs) with JAKis are quite impressive [33]. In particular, baricitinib showed significantly better performance than adalimumab in reducing pain [as measured by global visual analogue scale (VAS) pain] and fatigue [Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score] and improving quality of life [Health Assessment Questionnaire-Disability Index (HAQ-DI), Short Form 36]. A recent meta-analysis on pooled data from RCTs comparing different JAK inhibitors with TNFis, tocilizumab and abatacept, confirmed significantly better results with JAK inhibitors compared with bDMARDs in terms of pain relief, fatigue and general health [34]. These findings have been replicated in real-world populations with remarkable results. Spinelli et al. reported a significant reduction in pain (≥ 50%) as early as week 4 of treatment with baricitinib and tofacitinib, which was maintained over time and was associated with a significant improvement in overall health [35]. Similar results were found in another observational study, which also showed that pain reduction in patients with high levels of synovial and systemic inflammation at baseline was the same as in those with low or no inflammatory status [36]. Indeed, it has been hypothesized that the effect of JAKis on pain goes beyond their ability to inhibit inflammation, but the underlying mechanisms of this widespread analgesic effect are not fully understood. However, it may be related to their pleiotropic effects on several inflammatory cytokines and pathways, including IL-6, GM-CSF and IL-10, whose role in the induction of nociceptive and neuropathic pain has been extensively described [37]. As JAKis are small molecules, a direct role in the central nervous system has also been hypothesized. Studies in animal models have demonstrated their ability to cross the blood–brain barrier [38, 39]. However, evidence in humans is limited to date [40], and further developments in this area would be of great interest.
3.5 Retention Rate
In real-world clinical practice, an extremely useful outcome measure for assessing the efficacy and long-term tolerability of a drug is the drug retention rate. The largest body of data concerns the use of TNFis, with over 20 years of experience in this area. Registry data show that, 12 years after starting a first-line TNFi, an average of 23.4% of patients are still on therapy, with a significantly higher retention rate for etanercept than for monoclonal antibodies [41]. In the Rhumadata registry, TNFis was associated with a similar rate of treatment retention after 9 years compared with abatacept in b/tsDMARDs-naive patients [42]. Larger but shorter cohort data compared the persistence rate of different b/tsDMARDs. The Japanese ANSWER study evaluated the persistence rate according to the reasons for discontinuation of the different treatments and showed that anti-IL6Rs have a higher persistence rate than TNFis, abatacept and JAKis according to inefficacy. In contrast, abatacept has the highest persistence rate on a safety basis [43].
At an average of 2 years after starting treatment, the retention rates from different observational cohorts for baricitinib, tocilizumab and abatacept are 93.7%, 72.2% and 51.7%, respectively [44,45,46]. For tofacitinib, the average persistence rate was estimated to be 78.8% in both bDMARD-naive and b/tsDMARD-IR patients. Although the persistence rate in naive patients is not reported, the authors stated that no significant difference was found between the two patient categories [45].
3.6 Monotherapy
International guidelines recommend combination therapy with b- or tsDMARDs after failure of csDMARDs [7]. In fact, combination with MTX has been shown to be more effective than monotherapy [47,48,49]. This is particularly true for some bDMARDs, especially TNFis, which in combination with MTX show higher retention rates and better radiological outcomes than monotherapy [27, 33, 50,51,52,53]. Indeed, combination with MTX can reduce the immunogenicity of bDMARDs on the one hand [54, 55], and have a synergistic immunosuppressive effect on the other, depending on the mechanism of action of each drug [56]. Despite the recommendations, more than a third of patients who fail first-line therapy start b/tsDMARDs as monotherapy, while another third of patients discontinue or reduce MTX within the first 2 years of starting biologic therapy [52, 57]. Data from RCTs and real-world observational experience show that anti-IL6R drugs and JAKis are effective and have good retention rates even as monotherapy [25, 58,59,60,61,62,63], and are therefore preferred for all patients when combination therapy is inappropriate due to intolerance or contraindications [7].
4 Patient-Related Factors
4.1 Age
As the age of the general population increases, so does the age of patients with RA. Older people tend to be frailer and often have multiple comorbidities that need to be treated with polypharmacy. In this scenario, it is questionable whether age can be a discriminatory factor in the choice of therapy in RA patients. According to FDA and EMA recommendations, age over 65 years alone is sufficient criteria to start a first-line bDMARD after MTX failure [10, 11]. In these patients, JAK inhibitors can only be considered if other treatment options have failed or are contraindicated.
These indications are based on evidence from RCTs of an increased risk of adverse events (serious infections, herpes zoster, cardiovascular events and neoplasms) associated with the use of JAKis in patients over 65 years of age [8, 64,65,66,67,68,69]. With regard to the risk of serious infections, real-world data confirmed that the risk starts to increase in patients treated with tofacitinib after the age of 69 years compared with patients treated with bDMARDs, and the difference becomes clinically relevant in patients over 76 years [68, 70]. In contrast, data on cardiovascular (CV) risk are inconsistent, with large observational studies showing no increased risk in patients over 65 years on JAKis compared with TNFis [66, 71, 72]. Recently a group of Japanese authors evaluated the retention rate of JAKis in elderly (< 65 years) and very elderly (> 75 years) patients and showed no differences between the two groups, even when analysing the discontinuation rate due to adverse events [73]. However, the survival curve changed in patients treated with the approved dose compared with those treated with the reduced dose, showing a significantly higher retention rate with the reduced dose. The main reason for discontinuation in all patient categories was infection [73].
In clinical practice, it has been observed that age is one of the main factors affecting the choice of bDMARD, with abatacept and tocilizumab being the most commonly used in older patients [74]. Indeed, both abatacept and tocilizumab are also the drugs with the highest drug retention among patients aged 75 years and older. Abatacept in particular appears to be the b/tsDMARD with the lowest discontinuation rate due to adverse events, particularly in older patients [70, 75, 76]. In view of the good safety profile of abatacept, also in the long term, the British Society for Rheumatology recommends its use as a first-line bDMARD for patients at high risk of infection [77]. However, tocilizumab’s good persistence may be related to the possibility of using tocilizumab effectively even as monotherapy. Indeed, the use of methotrexate in elderly patients is often poorly tolerated or contraindicated due to polypharmacology and potential impaired renal function [78]. Higher disease activity and C-reactive protein (CRP) levels at baseline have also been reported in patients with elderly-onset RA, which could explain the good clinical response to tocilizumab in these patients [79]. Nevertheless, as tocilizumab has been shown to increase the risk of bowel perforation in patients with pre-existing diverticulitis, special care should be taken when treating elderly subjects, who are more likely to be affected by diverticulosis.
4.2 Sex
Several lines of evidence suggest that women tend to respond less to bDMARDs than men do, despite similar baseline disease activity between the sexes [80, 81]. This finding was recently confirmed by a post-hoc analysis of the NORD-STAR trial, a RCT comparing the efficacy and safety of three different bDMARDs (certolizumab, abatacept and tocilizumab) with conventional active therapy (csDMARDs plus oral or intra-articular corticosteroids) in patients with early RA [82, 83]. Response was lower in women than in men in each treatment arm, particularly in the tocilizumab group [83].
The influence of sex on the response to JAK inhibitors, however, seems to be limited to pain modulation. A greater effect on pain was observed in men than in women, but the overall response and thus the likelihood of achieving the therapeutic goal seems to be the same in both sexes [84].
In terms of safety, it has been shown that men with RA are more prone to adverse events, particularly serious infections, during biologic treatment [85] and also have a significantly higher 10-year risk of cardiovascular disease (CVD) than women [86].
These data suggest that sex may be a discriminatory factor in the choice of therapy. However, to date there is a lack of randomized trials specifically designed to test whether there are differences in the efficacy of different DMARDs between men and women [87].
4.3 Reproductive Health/Pregnancy and Lactation
Women are two to three times more likely to have RA than men, and the disease often starts during their childbearing years [88]. The choice of therapy must also take into account the patient’s current or future life plans. Biological drugs can be actively transported across the placenta via the Fc region of the monoclonal antibody. The only exception is certolizumab, which lacks Fc portion and is conjugated to a polyethylene glycol (PEG) molecule. Its unique structure makes it compatible with all three trimesters of pregnancy. More recently, the other TNFis have also been shown to be compatible with pregnancy, but it is recommended that they be discontinued between the first and second trimester, depending on their half-life, to ensure adequate immunization of the unborn child [89]. For the other bDMARDs, although there are no data to support their teratogenicity or increased risk of infection, it is recommended that they be discontinued at conception due to the paucity of evidence [89]. With regard to lactation, minimal transfer of bDMARDs into breastmilk has been reported [90]. However, many studies have shown that IgG antibodies are efficiently digested by the infant gut [91]. According to the latest guideline on prescribing in pregnancy and breastfeeding from the British Society of Rheumatology, all TNFis are compatible with breastmilk exposure. Indications also exist for other bDMARDs, but these are based on limited evidence. Data on the safety of JAKis during pregnancy and lactation are still too limited, and therefore, these should be avoided [89].
4.4 Compliance and Adherence to Treatment
In general, the preferred route of administration for patients is the least invasive, which is primarily the oral route [92]. The parenteral route of administration can also be easily associated with the occurrence of infusion reactions or drug injection-site symptoms, most of which are mild (i.e. pain, erythema, itching, swelling, and burning) but can still alter patient satisfaction with treatment and potentially lead to reduced adherence [93]. However, intravenous therapy may allow for closer and more careful monitoring of those patients who have demonstrated low adherence [94].
4.5 Comorbidities
4.5.1 Cardiovascular Disease (CVD)
Inflammation is a pathophysiological process highly correlated with atherogenesis and atherothrombosis [95]. It is known that people with RA have an increased risk of cardiovascular (CV) morbidity and mortality and that inflammation confers a 1.5-fold further increase in the CV risk related to traditional risk factors [96,97,98]. Recently, large RCTs have shown that modulation of inflammation could be an effective prevention strategy in the general population [99, 100] as well as in RA patients. In particular, several observational studies have shown how the use of TNFis correlates with a reduction in CV risk in RA patients [101, 102], and a recent study even demonstrated a reduction in inflammation at the vascular level that was independent of clinical response related to the decrease of disease activity [103]. However, in patients without RA, the ATTACH trial demonstrated higher rates of death or hospitalization in subjects with congestive heart failure (CHF) receiving infliximab, especially in the subgroup treated with a high dosage (10 mg/kg), which is not approved for RA treatment [104]. Another two similar RCTs with etanercept showed no differences in the rates of hospitalization or death between etanercept and placebo [105]. These findings led to the conclusion that TNFis may cause cardiomyocyte dysregulation in a decompensated heart [106]. For this reason the ACR suggested that the use of TNFis should be avoided or used with extreme caution in patients with chronic heart failure, especially in those with New York Heart Association (NYHA) class III or IV [107]. Actually, in patients with RA, there is some evidence supporting a protective role of TNFis against the development of heart disease [108,109,110], especially diastolic dysfunction, which is known to be strictly related to inflammatory cytokines, including TNFα [111].
Inhibition of IL6, a cytokine widely implicated in CV disease risk in both RA patients and the general population, has been shown in several clinical trials to alter the lipid profile, with increases in total cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides, but also to be associated with an increase in high-density lipoprotein (HDL) cholesterol levels and function [112] and a reduction in other pro-atherogenic proteins such as serum amyloid A, lipoprotein (a) and secretory phospholipase A2 [113, 114]. Nevertheless, the metabolic changes induced by IL-6 inhibition have been shown not to be associated with an increased CV risk, being comparable in fact to that associated with TNFis [115].
Abatacept appears to have a very good CV safety profile and has shown greater cardioprotection than TNFis and other bDMARDs in many studies [116, 117].
The impact of JAKis on CV disease is a current topic of debate and study. As mentioned above, concerns about the cardiovascular safety of JAKis arose in 2022 from a post-marketing safety surveillance study of tofacitinib (ORAL Surveillance), which enrolled patients with RA with an inadequate response to methotrexate who were over 50 years of age and had at least one cardiovascular risk factor [8]. The study showed an increased incidence of major adverse cardiovascular events (MACE) with tofacitinib compared with TNF inhibition based on a prespecified non-inferiority criterion. The risk of MACE was higher in patients older than 65 years, and patients enrolled in North America. A post-hoc analyses revealed that risk of MACE was primarily observed in patients with a history of atherosclerotic cardiovascular disease (ASCVD), while no differences between tofacitinib and TNFi were observed in those with prevalent risk factors but no history of ASCVD [64]. Despite the results of ORAL Surveillance, prior to this trial, data from several RCTs [118, 119], systematic reviews and meta-analyses [120] found no difference in the rate of CV events in patients receiving JAKis compared with placebo. Furthermore, recent real-world observational data have shown that CV risk is similar between JAK inhibitors and other bDMARDs [66, 72, 121, 122]. However, a numerical increase in cardiovascular events was observed in some studies when the analysis was restricted to patients with pre-existent cardiovascular risk factors [121, 122].
Pending more robust epidemiological data and studies clarifying the pathogenetic mechanism underlying the CV risk associated with JAKis, the most reasonable approach at present seems to be risk stratification on an individual patient basis, including medical history of ASCVD and the use of CV risk scores that have been validated in patients with RA [97, 123,124,125].
4.5.2 Venous Thromboembolic (VTE) Risk
Patients with RA are at increased risk of VTE [126, 127] due to systemic inflammation leading to endothelial dysfunction and vascular damage that may predispose patients to the development of venous and arterial thrombi [128]. While there has been no evidence from clinical trials or observational registries of an increased risk of VTE associated with the use of bDMARDs, warnings have recently been raised in patients treated with JAKis. Indeed, an increased incidence of VTE events has been described in association with tofacitinib and baricitinib, mostly at higher doses (10 mg twice daily (BID) and 4 mg once daily, respectively) [8, 9]. Also, a post-hoc analysis of the SELECT phase 3 clinical programme with upadacitinib revealed a higher incidence of thromboembolic events in patients with a higher CV risk [129]. Data from real-world practice are somewhat conflicting, but overall an increase in thrombophlebitis, deep vein thrombosis or pulmonary embolism has been observed in patients treated with JAKis compared with those treated with bDMARDs [72, 130]. Risk of VTE was highest among males and those with a previous history of VTE [130]. To date, no increased thromboembolic risk associated with using filgotinib was reported. As filgotinib has only recently been introduced, it is important to note that real-world studies do not include (or include a very small proportion of) patients treated with filgotinib.
4.5.3 Malignancies
Incidence of malignancies is overall increased in patients with RA compared with general population, mainly due to an increased occurrence of lung cancer and lymphoma [131,132,133]. Despite optimal disease control and application of a treat-to-target strategy, cancer still remains an important cause of death in RA [134]. A large amount of data has been reassuring on the cancer risks with the use of bDMARDs, particularly with TNFis and rituximab, even in the long term [135,136,137,138]. Some concerns exist about treatment with abatacept, which has shown conflicting results. Three studies found a statistically significant increased risk of cancer overall with abatacept [138,139,140], while two studies did not confirm these findings [141, 142]. However, an increased risk of melanoma and non-melanoma skin cancer was noted with abatacept [142, 143]. There is less data on the risk associated with anti-IL6R drugs, but evidence suggests they are not associated with an increased risk of cancer [138, 144].
Regarding JAKis, in the ORAL Surveillance trial, a higher incidence of both adjudicated malignancies excluding non-melanoma skin cancer (NMSC) and of NMSC was observed with combined tofacitinib doses in comparison to TNFis (4.2% and 2.2% for tofacitinib versus 2.9% and 1.1% for TNFis) [8]. Risk was highest in patients with history of ASCVD or increased CV risk [145]. To better estimate the association of JAKis with the incidence of malignancy, a large meta-analysis was performed including 78 clinical trials and long-term extension studies of all the approved JAKis in adults with all the licensed indications (RA, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease and atopic dermatitis) [146]. Results showed that JAKis are not associated with a higher incidence of malignancies compared with placebo or methotrexate. However, JAKis seemed to be associated with a higher incidence of cancer compared with TNFis [146]. Considering that treatment with TNFis resulted in a significant reduced cancer risk compared with placebo, it could be discussed whether JAKis are harmful or rather whether they are less protective than TNFis with regard to cancer risk. Real-world observational short-term studies have found no increased risk of cancer overall with JAKis compared with TNFis [138, 147, 148], with the only exception of NMSC [149].
The issue becomes even more complex and sensitive when the treatment has to be started in a patient who has already been diagnosed with cancer in the past. As a history of cancer is generally an exclusion criterion in RCTs, there is a lack of robust safety evidence for the use DMARDs in this population. Recommendations for treating RA in oncological patients are also based on low-grade evidence—mostly expert opinion [150]. However, reassuring data are emerging about a non-increased risk of tumour recurrence with the use of b/tsDMARDs [151]. A greater amount of evidence exists for TNFis and rituximab [152, 153].
4.5.4 Infections
Infections are a major cause of mortality in RA. The risk of serious infections (SIs) in RA, estimated to be 50% higher than in matched patients with non-inflammatory musculoskeletal conditions, may be explained by disease-related immune alterations, comorbidities and/or immunosuppressive medications [154]. An increased rate of serious infections has been reported for several biologicals as compared with placebo and conventional DMARDs [155, 156].
Recently, a scoping review and meta-analysis combined the results of 242 studies (trials and registries) involving a total of 293,431 patients to estimate the impact of biologic and targeted synthetic therapies on infectious complications in RA patients. The study showed that non-serious infections were much more common than serious infections during the use of b/tsDMARDs in RA. In fact, 30% of patients experienced an infection during a trial, compared with 2–3% who experienced a serious infection. There were no differences in the risk of serious infections between the different biologicals. However, the proportion of patients with serious infections was highest among rituximab users compared with other drug classes. TNFis were associated with an increased proportion of mycobacterial infections. Conversely, tofacitinib use was associated with an increased proportion of herpes zoster [157]. This study confirms what was already partly known. In fact, the use of anti-TNF drugs is notably associated with risk of reactivation of latent tuberculosis [158], while herpes zoster, or varicella zoster virus (VZV) reactivation has emerged as the most relevant infectious complication of JAKis. Data from safety analyses of tofacitinib, baricitinib and upadacitinib have reported increased rates compared with placebo, with a greater risk in patients prescribed higher doses [159,160,161]. Real-world data have also confirmed HZ as the most common adverse event with these drugs, with no significant difference between them. The risk is greatest in elderly patients, those concomitantly prescribed glucocorticoids or MTX and in Asian regions [162]. Multidermatomal or disseminated herpes has been rarely described, while no cases of visceral disease or death are known [162]. Uncomplicated HZ has also been reported in filgotinib clinical trials, particularly in patients older than 55 years. A recent network analysis of RCT data reported that filgotinib is associated with the lowest risk of HZ compared with other JAKi [163].
4.5.5 Obesity
Obesity is a common comorbidity in RA patients and has been shown to have a strong direct and indirect impact on disease activity. Indeed, obesity is an inflammatory state characterized by an excess of visceral adipose tissue, which is associated with elevated levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) [164]. Obese RA patients have higher disease activity at baseline, higher scores on patient global assessment, pain, disability and overall quality of life. Several studies (RCTs or observational trials) have evaluated the impact of obesity on the response to individual treatments [165]. For TNFis, a higher BMI at baseline has been observed to correlate with a lower likelihood of achieving and maintaining the therapeutic goals of remission or low disease activity (LDA) over time [166, 167]. In contrast, BMI does not appear to influence the therapeutic response to anti-IL6Rs and abatacept, which showed the same clinical response rate in both normal-weight and overweight patients [168,169,170,171]. It has been hypothesized that pathophysiological mechanisms intrinsic to the production of pro-inflammatory adipokines may explain the differential effect of obesity on the response to different anti-cytokine drugs (TNFis versus anti-IL6R) [172]. Less is known about how obesity affects response to JAKis. Results from post-hoc analyses of RCTs showed no significant difference in clinical response between overweight and normal-weight patients for tofacitinib, baricitinib, and filgotinib [173,174,175]. This finding has been confirmed in real life experience [176, 177].
5 Disease-Related Factors
5.1 Seropositivity
Patients with RA are commonly stratified according to rheumatoid factor and/or anti-citrullinated protein antibody (ACPA) positivity. The serological status appears to confer a different clinical course and prognosis between patients who are seropositive and those who are seronegative [178]. The antibody profile may also influence the clinical response to some DMARDs. In particular, drugs that affect the adaptive immune response by altering the antibody production process, i.e., rituximab and abatacept, are particularly effective in seropositive patients [18, 179, 180]. The effect of serological status on clinical response to other bDMARDs is less clear [181, 182]. Similarly, the clinical response of patients treated with JAKis does not appear to be influenced by their serological status [183, 184].
5.2 Extra-Articular Manifestations
5.2.1 Interstitial Lung Disease
The incidence of interstitial lung disease (ILD) in patients with RA varies from 10% to 20%, depending on the clinical or radiological definition. To date, there is limited and often conflicting data regarding the optimal management of patients with RA-ILD. Previously, it was reported that TNFis could lead to a worsening of lung function in patients already suffering from RA-ILD [185]. However, subsequent studies did not confirm these data and reported similar incidence and progression rates compared with other classes of bDMARDs [186]. Nevertheless, the use of non-anti-TNFi bDMARDs appears to be associated with a lower risk of ILD progression, acute flares and mortality compared with TNFis [187,188,189]. In particular, abatacept appears to be associated with a lower risk of infections, which are one of the main causes of progression and mortality in patients with RA-ILD [190].
The role of JAKis in RA-ILD remains to be clarified. Recently, very promising and reassuring data have emerged that seem to demonstrate their potential in reducing the occurrence of RA-ILD [191] and clinical and radiographic progression in already-affected patients [186, 192, 193]. In this context, two RCTs evaluating the efficacy and safety of tofacitinib in patients with RA-ILD (NCT04311567 and NCT052462) are ongoing.
6 Discussion/Current Gaps
More than 50% of patients do not respond to first-line treatment with methotrexate and are candidates for treatment escalation to biologic or targeted synthetic DMARDs. Undoubtedly, the recent positioning of international regulatory agencies regarding the safety profile of JAKis has been a major limitation for the prescription of this drug class in patients who are insufficiently responsive to MTX. In a cohort of 198 patients treated with JAKis, we demonstrated the presence of ineligibility criteria in 45.4% of the study population, confirming that an age of over 65 years and/or the presence of cardiovascular, oncological or thromboembolic risk factors is a fairly frequent occurrence in a real-life population of RA patients. In these cases, the choice of the first targeted therapy after MTX failure can only fall to a bDMARD, to be identified taking into consideration the different available mechanisms of action (MoAs). In the remaining segment of patients who do not carry such specific risk factors, the range of therapeutic options is broader and also includes the class of JAKis.
Ideally, the choice of drug should be made by selecting the treatment that provides the best clinical response with the least toxicity for each patient. Unfortunately, no clinical, radiological or biological markers have yet been identified that can predict treatment response. Great efforts are being made towards precision medicine approaches in RA, and one of the major challenges in this field is the high degree of heterogeneity of the disease. In recent years, the study of synovial tissue and the use of multi-omics methods allowed the identification of several histological pathotypes whose cellular and molecular signature may be able to predict response to treatment [3, 194]. In addition, it has been possible to identify several synovial effector cells that may constitute potential therapeutic targets [195, 196]. The improved ability to access synovial tissue with minimally invasive techniques and to study its cellular and molecular pathways has allowed the first biopsy-driven clinical trials, whose results are paving the way for interesting new perspectives [197, 198]. It is reasonable to imagine that the design of innovative clinical trials, specifically designed not only to identify biomarkers but also to elucidate the mechanisms of response or non-response to different drugs, will lead to the selection of the right therapy for each individual patient a priori, thereby increasing the response and persistence rates of individual treatments [2]. Until the development of a true precision medicine, therapeutic decisions can still be based on a personalized approach, taking into account the intrinsic properties of the drug, the characteristics of the patient (for example, age, sex, co-morbidity and the patient’s preference for the route of administration) and those of the disease (for example, serology status and the presence of extra-articular manifestations), in accordance with international recommendations. However, all of this cannot ignore the economic aspect. Since the patent expiry of the first TNFis, the economic gap in terms of annual cost between biosimilar and branded drugs is still very wide, albeit with progressive levelling down of the overall price of targeted products. In this scenario and in the absence of strong clinical drivers or biomarkers to guide the therapeutic choice elsewhere, the use of a TNFi as first-line targeted therapy still represents the most proper treatment choice for the vast majority of MTX-IR patients.
Cases in which to deviate from this approach and prescribe a JAKi as first-line targeted therapy may include needle-phobic patients in whom the use of an oral rather than parenteral product could significantly increase therapeutic adherence. In addition, patients intolerant to concomitant MTX may benefit from drugs such as JAKis that have demonstrated clinical efficacy as monotherapy comparable to that in combination with csDMARDs. Finally, the presence of a prominent pain component in the clinical picture with a marked elevation of PRO scores may represent a driver towards the early use of JAKis upon MTX failure.
References
Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–48. https://doi.org/10.1016/S0140-6736(17)31491-5.
Pitzalis C, Choy EHS, Buch MH. Transforming clinical trials in rheumatology: towards patient-centric precision medicine. Nat Rev Rheumatol. 2020;16(10):590–9. https://doi.org/10.1038/s41584-020-0491-4.
Lewis MJ, Barnes MR, Blighe K, et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 2019;28(9):2455-2470.e5. https://doi.org/10.1016/j.celrep.2019.07.091.
Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(1):17–33. https://doi.org/10.1038/s41584-020-00541-7.
Lalor AF, Brooker JE, Rozbroj T, et al. Factors influencing clinician prescribing of disease-modifying anti-rheumatic drugs for inflammatory arthritis: a systematic review and thematic synthesis of qualitative studies. Semin Arthritis Rheum. 2022;55: 151988. https://doi.org/10.1016/j.semarthrit.2022.151988.
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. https://doi.org/10.1136/annrheumdis-2019-216655.
Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. https://doi.org/10.1136/ard-2022-223356.
Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–26. https://doi.org/10.1056/NEJMoa2109927.
Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2023;10(1):201–23. https://doi.org/10.1007/s40744-022-00505-1.
FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed 7 Jan 2024.
EMA. Janus kinase inhibitors (JAKi). European Medicines Agency. 2022. https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki. Accessed 28 Nov 2023.
Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther. 2020;7(3):473–516. https://doi.org/10.1007/s40744-020-00219-2.
Bonelli M, Kerschbaumer A, Kastrati K, et al. Selectivity, efficacy and safety of JAKinibs: new evidence for a still evolving story. Ann Rheum Dis. 2023. https://doi.org/10.1136/ard-2023-223850.
Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG. Upadacitinib and filgotinib: the role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context. 2019;8: 212595. https://doi.org/10.7573/dic.212595.
Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80(7):865–75. https://doi.org/10.1136/annrheumdis-2020-219012.
Strand V, Goncalves J, Isaacs JD. Immunogenicity of biologic agents in rheumatology. Nat Rev Rheumatol. 2021;17(2):81–97. https://doi.org/10.1038/s41584-020-00540-8.
Gehin JE, Goll GL, Brun MK, Jani M, Bolstad N, Syversen SW. Assessing immunogenicity of biologic drugs in inflammatory joint diseases: progress towards personalized medicine. BioDrugs. 2022;36(6):731–48. https://doi.org/10.1007/s40259-022-00559-1.
Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med. 2013;173(15):1416–28. https://doi.org/10.1001/jamainternmed.2013.7430.
Pecoraro V, De Santis E, Melegari A, Trenti T. The impact of immunogenicity of TNFα inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis. Autoimmun Rev. 2017;16(6):564–75. https://doi.org/10.1016/j.autrev.2017.04.002.
Bergstra SA, Branco JC, Vega-Morales D, et al. Inequity in access to bDMARD care and how it influences disease outcomes across countries worldwide: results from the METEOR-registry. Ann Rheum Dis. 2018;77(10):1413–20. https://doi.org/10.1136/annrheumdis-2018-213289.
Ascef B de O, Almeida MO, Medeiros-Ribeiro AC de, Oliveira de Andrade DC, Oliveira Junior HA de, de Soárez PC. Therapeutic equivalence of biosimilar and reference biologic drugs in rheumatoid arthritis: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(5):e2315872. https://doi.org/10.1001/jamanetworkopen.2023.15872.
Van Der Togt CJT, Van Den Bemt B, Aletaha D, et al. Points to consider for cost-effective use of biological and targeted synthetic DMARDs in inflammatory rheumatic diseases: results from an umbrella review and international Delphi study. RMD Open. 2023;9(1): e002898. https://doi.org/10.1136/rmdopen-2022-002898.
Brkic A, Diamantopoulos AP, Haavardsholm EA, et al. Exploring drug cost and disease outcome in rheumatoid arthritis patients treated with biologic and targeted synthetic DMARDs in Norway in 2010–2019—a country with a national tender system for prescription of costly drugs. BMC Health Serv Res. 2022;22(1):48. https://doi.org/10.1186/s12913-021-07425-w.
Kay J, Schoels MM, Dörner T, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77(2):165–74. https://doi.org/10.1136/annrheumdis-2017-211937.
Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–68. https://doi.org/10.1016/S0140-6736(17)31618-5.
Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80(7):848–58. https://doi.org/10.1136/annrheumdis-2020-219214.
Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–62. https://doi.org/10.1056/NEJMoa1608345.
Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol (Hoboken, NJ). 2019;71(11):1788–800. https://doi.org/10.1002/art.41032.
Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383(16):1511–21. https://doi.org/10.1056/NEJMoa2008250.
Pappas DA, St John G, Etzel CJ, et al. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann Rheum Dis. 2021;80(1):96–102. https://doi.org/10.1136/annrheumdis-2020-217209.
Pappas DA, O’Brien J, Guo L, et al. Treatment patterns and clinical outcomes in patients with rheumatoid arthritis initiating etanercept, adalimumab, or Janus kinase inhibitor as first-line therapy: results from the real-world CorEvitas RA Registry. Arthritis Res Ther. 2023;25(1):166. https://doi.org/10.1186/s13075-023-03120-9.
de Castro CT, de Queiroz MJ, Albuquerque FC, et al. Real-world effectiveness of biological therapy in patients with rheumatoid arthritis: systematic review and meta-analysis. Front Pharmacol. 2022;13: 927179. https://doi.org/10.3389/fphar.2022.927179.
Strand V. Patient-reported outcomes and realistic clinical endpoints for JAK inhibitors in rheumatoid arthritis. Expert Rev Clin Immunol. 2022;18(3):193–205. https://doi.org/10.1080/1744666X.2022.2049242.
Tóth L, Juhász MF, Szabó L, et al. Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: a systematic review and meta-analysis of 24,135 patients. Int J Mol Sci. 2022;23(3):1246. https://doi.org/10.3390/ijms23031246.
Spinelli FR, Garufi C, Ceccarelli F, et al. FRI0134 effect of JAK inhibitors on pain and quality of life in rheumatoid arthritis patients. Ann Rheum Dis. 2020;79:649.1-649. https://doi.org/10.1136/annrheumdis-2020-eular.4903.
De Stefano L, Bozzalla Cassione E, Bottazzi F, et al. Janus kinase inhibitors effectively improve pain across different disease activity states in rheumatoid arthritis. Intern Emerg Med. 2023;18(6):1733–40. https://doi.org/10.1007/s11739-023-03350-4.
Sunzini F, Schrepf A, Clauw DJ, Basu N. The biology of pain: through the rheumatology lens. Arthritis Rheumatol. 2023;75(5):650–60. https://doi.org/10.1002/art.42429.
Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(2):460-475.e21. https://doi.org/10.1016/j.cell.2020.11.007.
Fukuyama T, Tschernig T, Qi Y, Volmer DA, Bäumer W. Aggression behaviour induced by oral administration of the Janus-kinase inhibitor tofacitinib, but not oclacitinib, under stressful conditions. Eur J Pharmacol. 2015;764:278–82. https://doi.org/10.1016/j.ejphar.2015.06.060.
Jang Y, Lee WJ, Lee HS, Chu K, Lee SK, Lee ST. Tofacitinib treatment for refractory autoimmune encephalitis. Epilepsia. 2021;62(4):e53–9. https://doi.org/10.1111/epi.16848.
Favalli EG, Pregnolato F, Biggioggero M, et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res. 2016;68(4):432–9. https://doi.org/10.1002/acr.22788.
Choquette D, Bessette L, Alemao E, et al. Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res Ther. 2019;21(1):138. https://doi.org/10.1186/s13075-019-1917-8.
Ebina K, Etani Y, Maeda Y, et al. Drug retention of biologics and Janus kinase inhibitors in patients with rheumatoid arthritis: the ANSWER cohort study. RMD Open. 2023;9(3): e003160. https://doi.org/10.1136/rmdopen-2023-003160.
Baldi C, Berlengiero V, Falsetti P, et al. Baricitinib retention rate: “real-life” data from a mono-centric cohort of patients affected by rheumatoid arthritis. Front Med (Lausanne). 2023;10:1176613. https://doi.org/10.3389/fmed.2023.1176613.
Paroli M, Becciolini A, Bravi E, et al. Long-term retention rate of tofacitinib in rheumatoid arthritis: an Italian multicenter retrospective cohort study. Medicina. 2023;59(8):1480. https://doi.org/10.3390/medicina59081480.
Yazici A, Özdemir Işık Ö, Dalkılıç E, et al. A national, multicenter, secondary data use study evaluating efficacy and retention of first-line biologic treatment with tocilizumab in patients with rheumatoid arthritis in real-life setting: results from TURKBIO registry. Sci Rep. 2022;12(1):21972. https://doi.org/10.1038/s41598-022-26106-0.
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55(3):523–34. https://doi.org/10.1093/rheumatology/kev374.
Silvagni E, Bortoluzzi A, Carrara G, Zanetti A, Govoni M, Scirè CA. Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage On Rheumatic Diseases study on health care administrative databases. BMJ Open. 2018;8(9): e021447. https://doi.org/10.1136/bmjopen-2017-021447.
Emery P, Pope JE, Kruger K, et al. Efficacy of monotherapy with biologics and JAK Inhibitors for the treatment of rheumatoid arthritis: a systematic review. Adv Ther. 2018;35(10):1535–63. https://doi.org/10.1007/s12325-018-0757-2.
Skácelová M, Nekvindová L, Mann H, et al. The beneficial effect of csDMARDs co-medication on drug persistence of first-line TNF inhibitor in rheumatoid arthritis patients: data from Czech ATTRA registry. Rheumatol Int. 2022;42(5):803–14. https://doi.org/10.1007/s00296-021-05072-2.
Boone NW, Sepriano A, van der Kuy PH, Janknegt R, Peeters R, Landewé RBM. Cotreatment with methotrexate in routine care patients with rheumatoid arthritis receiving biological treatment yields better outcomes over time. RMD Open. 2019;5(1): e000836. https://doi.org/10.1136/rmdopen-2018-000836.
Gabay C, Riek M, Scherer A, Finckh A, on behalf of the SCQM collaborating physicians. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664–72. https://doi.org/10.1093/rheumatology/kev019.
van der Heijde D, Breedveld FC, Kavanaugh A, et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol. 2010;37(11):2237–46. https://doi.org/10.3899/jrheum.100208.
Jani M, Barton A, Warren RB, Griffiths CEM, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology. 2014;53(2):213–22. https://doi.org/10.1093/rheumatology/ket260.
Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2013;72(12):1947–55. https://doi.org/10.1136/annrheumdis-2012-202220.
O’Mahony A, Berg EL, John MR, Ganeshalingam K, Choy EH. THU0526 Tocilizumab is less dependent than adalimumab on supplementary effects of methotrexate for immunoregulation: a Biomap® profiling study. Ann Rheum Dis. 2014;73(Suppl 2):365–365. https://doi.org/10.1136/annrheumdis-2014-eular.3386.
Jørgensen TS, Kristensen LE, Christensen R, et al. Effectiveness and drug adherence of biologic monotherapy in routine care of patients with rheumatoid arthritis: a cohort study of patients registered in the Danish biologics registry. Rheumatology. 2015;54(12):2156–65. https://doi.org/10.1093/rheumatology/kev216.
Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76(5):840–7. https://doi.org/10.1136/annrheumdis-2016-210310.
Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50. https://doi.org/10.1016/S0140-6736(13)60250-0.
Batticciotto A, Ravasio R, Riva M, Sarzi-Puttini P. Efficacy and treatment costs of monotherapy with bDMARDs in the treatment of rheumatoid arthritis in patients intolerant to or inappropriate to continue treatment with methotrexate. Adv Ther. 2016;33(8):1360–73. https://doi.org/10.1007/s12325-016-0372-z.
Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol (Hoboken, NJ). 2017;69(3):506–17. https://doi.org/10.1002/art.39953.
Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86. https://doi.org/10.1056/NEJMoa1310476.
Migliore A, Bizzi E, Egan CG, Bernardi M, Petrella L. Efficacy of biological agents administered as monotherapy in rheumatoid arthritis: a Bayesian mixed-treatment comparison analysis. Ther Clin Risk Manag. 2015;11:1325–35. https://doi.org/10.2147/TCRM.S89678.
Charles-Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis. 2023;82(1):119–29. https://doi.org/10.1136/ard-2022-222259.
Kristensen LE, Danese S, Yndestad A, et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance. Ann Rheum Dis. 2023;82(7):901–10. https://doi.org/10.1136/ard-2022-223715.
Meissner Y, Schäfer M, Albrecht K, et al. Risk of major adverse cardiovascular events in patients with rheumatoid arthritis treated with conventional synthetic, biologic and targeted synthetic disease-modifying antirheumatic drugs: observational data from the German RABBIT register. RMD Open. 2023;9(4): e003489. https://doi.org/10.1136/rmdopen-2023-003489.
Buch MH, Gómez-Puerta JA, Burmester GR, et al. POS0308 long-term clinical profile of filgotinib (FIL) in patients (PTS) with rheumatoid arthritis (RA) by cardiovascular (CV) RISK factors: a post hoc subgroup analysis. In: Scientific Abstracts. BMJ Publishing Group Ltd and European League Against Rheumatism; 2023:397.1–398. https://doi.org/10.1136/annrheumdis-2023-eular.1552.
Riek M, Scherer A, Möller B, et al. Serious infection risk of tofacitinib compared to biologics in patients with rheumatoid arthritis treated in routine clinical care. Sci Rep. 2023;13(1):17776. https://doi.org/10.1038/s41598-023-44841-w.
Rubbert-Roth A, Kakehasi AM, Takeuchi T, et al. Malignancy in the upadacitinib clinical trials for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and non-radiographic axial spondyloarthritis. Rheumatol Ther. 2023. https://doi.org/10.1007/s40744-023-00621-6.
Kubo S, Miyazaki Y, Todoroki Y, et al. Generation-dependent retention rates and reasons for discontinuation of molecular targeted therapies in patients with rheumatoid arthritis: from FIRST registry. Rheumatol Ther. 2023;10(6):1705–23. https://doi.org/10.1007/s40744-023-00603-8.
Aymon R, Mongin D, Bergstra SA, et al. OP0219 incidence of major adverse cardiovascular events in patients with rheumatoid arthritis treated with JAK-inhibitors compared to bDMARDS: data from an international collaboration of registries (the “JAK-POT” study). In: Scientific Abstracts. BMJ Publishing Group Ltd and European League Against Rheumatism; 2023:143.2–145. https://doi.org/10.1136/annrheumdis-2023-eular.660.
Hoisnard L, Pina Vegas L, Dray-Spira R, Weill A, Zureik M, Sbidian E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis. 2023;82(2):182–8. https://doi.org/10.1136/ard-2022-222824.
Temmoku J, Miyata M, Suzuki E, et al. Drug retention rates and the safety of Janus kinase inhibitors in elderly patients with rheumatoid arthritis. J Clin Med. 2023;12(14):4585. https://doi.org/10.3390/jcm12144585.
Monti S, Klersy C, Gorla R, et al. Factors influencing the choice of first- and second-line biologic therapy for the treatment of rheumatoid arthritis: real-life data from the Italian LORHEN Registry. Clin Rheumatol. 2017;36(4):753–61. https://doi.org/10.1007/s10067-016-3528-y.
Temmoku J, Miyata M, Suzuki E, et al. Comparing the effectiveness and safety of abatacept and tocilizumab in elderly patients with rheumatoid arthritis. PLoS ONE. 2022;17(9): e0274775. https://doi.org/10.1371/journal.pone.0274775.
Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis – the ANSWER cohort study. PLoS ONE. 2019;14(5): e0216624. https://doi.org/10.1371/journal.pone.0216624.
Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology. 2019;58(2):e3–42. https://doi.org/10.1093/rheumatology/key208.
Bernatsky S, Feldman DE. Discontinuation of methotrexate therapy in older patients with newly diagnosed rheumatoid arthritis. Drugs Aging. 2008;25(10):879–84. https://doi.org/10.2165/00002512-200825100-00007.
Pavlov-Dolijanovic S, Bogojevic M, Nozica-Radulovic T, Radunovic G, Mujovic N. Elderly-onset rheumatoid arthritis: characteristics and treatment options. Medicina. 2023;59(10):1878. https://doi.org/10.3390/medicina59101878.
Jawaheer D, Maranian P, Park G, Lahiff M, Amjadi SS, Paulus HE. Disease progression and treatment responses in a prospective DMARD-naive seropositive early rheumatoid arthritis cohort: does gender matter? J Rheumatol. 2010;37(12):2475–85. https://doi.org/10.3899/jrheum.091432.
Jawaheer D, Olsen J, Hetland ML. Sex differences in response to anti-tumor necrosis factor therapy in early and established rheumatoid arthritis—results from the DANBIO Registry. J Rheumatol. 2012;39(1):46–53. https://doi.org/10.3899/jrheum.110548.
Hetland ML, Haavardsholm EA, Rudin A, et al. Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial. Br Med J. 2020;371: m4328. https://doi.org/10.1136/bmj.m4328.
Lend K, van Vollenhoven RF, Lampa J, et al. Sex differences in remission rates over 24 weeks among three different biological treatments compared to conventional therapy in patients with early rheumatoid arthritis (NORD-STAR): a post-hoc analysis of a randomised controlled trial. Lancet Rheumatol. 2022;4(10):e688–98. https://doi.org/10.1016/S2665-9913(22)00186-2.
Spinelli FR, Chimenti MS, Vadacca M, et al. Sat0153 gender does not influence clinical response to jak inhibitors in rheumatoid arthritis: an Italian multicentre analysis. Ann Rheum Dis. 2020;79(Suppl 1):1016–7. https://doi.org/10.1136/annrheumdis-2020-eular.5978.
Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11(1):R7. https://doi.org/10.1186/ar2591.
Targońska-Stępniak B, Biskup M, Biskup W, Majdan M. Gender differences in cardiovascular risk profile in rheumatoid arthritis patients with low disease activity. Biomed Res Int. 2019;2019: e3265847. https://doi.org/10.1155/2019/3265847.
Sepriano A, Nikiphorou E. Precision medicine in rheumatoid arthritis: unravelling sex-driven differences in response to treatment. Lancet Rheumatol. 2022;4(10):e650–1. https://doi.org/10.1016/S2665-9913(22)00223-5.
Finckh A, Gilbert B, Hodkinson B, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602. https://doi.org/10.1038/s41584-022-00827-y.
Russell MD, Dey M, Flint J, et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology. 2023;62(4):e48–88. https://doi.org/10.1093/rheumatology/keac551.
Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology. 2018;155(3):696–704. https://doi.org/10.1053/j.gastro.2018.05.040.
Demers-Mathieu V, Underwood MA, Beverly RL, Nielsen SD, Dallas DC. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients. 2018;10(5):631. https://doi.org/10.3390/nu10050631.
Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–99. https://doi.org/10.2147/PPA.S101821.
Chowdhury T, Dutta J, Noel P, et al. an overview on causes of nonadherence in the treatment of rheumatoid arthritis: its effect on mortality and ways to improve adherence. Cureus. 2022. https://doi.org/10.7759/cureus.24520.
Mena-Vazquez N, Manrique-Arija S, Yunquera-Romero L, et al. Adherence of rheumatoid arthritis patients to biologic disease-modifying antirheumatic drugs: a cross-sectional study. Rheumatol Int. 2017;37(10):1709–18. https://doi.org/10.1007/s00296-017-3758-6.
Weber BN, Giles JT, Liao KP. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat Rev Rheumatol. 2023;19(7):417–28. https://doi.org/10.1038/s41584-023-00969-7.
Wang M, Chao C, Mei K, et al. Relationship between rheumatoid arthritis and cardiovascular comorbidity, causation or co-occurrence: a Mendelian randomization study. Front Cardiovasc Med. 2023;10:1099861. https://doi.org/10.3389/fcvm.2023.1099861.
Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a Consortium of Rheumatology Researchers of North America Registry study. Arthritis Rheumatol. 2015;67(8):1995–2003. https://doi.org/10.1002/art.39195.
Myasoedova E, Chandran A, Ilhan B, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis. 2016;75(3):560–5. https://doi.org/10.1136/annrheumdis-2014-206411.
Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505. https://doi.org/10.1056/NEJMoa1912388.
Hu S, Lin C, Cai X, et al. The biological disease-modifying antirheumatic drugs and the risk of cardiovascular events: a systematic review and meta-analysis. Mediat Inflamm. 2021;2021:7712587. https://doi.org/10.1155/2021/7712587.
Sattin M, Towheed T. The effect of TNFα-inhibitors on cardiovascular events in patients with rheumatoid arthritis: an updated systematic review of the literature. Curr Rheumatol Rev. 2016;12(3):208–22.
Solomon DH, Giles JT, Liao KP, et al. Reducing cardiovascular risk with immunomodulators: a randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(3):324–30. https://doi.org/10.1136/ard-2022-223302.
Mann DL, McMurray JJV, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure. Circulation. 2004;109(13):1594–602. https://doi.org/10.1161/01.CIR.0000124490.27666.B2.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure. Circulation. 2003;107(25):3133–40. https://doi.org/10.1161/01.CIR.0000077913.60364.D2.
Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92(5):2303–12. https://doi.org/10.1172/JCI116834.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73(7):924–39. https://doi.org/10.1002/acr.24596.
Chen HK, Shao SC, Weng MY, et al. Risk of heart failure in rheumatoid arthritis patients treated with tumor necrosis factor-α inhibitors. Clin Pharmacol Ther. 2021;110(6):1595–603. https://doi.org/10.1002/cpt.2415.
Vizzardi E, Cavazzana I, Franceschini F, et al. Left ventricular function in rheumatoid arthritis during anti-TNF-α treatment: a speckle tracking prospective echocardiographic study. Monaldi Arch Chest Dis. 2016;84(1–2):716. https://doi.org/10.4081/monaldi.2015.716.
Listing J, Strangfeld A, Kekow J, et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58(3):667–77. https://doi.org/10.1002/art.23281.
Schau T, Gottwald M, Arbach O, et al. Increased prevalence of diastolic heart failure in patients with rheumatoid arthritis correlates with active disease, but not with treatment type. J Rheumatol. 2015;42(11):2029–37. https://doi.org/10.3899/jrheum.141647.
Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1806–12. https://doi.org/10.1136/annrheumdis-2015-207872.
McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74(4):694–702. https://doi.org/10.1136/annrheumdis-2013-204345.
Pierini FS, Botta E, Soriano ER, et al. Effect of tocilizumab on LDL and HDL characteristics in patients with rheumatoid arthritis. An observational study. Rheumatol Ther. 2021;8(2):803–15. https://doi.org/10.1007/s40744-021-00304-0.
Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72(1):31–40. https://doi.org/10.1002/art.41095.
Jin Y, Kang EH, Brill G, Desai RJ, Kim SC. Cardiovascular (CV) risk after initiation of abatacept versus TNF inhibitors in rheumatoid arthritis patients with and without baseline CV disease. J Rheumatol. 2018;45(9):1240–8. https://doi.org/10.3899/jrheum.170926.
Ozen G, Pedro S, Michaud K. The risk of cardiovascular events associated with disease-modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol. 2021;48(5):648–55. https://doi.org/10.3899/jrheum.200265.
Charles-Schoeman C, Choy E, McInnes IB, et al. MACE and VTE across upadacitinib clinical trial programmes in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. RMD Open. 2023;9(4): e003392. https://doi.org/10.1136/rmdopen-2023-003392.
Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46(3):261–71. https://doi.org/10.1016/j.semarthrit.2016.05.014.
Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019;78(8):1048–54. https://doi.org/10.1136/annrheumdis-2018-214846.
Bower H, Frisell T, di Giuseppe D, Delcoigne B, Askling J. Comparative cardiovascular safety with Janus kinase inhibitors and biological disease-modifying antirheumatic drugs as used in clinical practice: an observational cohort study from Sweden in patients with rheumatoid arthritis. RMD Open. 2023;9(4): e003630. https://doi.org/10.1136/rmdopen-2023-003630.
Frisell T, Bower H, Morin M, et al. Safety of biological and targeted synthetic disease-modifying antirheumatic drugs for rheumatoid arthritis as used in clinical practice: results from the ARTIS programme. Ann Rheum Dis. 2023;82(5):601–10. https://doi.org/10.1136/ard-2022-223762.
Favalli EG, Cincinelli G, Germinario S, et al. The impact of EMA recommendations on the real-life use of Janus kinases inhibitors for rheumatoid arthritis: the Expanded Risk Score in RA as a tool to quantify the risk of cardiovascular events. Front Immunol. 2023;14:1225160. https://doi.org/10.3389/fimmu.2023.1225160.
Cacciapaglia F, Spinelli FR, Piga M, et al. Estimated 10-year cardiovascular risk in a large Italian cohort of rheumatoid arthritis patients: data from the Cardiovascular Obesity and Rheumatic DISease (CORDIS) Study Group. Eur J Intern Med. 2022;96:60–5. https://doi.org/10.1016/j.ejim.2021.10.001.
Ciccia F, Caporali R. Proposals for the rheumatological use of JAK inhibitors. Nat Rev Rheumatol. 2024;20(2):65–6. https://doi.org/10.1038/s41584-023-01068-3.
Choi HK, Rho YH, Zhu Y, Cea-Soriano L, Aviña-Zubieta JA, Zhang Y. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2013;72(7):1182–7. https://doi.org/10.1136/annrheumdis-2012-201669.
Chung WS, Peng CL, Lin CL, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. 2014;73(10):1774–80. https://doi.org/10.1136/annrheumdis-2013-203380.
van den Oever IAM, Sattar N, Nurmohamed MT. Thromboembolic and cardiovascular risk in rheumatoid arthritis: role of the haemostatic system. Ann Rheum Dis. 2014;73(6):954–7. https://doi.org/10.1136/annrheumdis-2013-204767.
Fleischmann R, Curtis JR, Charles-Schoeman C, et al. Safety profile of upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the SELECT phase III rheumatoid arthritis clinical programme. Ann Rheum Dis. 2023;82(9):1130–41. https://doi.org/10.1136/ard-2023-223916.
Molander V, Bower H, Frisell T, Delcoigne B, Di Giuseppe D, Askling J. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(2):189–97. https://doi.org/10.1136/ard-2022-223050.
Beydon M, Pinto S, Rycke YD, et al. Risk of cancer for patients with rheumatoid arthritis versus general population: a national claims database cohort study. Lancet Reg Health Europe. 2023. https://doi.org/10.1016/j.lanepe.2023.100768.
Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–44. https://doi.org/10.1001/archinte.165.20.2337.
Wu X, Peng H, Wen Y, et al. Rheumatoid arthritis and risk of lung cancer: meta-analysis and Mendelian randomization study. Semin Arthritis Rheum. 2021;51(3):565–75. https://doi.org/10.1016/j.semarthrit.2021.03.015.
Heckert SL, Maassen JM, le Cessie S, et al. Long-term mortality in treated-to-target RA and UA: results of the BeSt and IMPROVED cohort. Ann Rheum Dis. 2023. https://doi.org/10.1136/ard-2023-224814.
Dreyer L, Mellemkjær L, Andersen AR, et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides—a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79–82. https://doi.org/10.1136/annrheumdis-2012-201969.
Aaltonen KJ, Joensuu JT, Virkki L, et al. Rates of serious infections and malignancies among patients with rheumatoid arthritis receiving either tumor necrosis factor inhibitor or rituximab therapy. J Rheumatol. 2015;42(3):372–8. https://doi.org/10.3899/jrheum.140853.
van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol. 2015;42(10):1761–6. https://doi.org/10.3899/jrheum.150051.
Huss V, Bower H, Wadström H, Frisell T, Askling J, The ARTIS Group. Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice. Rheumatology. 2022;61(5):1810–8. https://doi.org/10.1093/rheumatology/keab570.
Montastruc F, Renoux C, Dell’Aniello S, et al. Abatacept initiation in rheumatoid arthritis and the risk of cancer: a population-based comparative cohort study. Rheumatology (Oxford). 2019;58(4):683–91. https://doi.org/10.1093/rheumatology/key352.
Simon TA, Boers M, Hochberg M, et al. Comparative risk of malignancies and infections in patients with rheumatoid arthritis initiating abatacept versus other biologics: a multi-database real-world study. Arthritis Res Ther. 2019;21(1):228. https://doi.org/10.1186/s13075-019-1992-x.
Ozen G, Pedro S, Schumacher R, Simon TA, Michaud K. Safety of abatacept compared with other biologic and conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: data from an observational study. Arthritis Res Ther. 2019;21(1):141. https://doi.org/10.1186/s13075-019-1921-z.
de Germay S, Bagheri H, Despas F, Rousseau V, Montastruc F. Abatacept in rheumatoid arthritis and the risk of cancer: a world observational post-marketing study. Rheumatology (Oxford). 2020;59(9):2360–7. https://doi.org/10.1093/rheumatology/kez604.
Wadström H, Frisell T, Askling J, For the Anti-Rheumatic Therapy in Sweden (ARTIS) Study Group. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605–12. https://doi.org/10.1001/jamainternmed.2017.4332.
Fleischmann R, Genovese MC, Lin Y, et al. Long-term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years’ follow-up. Rheumatology. 2020;59(2):292–302. https://doi.org/10.1093/rheumatology/kez265.
Curtis JR, Yamaoka K, Chen YH, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2023;82(3):331–43. https://doi.org/10.1136/ard-2022-222543.
Russell MD, Stovin C, Alveyn E, et al. JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications. Ann Rheum Dis. 2023;82(8):1059–67. https://doi.org/10.1136/ard-2023-224049.
Song YJ, Cho SK, You SH, et al. Association between malignancy risk and Janus kinase inhibitors versus tumour necrosis factor inhibitors in Korean patients with rheumatoid arthritis: a nationwide population-based study. RMD Open. 2022;8(2): e002614. https://doi.org/10.1136/rmdopen-2022-002614.
Uchida T, Iwamoto N, Fukui S, et al. Comparison of risks of cancer, infection, and MACEs associated with JAK inhibitor and TNF inhibitor treatment: a multicentre cohort study. Rheumatology. 2023;62(10):3358–65. https://doi.org/10.1093/rheumatology/kead079.
Huss V, Bower H, Hellgren K, et al. Cancer risks with JAKi and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis or psoriatic arthritis: a national real-world cohort study. Ann Rheum Dis. 2023;82(7):911–9. https://doi.org/10.1136/ard-2022-223636.
Lopez-Olivo MA, Colmegna I, Karpes Matusevich AR, et al. Systematic review of recommendations on the use of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and cancer. Arthritis Care Res (Hoboken). 2020;72(3):309–18. https://doi.org/10.1002/acr.23865.
Molina-Collada J, Alonso F, Otero L, et al. Cancer risk with biologic and targeted synthetic DMARDs in patients with rheumatic diseases and previous malignancies: results from the BIOBADASER register. Semin Arthritis Rheum. 2023;64: 152341. https://doi.org/10.1016/j.semarthrit.2023.152341.
Sebbag E, Collada JM, Lauper K, et al. Pos1069 systematic literature review informing the eular points to consider task force on the initiation of targeted therapies in patients with inflammatory arthritides and a history of cancer. Ann Rheum Dis. 2023;82(Suppl 1):856–856. https://doi.org/10.1136/annrheumdis-2023-eular.4884.
Regierer AC, Strangfeld A. Rheumatoid arthritis treatment in patients with a history of cancer. Curr Opin Rheumatol. 2018;30(3):288–94. https://doi.org/10.1097/BOR.0000000000000492.
Mehta B, Pedro S, Ozen G, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5(1): e000935. https://doi.org/10.1136/rmdopen-2019-000935.
Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011(2): CD008794. https://doi.org/10.1002/14651858.CD008794.pub2.
Singh JA, Cameron C, Noorbaloochi S, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–65. https://doi.org/10.1016/s0140-6736(14)61704-9.
Bergmans BJM, Gebeyehu BY, van Puijenbroek EP, et al. Infections in biological and targeted synthetic drug use in rheumatoid arthritis: where do we stand? A scoping review and meta-analysis. Rheumatol Ther. 2023;10(5):1147–65. https://doi.org/10.1007/s40744-023-00571-z.
Cantini F, Niccoli L, Capone A, Petrone L, Goletti D. Risk of tuberculosis reactivation associated with traditional disease modifying anti-rheumatic drugs and non-anti-tumor necrosis factor biologics in patients with rheumatic disorders and suggestion for clinical practice. Expert Opin Drug Saf. 2019;18(5):415–25. https://doi.org/10.1080/14740338.2019.1612872.
Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81(3):335–43. https://doi.org/10.1136/annrheumdis-2021-221276.
Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76(7):1253–62. https://doi.org/10.1136/annrheumdis-2016-210457.
Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021;80(3):304–11. https://doi.org/10.1136/annrheumdis-2020-218510.
Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(10):2675–84. https://doi.org/10.1002/art.38745.
Alves C, Penedones A, Mendes D, Marques FB. The risk of infections associated with jak inhibitors in rheumatoid arthritis: a systematic review and network meta-analysis. J Clin Rheumatol. 2022;28(2):e407–14. https://doi.org/10.1097/RHU.0000000000001749.
Poudel D, George MD, Baker JF. The impact of obesity on disease activity and treatment response in rheumatoid arthritis. Curr Rheumatol Rep. 2020;22(9):56. https://doi.org/10.1007/s11926-020-00933-4.
Gialouri CG, Pappa M, Evangelatos G, Nikiphorou E, Fragoulis GE. Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review. Autoimmun Rev. 2023;22(7): 103357. https://doi.org/10.1016/j.autrev.2023.103357.
Hamann PDH, Pauling JD, McHugh N, Shaddick G, Hyrich K, The BSRBR-RA Contributors Group. Predictors, demographics and frequency of sustained remission and low disease activity in anti-tumour necrosis factor–treated rheumatoid arthritis patients. Rheumatology. 2019;58(12):2162–9. https://doi.org/10.1093/rheumatology/kez188.
Elalouf O, Lidar M, Reitblat T, et al. High body mass index is associated with shorter retention of tumor necrosis factor-alpha blocker treatment in rheumatoid arthritis. BTT. 2021;15:279–87. https://doi.org/10.2147/BTT.S290169.
Burmester GR, Rubbert-Roth A, Cantagrel A, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73(1):69–74. https://doi.org/10.1136/annrheumdis-2013-203523.
Huang H, Cen H, Zhou L, et al. Body mass index and clinical response to tocilizumab in patients with rheumatoid arthritis. Arch Rheumatol. 2019;34(4):406–13. https://doi.org/10.5606/ArchRheumatol.2019.7146.
Iannone F, Ferraccioli G, Sinigaglia L, et al. Real-world experience of tocilizumab in rheumatoid arthritis: sub-analysis of data from the Italian biologics’ register GISEA. Clin Rheumatol. 2018;37(2):315–21. https://doi.org/10.1007/s10067-017-3846-8.
Di Carlo M, Salaffi F, Gremese E, Iannone F, Lapadula G, Ferraccioli G. Body mass index as a driver of selection of biologic therapy in rheumatoid arthritis. Results from the US-CLARA study. Eur J Intern Med. 2019;66:57–61. https://doi.org/10.1016/j.ejim.2019.05.017.
Novella-Navarro M, Genre F, Hernández-Breijo B, et al. Obesity and response to biological therapy in rheumatoid arthritis: the role of body mass index and adipose tissue cytokines. Clin Exp Rheumatol. 2022;40(9):1726–32. https://doi.org/10.55563/clinexprheumatol/a9gskx.
Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–24. https://doi.org/10.1016/S0140-6736(18)31116-4.
Balsa A, Wassenberg S, Tanaka Y, et al. Effect of filgotinib on body mass index (BMI) and effect of baseline BMI on the efficacy and safety of filgotinib in rheumatoid arthritis. Rheumatol Ther. 2023;10(6):1555–74. https://doi.org/10.1007/s40744-023-00599-1.
Dikranian AH, Gonzalez-Gay MA, Wellborne F, et al. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by baseline body mass index: an analysis of pooled data from phase 3 studies. RMD Open. 2022;8(1): e002103. https://doi.org/10.1136/rmdopen-2021-002103.
Meißner Y, Baganz L, Schneider M, Schwarze I. Baricitinib and tofacitinib in real life—does obesity impact response to Janus kinase inhibitor therapy in rheumatoid arthritis? ACR Meeting Abstracts. https://acrabstracts.org/abstract/baricitinib-and-tofacitinib-in-real-life-does-obesity-impact-response-to-janus-kinase-inhibitor-therapy-in-rheumatoid-arthritis/. Accessed 2 May 2024.
Spinelli FR, Ceccarelli F, Garufi C, et al. Effectiveness and safety of baricitinib in rheumatoid arthritis: a monocentric, longitudinal, real-life experience. Clin Exp Rheumatol. 2021;39(3):525–31. https://doi.org/10.55563/clinexprheumatol/lfg83z.
Bugatti S, Manzo A, Montecucco C, Caporali R. The clinical value of autoantibodies in rheumatoid arthritis. Front Med (Lausanne). 2018;5:339. https://doi.org/10.3389/fmed.2018.00339.
Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75(4):709–14. https://doi.org/10.1136/annrheumdis-2015-207942.
Harrold LR, Connolly SE, Wittstock K, et al. Baseline anti-citrullinated protein antibody status and response to abatacept or non-TNFi biologic/targeted-synthetic DMARDs: US observational study of patients with RA. Rheumatol Ther. 2022;9(2):465–80. https://doi.org/10.1007/s40744-021-00401-0.
Lv Q, Yin Y, Li X, et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFα agent treatment in patients with rheumatoid arthritis: a meta-analysis. PLoS ONE. 2014;9(2): e89442. https://doi.org/10.1371/journal.pone.0089442.
Pers YM, Fortunet C, Constant E, et al. Predictors of response and remission in a large cohort of rheumatoid arthritis patients treated with tocilizumab in clinical practice. Rheumatology. 2014;53(1):76–84. https://doi.org/10.1093/rheumatology/ket301.
Bird P, Hall S, Nash P, et al. Treatment outcomes in patients with seropositive versus seronegative rheumatoid arthritis in phase III randomised clinical trials of tofacitinib. RMD Open. 2019;5(1): e000742. https://doi.org/10.1136/rmdopen-2018-000742.
Martinez-Molina C, Gich I, Diaz-Torné C, et al. Patient-related factors influencing the effectiveness and safety of Janus kinase inhibitors in rheumatoid arthritis: a real-world study. Sci Rep. 2024;14(1):172. https://doi.org/10.1038/s41598-023-50379-8.
Ostör AJK, Chilvers ER, Somerville MF, et al. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. 2006;33(3):622–8.
Yuan H, Cui S, Yang L, et al. Efficacy of non-conventional synthetic DMARDs for patients with rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. RMD Open. 2023;9(4): e003487. https://doi.org/10.1136/rmdopen-2023-003487.
Vicente-Rabaneda EF, Atienza-Mateo B, Blanco R, et al. Efficacy and safety of abatacept in interstitial lung disease of rheumatoid arthritis: s systematic literature review. Autoimmun Rev. 2021;20(6): 102830. https://doi.org/10.1016/j.autrev.2021.102830.
Mena-Vázquez N, Godoy-Navarrete FJ, Manrique-Arija S, et al. Non-anti-TNF biologic agents are associated with slower worsening of interstitial lung disease secondary to rheumatoid arthritis. Clin Rheumatol. 2021;40(1):133–42. https://doi.org/10.1007/s10067-020-05227-9.
Manfredi A, Luppi F, Cassone G, Vacchi C, Salvarani C, Sebastiani M. Pathogenesis and treatment of idiopathic and rheumatoid arthritis-related interstitial pneumonia. The possible lesson from COVID-19 pneumonia. Expert Rev Clin Immunol. 2020;16(8):751–70. https://doi.org/10.1080/1744666X.2020.1803064.
Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2014;43(5):613–26. https://doi.org/10.1016/j.semarthrit.2013.09.005.
Baker MC, Liu Y, Lu R, Lin J, Melehani J, Robinson WH. Incidence of interstitial lung disease in patients with rheumatoid arthritis treated with biologic and targeted synthetic disease-modifying antirheumatic drugs. JAMA Netw Open. 2023;6(3): e233640. https://doi.org/10.1001/jamanetworkopen.2023.3640.
Tardella M, Di Carlo M, Carotti M, Ceccarelli L, Giovagnoni A, Salaffi F. A retrospective study of the efficacy of JAK inhibitors or abatacept on rheumatoid arthritis-interstitial lung disease. Inflammopharmacol. 2022;30(3):705–12. https://doi.org/10.1007/s10787-022-00936-w.
Vacchi C, Manfredi A, Cassone G, et al. Tofacitinib for the treatment of severe interstitial lung disease related to rheumatoid arthritis. Case Rep Med. 2021;2021: e6652845. https://doi.org/10.1155/2021/6652845.
Humby F, Lewis M, Ramamoorthi N, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis. 2019;78(6):761–72. https://doi.org/10.1136/annrheumdis-2018-214539.
Zhang F, Jonsson AH, Nathan A, et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature. 2023;623(7987):616–24. https://doi.org/10.1038/s41586-023-06708-y.
Bhamidipati K, Wei K. Precision medicine in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2022;36(1): 101742. https://doi.org/10.1016/j.berh.2022.101742.
Humby F, Durez P, Buch MH, et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet (London, England). 2021;397:10271. https://doi.org/10.1016/S0140-6736(20)32341-2.
Rivellese F, Nerviani A, Giorli G, et al. Stratification of biological therapies by pathobiology in biologic-naive patients with rheumatoid arthritis (STRAP and STRAP-EU): two parallel, open-label, biopsy-driven, randomised trials. Lancet Rheumatology. 2023;5(11):e648–59. https://doi.org/10.1016/S2665-9913(23)00241-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.G.F. has received consulting fees and/or honoraria from Alfasigma, AbbVie, BMS, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer and UCB. G.M. has received consulting fees and/or honoraria from Janssen and Lilly. R.C. has received consulting fees and/or honoraria from AbbVie, Amgen, BMS, Celltrion, Fresenius, Galapagos, Janssen, Lilly, Novartis, Pfizer and UCB.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Ethics approval (appropriate approvals or waivers)
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials (data transparency)
No data were used for the research described in the article.
Code availability (software application or custom code)
Not applicable.
Author contributions
E.G.F. and G.M. conceived, designed and wrote the manuscript; collected data; and performed literature analysis and interpretation. R.C. supervised the drafting and contributed to the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Favalli, E.G., Maioli, G. & Caporali, R. Biologics or Janus Kinase Inhibitors in Rheumatoid Arthritis Patients Who are Insufficient Responders to Conventional Anti-Rheumatic Drugs. Drugs (2024). https://doi.org/10.1007/s40265-024-02059-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02059-8