Abstract
Increasing evidence has implicated lipoprotein(a) [Lp(a)] in the causality of atherosclerosis and calcific aortic stenosis. This has stimulated immense interest in developing novel approaches to integrating Lp(a) into the setting of cardiovascular prevention. Current guidelines advocate universal measurement of Lp(a) levels, with the potential to influence cardiovascular risk assessment and triage of higher-risk patients to use of more intensive preventive therapies. In parallel, considerable activity has been undertaken to develop novel therapeutics with the potential to achieve selective and substantial reductions in Lp(a) levels. Early studies of antisense oligonucleotides (e.g., mipomersen, pelacarsen), RNA interference (e.g., olpasiran, zerlasiran, lepodisiran) and small molecule inhibitors (e.g., muvalaplin) have demonstrated effective Lp(a) lowering and good tolerability. These agents are moving forward in clinical development, in order to determine whether Lp(a) lowering reduces cardiovascular risk. The results of these studies have the potential to transform our approach to the prevention of cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lipoprotein(a) [Lp(a)] plays a causal role in cardiovascular disease. |
Apart from apheresis, there are currently no effective approaches for Lp(a) lowering. |
Novel therapies robustly lower Lp(a) and are being evaluated with regard to their effect on clinical outcomes. |
1 Introduction
A generation of clinical trials have consistently demonstrated that lowering levels of low-density lipoprotein cholesterol (LDL-C) reduces the risk of cardiovascular events [1]. These studies have prompted successive updates to treatment guidelines for cardiovascular prevention and advocate achieving very low LDL-C levels in the highest risk patients [2]. However, many patients continue to experience clinical events, despite the use of intensive lipid-lowering therapy [3]. This supports the need to develop new therapies, targeting alternative factors, in order to tackle this residual risk more effectively. Additional atherogenic lipid factors, such as lipoprotein(a) [Lp(a)] have emerged as potential important factors in efforts to achieve more effective prevention of cardiovascular disease.
2 Lipoprotein(a) [Lp(a)] and Cardiovascular Risk
First discovered in the 1960s, Lp(a) has been increasingly recognised as a lipoprotein with considerable potential to promote cardiovascular risk [4]. Lipoprotein(a) is formed in the liver by covalent bonding of apolipoprotein(a) [apo(a)] to apolipoprotein B-100 (apoB) to produce an LDL-like particle [5, 6]. This process involves non-covalent binding of apo(a) Kringle IV domains 7 and 8 to apoB lysine residues, followed by a covalent disulfide bond [5, 6]. Kringle IV contains ten subtypes, with one copy of 1 and 3-10, while domain 2 demonstrates considerable heterogeneity, ranging in number from 1 to 40 copies [7, 8]. This variability in the number of Kringle IV repeats and isoform size is strongly regulated by the LPA gene. This translates to an impact on production within the liver and circulating Lp(a) concentrations, with those individuals with smaller apo(a) isoforms demonstrating higher circulating Lp(a) levels [9].
Preclinical studies have demonstrated a number of functional properties of Lp(a), which may contribute to a potential mechanistic role in atherosclerotic cardiovascular disease. These include its ability to behave like any apoB containing lipoprotein, to enter the artery wall, undergo oxidative modification and promote foam cell formation [10]. The atherogenicity of Lp(a) is estimated to be more than six times more potent than LDL, on a molar basis [11]. Lysine binding sites within the artery wall promote greater retention of apo(a) within the artery wall [12]. Additional accumulation of inflammatory cells is promoted by the oxidized phospholipid content of Lp(a) [13]. More vulnerable lesions appear to contain greater amounts of Lp(a) and oxidized phospholipids [14, 15]. These studies have also proposed potential effects on the thrombotic cascade, via an increase in endothelial expression of plasminogen activator inhibitor, tissue factor pathway inhibitor activity and platelet responsiveness, combined with both decreases in plasminogen activation and fibrin degradation [8, 16]. It is also well established that apo(a) demonstrates homology to plasminogen, having evolved from the same gene [6]. Apo(a), like plasminogen, can bind to exposed lysine-rich endothelium within the vasculature and on heart valves, resulting in a theoretical impact on fibrinolysis [12]. Stimulation of calcification within both the vessel wall and aortic valve by apo(a) provides further potential to promote cardiovascular disease [8]. The concomitant presence of autotaxin with Lp(a) may more efficiently deliver oxidized phospholipids into valve tissue, where they can promote inflammatory and calcific effects that underscore the development of aortic stenosis [17, 18].
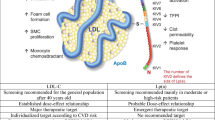
The association between Lp(a) levels and the clinical risk of atherosclerotic cardiovascular disease is strongly supported by observations from cohort and genomic studies. A curvilinear relationship has been consistently demonstrated between increasing Lp(a) levels and the risk of myocardial infarction, ischaemic stroke, abdominal aortic aneurysm and peripheral arterial disease, which persists after controlling for traditional risk factors, particularly at levels greater than 30 mg/dL [19,20,21,22]. A more linear relationship has been reported in the primary prevention setting, whereas the association between Lp(a) and subsequent clinical events appears to plateau at very high Lp(a) levels in those with manifest cardiovascular disease [23]. The potential for Lp(a) levels to reclassify cardiovascular risk in up to 40% of individuals, previously identified on the basis of assessment of traditional risk factors, has led to its inclusion in current prevention guidelines as a risk enhancer, with the ability to triage patients to use of more intensive risk factor modification [24]. Positron emission tomography imaging studies in humans have demonstrated the presence of greater carotid and aortic wall inflammatory activity in participants with higher Lp(a) levels [25]. Additional reports of an association with heart failure are likely to reflect the presence of proinflammatory effects of Lp(a) [26,27,28]. Genomic studies have demonstrated that the presence of LPA variants (rs10455872, rs3798220), the number of pathogenic alleles and Mendelian randomization each demonstrate an association between Lp(a) and the risk of atherosclerotic cardiovascular disease [29,30,31,32]. Furthermore, the findings of Mendelian randomization establish that Lp(a) plays a causal role in atherosclerotic disease, with estimates that a reduction in Lp(a) levels by 50–100 mg/dL may be required to achieve a 15 –20% decrease in the risk of major adverse cardiovascular events [32, 33].
Similar findings have been observed with regard to aortic valve disease, with evidence of an association between higher levels of Lp(a) and oxidized phospholipids and the presence of calcific aortic stenosis, accelerated progression on serial echocardiography and a greater need for aortic valve replacement [34, 35]. Genomic studies have also reported that the presence of the LPA polymorphism rs10455872 is associated with a greater risk of aortic stenosis [36, 37], with evidence of causality on Mendelian randomization [38]. The totality of these findings with regard to a potential role of Lp(a) in vascular and valvular disease has led to major efforts to develop therapeutic approaches to reduce its circulating levels.
3 Existing Therapies and Lp(a) Reduction
While the presence of an elevated Lp(a) level identifies an individual who may benefit from more intensive risk factor modification – with evidence of lower event rates in high Lp(a) patients with better control of LDL cholesterol and blood pressure [24], the use of therapies to directly lower Lp(a) levels has proven challenging. Recent evidence from genomic and cohort studies suggest that aspirin may reduce cardiovascular risk in people with very high Lp(a) levels, greater than 50 mg/dL [39, 40]. For decades, oestrogen and nicotinic acid have been reported to decrease Lp(a), although neither have been demonstrated to reduce cardiovascular risk in contemporary trials [24]. Statins are commonly employed to lower cardiovascular risk, although they may raise Lp(a) levels by up to 10%, although the underlying mechanism remains uncertain [41]. Pharmacological inhibitors of proprotein convertase subtilisin Kexin type 9 (PCSK9) have been reported to decrease Lp(a) by 25–30%, with post hoc evidence from large clinical outcome trials that independently this is associated with their clinical benefit [42, 43]. Early experience with inclisiran demonstrates Lp(a) lowering by up to 26% [44]. In limited settings, apheresis has been employed to treat very–high-risk patients with markedly elevated Lp(a) levels [45, 46]. However, the field has lacked the presence of therapeutic agents which selectively and robustly decrease Lp(a) levels (Table 1).
4 Antisense Oligonucleotides
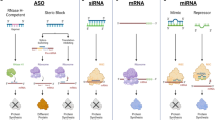
Novel therapeutics have focused on interrupting hepatic production of Lp(a) by use of agents that directly target RNA. Early approaches with mipomersen, an antisense oligonucleotide targeting apoB, demonstrated reductions in circulating Lp(a) levels by 25% due to reduced hepatic assembly [47]. More contemporary agents have directly targeted apo(a) production and have employed the N-acetyl-galactosamine (GalNac) molecule to selectively deliver therapeutics to the hepatocyte, with the potential for greater efficacy and tolerability. Pelacarsen is an antisense agent targeting apo(a), conjugated to GalNac, with early clinical studies demonstrating dose-dependent lowering of Lp(a) by 66–92% and good tolerability, with a small incidence of mild injection-site reactions [48, 49]. The Assessing the Impact of Lipoprotein (a) Lowering with TQJ230 on Major Cardiovascular Events in Patients with CVD (Lp(a) HORIZON) study, is the first large clinical outcomes trial in the Lp(a) field, comparing the effects of treatment with monthly subcutaneous administration of pelacarsen 80 mg or matching placebo on the incidence of cardiovascular death, myocardial infarction, stroke or urgent coronary revascularization. This trial is being performed in patients aged 18–80 years, with clinically manifest atherosclerotic cardiovascular disease and Lp(a) levels ≥ 70 mg/dL, with a prespecified evaluation of efficacy in those with baseline levels ≥ 90 mg/dL [50]. This trial is likely to be the first to demonstrate the potential impact of robust Lp(a) lowering on cardiovascular risk. An additional study is evaluating the impact of pelacarsen on the change in peak aortic jet velocity and aortic valve calcium score over a 3-year period, in 502 patients, aged 50–80 years, with mild or moderate calcific aortic valve stenosis and an Lp(a) ≥ 175 nmol/L [51]. While the degree of Lp(a) lowering with pelacarsen is less than that observed with other injectable agents, the HORIZON trial is the first study to evaluate the impact of Lp(a) lowering and will establish the relationship between the degree of Lp(a) lowering with both cardiovascular benefit and safety.
5 RNA Interference
The discovery of endogenous RNA interference highlighted factors playing an important role in the regulation of protein expression, while also unlocking the therapeutic potential of agents that could be developed and then directly targeted to organs where they could produce a similar function. The development of short RNA interfering (siRNA) agents targeting a range of atherogenic lipid factors has been advanced by a similar ability to employ GalNac to specifically deliver these therapeutics to the liver. A number of clinical development programmes are investigating the impact of RNA interference agents on apo(a) expression within the liver.
Olpasiran was investigated in a dose-finding trial of patients with established atherosclerotic cardiovascular disease and an Lp(a) level >150 nmol/L. After 36 weeks of treatment, placebo-adjusted reductions in Lp(a) were observed by 70.5% with 10 mg administered every 12 weeks, 97.4% with 75 mg administered every 12 weeks, 101.1% with 225 mg administered every 12 weeks and 100.5% with 225 mg administered every 24 weeks. Apart from injection-site reactions, administration of olpasiran was well tolerated [52]. Long-term follow up of these patients revealed that Lp(a) lowering of more than 40% remained evident for more than 12 months after the final administration of the olpasiran 225 mg dose [53]. The Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction (OCEAN(a)-Outcomes) study is currently evaluating the effect of treatment of olpasiran or matching placebo administered every 12 weeks on the composite incidence of death due to coronary heart disease, myocardial infarction or urgent coronary revascularization in 7000 patients aged 18–85 years, with a history of atherosclerotic cardiovascular disease and Lp(a) ≥ 200 nmol/L [54].
Zerlasiran is a 19-mer siRNA covalently linked to a tri-antennary GalNac moiety. An early single-ascending dose study in adults with Lp(a) levels ≥ 150 nmol/L demonstrated good tolerability with zerlasiran and reductions in Lp(a) by 46% with 30 mg, 86% with 100 mg, 96% with 300 mg and 98% with 600 mg, persisting for up to 150 days, compared with a 10% reduction with placebo [55]. A multiple-dose study has been performed in patients with established atherosclerotic cardiovascular disease and Lp(a) levels ≥ 150 nmol/L, with evidence of maximal Lp(a) lowering by 60% with 200 mg, 90% with 300 mg and 89% with 450 mg. Considerable durability of effect was demonstrated with persistent reductions of Lp(a) by 60%, 90% and 89%, 201 days after administration of each dose [56]. This agent has not progressed to the stage of a large cardiovascular outcomes trial.
Lepodisiran is a Dicer substrate siRNA with a tetraloop structure, which contains three GalNac conjugates. An early single-ascending dose study, performed in adults without cardiovascular disease and with Lp(a) ≥ 75 nmol/L demonstrated that lepodisiran was well tolerated and produced reductions in Lp(a) by 41% with 4 mg, 59% with 12 mg, 76% with 32 mg, 90% with 96 mg, 96% with 304 mg and 97 mg with 608 mg at 148 days, compared with a 5% reduction with placebo. After 337 days, Lp(a) levels remained 94% lower compared with baseline in the lepodisiran 608 mg dose group [57]. This agent is currently being evaluated in a larger and longer study [58] and has recently progressed to the stage of a large cardiovascular outcomes trial. This study will recruit 12,500 patients with a Lp(a) ≥ 175 nmol/L and either established atherosclerotic cardiovascular disease (age >18 years) or high risk primary prevention (defined as (1) atherosclerotic disease without a clinical event, (2) familial hypercholesterolemia or (3) presence of multiple risk factors, age >55 years) and evaluate the impact of lepodisiran on the composite endpoint of cardiovascular death, myocardial infarction, stroke or coronary revascularization [59]. The degree of Lp(a) lowering with these siRNA agents is greater than that observed with the antisense approach and the impact appears to be more durable, permitting less frequent administration. Whether nearly complete reduction in Lp(a) level will produce greater clinical benefit, without safety issues, will need to be determined by the outcomes trials.
6 Small Molecule Inhibitors
While there has been interest in the development of small molecule inhibitors of Lp(a), the complex folding structure of apo(a) has presented a considerable challenge for medicinal chemistry. Muvalaplin has evolved as the first-in-class small molecule inhibitor of Lp(a) formation, disrupting the initial non-covalent interaction between apo(a) Kringle IV domains 7 and 8 and apoB [60]. The ability to disrupt this interaction mimics naturally occurring genetic apo(a) variants that prevent interaction with apoB and result in low Lp(a) levels [61, 62]. Preclinical studies of muvalaplin demonstrated a decrease in Lp(a) formation assembly reactions in cell models and reductions in Lp(a) levels in non-human primates [60]. Early findings from a Phase 1 single-ascending dose study in humans, regardless of Lp(a) level, and a multiple ascending dose study of participants with Lp(a) levels ≥ 30 mg/dL demonstrated good tolerability and placebo-adjusted Lp(a) lowering up to 65% [60].
No discernible effects were observed on thrombotic and fibrinolytic markers, which was an important focus of evaluation given the homology between apo(a) and plasminogen [60]. While it has been observed that multivalent molecules, similar to muvalaplin, bind plasminogen Kringle IV domains and reduce plasminogen activity in rats. Comparison of plasminogen protein sequences reveals less targeted interaction motifs in human compared with rat plasminogen, suggesting that muvalaplin is less likely to modulate plasminogen in humans [60].
The findings of these early studies of muvalaplin are interesting. They demonstrate the ability for oral therapeutics to produce robust reductions in Lp(a) levels. While the degree of Lp(a) lowering is less than that observed with parenteral agents, muvalaplin does not require injection, which may be preferable for many patients. It is also noteworthy that currently used immunoturbidometric Lp(a) assays may underestimate the degree of Lp(a) lowering with this agent as they will measure apo(a) bound to both apoB and muvalaplin, the latter thought to have no functional effect. Accordingly, advances in assay technology may ultimately identify that muvalaplin produces greater reductions in functional Lp(a) than currently observed. Ultimately, the ability of muvalaplin to reduce the risk of cardiovascular events in association with the demonstration of long-term safety and tolerability will also require its evaluation in a large clinical outcomes trial. To date, muvalaplin represents the only oral agent dedicated to Lp(a) lowering, albeit to a lesser degree than the injectable therapies. The ongoing clinical development plan of this agent remains unknown.
7 Gene Editing
The therapeutic potential of gene editing has transformed hope for treatment of patients with a range of uncommon diseases, where the ability to specifically knockdown protein expression may alter the natural history and clinical outcomes. This has now advanced to the potential to treat several forms of dyslipidaemia, with early evidence of LDL-C lowering in humans treated with gene editing that targets PCSK9. This technology is also being directed towards the potential to reduce hepatic apo(a) synthesis. Early evidence in non-human primates demonstrated that Cas9 mRNA and guide RNA encapsulated within a lipid nanoparticle (CTX320) produced dose-dependent (0.5, 1.5 and 3 mg/kg) lowering of Lp(a) by 20%, 80% and 90%, respectively [63]. Such agents will need to progress through the typical stages of drug development to understand their therapeutic potential, from both an efficacy and safety perspective.
8 Additional Considerations
A number of additional points should be considered in parallel with development of pharmacologic therapies that lower Lp(a) levels. A range of biochemical assays have been employed to measure circulating Lp(a) levels, with the consensus view advocating for a move from mass- to molar-based assessments [64]. This reduces the influence of apo(a) isoform size impacting Lp(a) measurements, although there is no straightforward method for easy conversion between different assays [64]. If there were to be a standardized approach to Lp(a) measurement, there are considerable barriers to access Lp(a) testing, which require out-of-pocket costs paid for by patients in many countries. It is also uncertain what the physiological significance of Lp(a) is and whether substantial therapeutic lowering will result in unexpected complications. Studies have demonstrated an association between low Lp(a) levels and an increased prevalence of diabetes, which may reflect insulin resistance [65]. Accordingly, it will be critical to evaluate safety in long-term studies of Lp(a) inhibitors. It is possible that Lp(a) lowering may also be beneficial in renal disease (where Lp(a) levels can be elevated) and in the setting of fatty liver, although these are yet to be tested. It is also noteworthy that the current cardiovascular outcomes trials are being conducted predominantly in patients with manifest atherosclerotic cardiovascular disease, although lepodisiran is also being evaluated in high-risk primary prevention patients. If the early trials prove to demonstrate a reduction in cardiovascular risk, it is likely that future studies will attempt to evaluate the impact of Lp(a) lowering in lower risk, asymptomatic people.
9 Summary
Since t’s discovery in 1963, Lp(a) has challenged approaches to cardiovascular prevention due to the lack of effective therapies that will selectively lower its levels. Recent advances have raised the possibility that a number of therapeutic strategies may not only produce substantial reductions in Lp(a) levels, but may ultimately translate to a reduction in cardiovascular events, although this must be balanced by robust evaluations of the safety of substantial Lp(a) lowering, with a specific focus on the incidence of new diagnoses of diabetes. Current programmes are largely focusing on their application in patients with atherosclerotic cardiovascular disease, although further studies should consider how best to use these agents in other settings, such as aortic valve disease. While the current utility of Lp(a) in clinical practice is to reclassify risk and triage patients to more intensive risk factor modification, the future may permit specific treatment of Lp(a). We eagerly await the results of large clinical development programmes to determine whether Lp(a) will truly transform our approach to the prevention of cardiovascular disease.
References
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8.
Berg K. A New Serum Type System in Man–the Lp System. Acta Pathol Microbiol Scand. 1963;59:369–82.
Berg K. A new serum type system in man—the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–82.
McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330(6144):132–7.
Marcovina SM, Albers JJ, Gabel B, Koschinsky ML, Gaur VP. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin Chem. 1995;41(2):246–55.
Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57(8):1339–59.
Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273(1):6–30.
Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(12):2311–6.
Bjornson E, Adiels M, Taskinen MR, Burgess S, Chapman MJ, Packard CJ, et al. Lipoprotein(a) is markedly more atherogenic than LDL: an apolipoprotein B-based genetic analysis. J Am Coll Cardiol. 2024;83(3):385–95.
Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711.
van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–24.
Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol. 2014;63(19):1961–71.
van Dijk RA, Kolodgie F, Ravandi A, Leibundgut G, Hu PP, Prasad A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53(12):2773–90.
Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57(5):745–57.
Nsaibia MJ, Mahmut A, Boulanger MC, Arsenault BJ, Bouchareb R, Simard S, et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med. 2016;280(5):509–17.
Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lepine JL, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132(8):677–90.
Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23.
Kostner GM, Avogaro P, Cazzolato G, Marth E, Bittolo-Bon G, Qunici GB. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 1981;38(1–2):51–61.
Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53.
Verbeek R, Boekholdt SM, Stoekenbroek RM, Hovingh GK, Witztum JL, Wareham NJ, et al. Population and assay thresholds for the predictive value of lipoprotein (a) for coronary artery disease: the EPIC-Norfolk Prospective Population Study. J Lipid Res. 2016;57(4):697–705.
Berman AN, Biery DW, Besser SA, Singh A, Shiyovich A, Weber BN, et al. Lipoprotein(a) and major adverse cardiovascular events in patients with or without baseline atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2024;83(9):873–86.
Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64(9):851–60.
Stiekema LCA, Stroes ESG, Verweij SL, Kassahun H, Chen L, Wasserman SM, et al. Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J. 2019;40(33):2775–81.
Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, et al. The association of lipoprotein(a) with incident heart failure hospitalization: atherosclerosis risk in communities study. Atherosclerosis. 2017;262:131–7.
Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4(1):78–87.
Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai MY. Lp(a) [Lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups. Arterioscler Thromb Vasc Biol. 2018;38(10):2498–504.
Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33.
Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–9.
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–28.
Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619–27.
Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40(1):255–66.
Capoulade R, Chan KL, Yeang C, Mathieu P, Bosse Y, Dumesnil JG, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236–46.
Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121(2):306–14.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–7.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–12.
Arsenault BJ, Boekholdt SM, Dube MP, Rheaume E, Wareham NJ, Khaw KT, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304–10.
Lacaze P, Bakshi A, Riaz M, Polekhina G, Owen A, Bhatia HS, et al. Aspirin for primary prevention of cardiovascular events in relation to lipoprotein(a) genotypes. J Am Coll Cardiol. 2022;80(14):1287–98.
Bhatia HS, Trainor P, Carlisle S, Tsai MY, Criqui MH, DeFilippis A, et al. Aspirin and cardiovascular risk in individuals with elevated lipoprotein(a): the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2024;13(3): e033562.
Yeang C, Hung MY, Byun YS, Clopton P, Yang X, Witztum JL, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10(3):594–603.
O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–92.
Szarek M, Bittner VA, Aylward P, Baccara-Dinet M, Bhatt DL, Diaz R, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41(44):4245–55.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Moriarty PM, Hemphill L. Lipoprotein apheresis. Endocrinol Metab Clin N Am. 2016;45(1):39–54.
Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, et al. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of follow-up and apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36(9):2019–27.
Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011–21.
Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–55.
Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386(10002):1472–83.
2019 [cited 2021 January 3, 2021]; Available from: https://clinicaltrials.gov/ct2/show/NCT04023552?term=HORIZON&cond=Lp%28a%29&draw=2&rank=1.
2024 [cited 2024 April 24]; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05646381.
2020 [cited 2021 January 3, 2021]; Available from: https://doi.org/10.1161/circ.142.suppl_3.13951.
2023 [cited 2024 March 2]; Available from: https://www.ecrjournal.com/video-index/esc-23-oceana-dose-olpasiran-patients-atherosclerotic-cvd-and-elevated-lpa.
2024 [cited 2024 March 2]; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05581303.
Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein(a) production in individuals with elevated plasma lipoprotein(a) levels. JAMA. 2022;327(17):1679–87.
2024 [cited 2024 March 2]; Available from: https://www.clinicaltrials.gov/study/NCT05537571?intr=sln360&rank=1
Nissen SE, Linnebjerg H, Shen X, Wolski K, Ma X, Lim S, et al. Lepodisiran, an extended-duration short interfering RNA targeting lipoprotein(a): a randomized dose-ascending clinical trial. JAMA. 2023;330(21):2075–83.
2024 [cited 2024 March 2]; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05565742.
2024 [cited 2024 April 24]; Available from: https://clinicaltrials.gov/study/NCT06292013?intr=lepodisiran&rank=1.
Nicholls SJ, Nissen SE, Fleming C, Urva S, Suico J, Berg PH, et al. Muvalaplin, an oral small molecule inhibitor of lipoprotein(a) formation: a randomized clinical trial. JAMA. 2023;330(11):1042–53.
Ogorelkova M, Gruber A, Utermann G. Molecular basis of congenital lp(a) deficiency: a frequent apo(a) “null” mutation in caucasians. Hum Mol Genet. 1999;8(11):2087–96.
Ogorelkova M, Kraft HG, Ehnholm C, Utermann G. Single nucleotide polymorphisms in exons of the apo(a) kringles IV types 6 to 10 domain affect Lp(a) plasma concentrations and have different patterns in Africans and Caucasians. Hum Mol Genet. 2001;10(8):815–24.
2023 [cited 2024 March 2]; Available from: /https://doi.org/10.1161/circ.148.suppl_1.17013.
Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–46.
Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56(8):1252–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflict of Interest
S.J.N. has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Cyclarity, Eli Lilly, Esperion, Resverlogix, New Amsterdam Pharma, Novartis, InfraReDx and Sanofi-Regeneron and is also a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi- Regeneron, Vaxxinity, CSL Sequiris, and Novo Nordisk.
Authors' Contributions
SJN wrote, edited and reviewed this manuscript.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated for this manuscript.
Code Availability
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nicholls, S.J. Therapeutic Potential of Lipoprotein(a) Inhibitors. Drugs 84, 637–643 (2024). https://doi.org/10.1007/s40265-024-02046-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-024-02046-z