Abstract
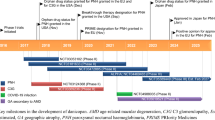
Crovalimab (派圣凯®; PiaSky) is a humanized, anti-complement component C5 (anti-C5) recycling monoclonal antibody developed by Chugai Pharmaceutical, in collaboration with Roche, which is being investigated for the treatment of complement-mediated diseases, including paroxysmal nocturnal haemoglobinuria (PNH), atypical haemolytic uremic syndrome, lupus nephritis and sickle cell disease. Crovalimab targets C5, inhibiting its cleavage to C5a and C5b, thus blocking the terminal complement pathway and preventing intravascular haemolysis in PNH. Crovalimab is designed to bind to the antigen repeatedly, resulting in sustained complement inhibition at a lower dosage, and allowing for once-monthly subcutaneous administration. In February 2024, subcutaneous crovalimab received its first approval in China for the treatment of adolescents and adults (aged ≥ 12 years) with PNH who have not been previously treated with complement inhibitors. Crovalimab has since been approved in Japan in March for use in the treatment of PNH, including in treatment-naïve and previously treated patients. Crovalimab is also under regulatory review for the treatment of naïve and previously treated patients with PNH in multiple countries, including the USA and the European Union. This article summarizes the milestones in the development of crovalimab leading to this first approval in China for the treatment of PNH.
Similar content being viewed by others
Data availability
Not applicable.
References
Kulasekararaj AG, Brodsky RA, Nishimura JI, et al. The importance of terminal complement inhibition in paroxysmal nocturnal hemoglobinuria. Ther Adv Hematol. 2022;13:20406207221091050.
Oliver M, Patriquin CJ. Paroxysmal nocturnal hemoglobinuria: current management, unmet needs, and recommendations. J Blood Med. 2023;14:613–28.
Panse J. Paroxysmal nocturnal hemoglobinuria: where we stand. Am J Hematol. 2023;98(Suppl 4):S20–32.
National Medical Products Administration. NMPA approval notice. 2024. https://www.nmpa.gov.cn/zhuanti/zt2023/ypgzhlfzh/shypqxgg/ggjzcx/20240207173310135.html. Accessed 29 Feb 2024.
Roche. Crovalimab: Chinese prescribing information. 2024. https://assets.roche.com/f/249469/x/dc4cbe0f74/kefalidankangzhusheye_20240206.pdf. Accessed 27 Feb 2024.
Chugai Pharmaceutical. Chugai obtains regulatory approval for “Piasky 340mg” for paroxysmal nocturnal hemoglobinuria in Japan [media release]. 26 Mar 2024. https://www.chugai-pharm.co.jp/english/news/.
Fukuzawa T, Sampei Z, Haraya K, et al. Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Sci Rep. 2017;7(1):1080.
Sampei Z, Haraya K, Tachibana T, et al. Antibody engineering to generate SKY59, a long-acting anti-C5 recycling antibody. PLoS ONE. 2018;13(12): e0209509.
Zelek WM, Stott M, Walters D, et al. Characterizing a pH-switch anti-C5 antibody as a tool for human and mouse complement C5 purification and cross-species inhibition of classical and reactive lysis. Immunology. 2018;155(3):396–403.
Brocchieri C, Beveridge L, Buri M, et al. Rapid complement inhibition with the C5 inhibitor crovalimab: a timing analysis using animal model and COMPOSER trial data [abstract no. 4033 plus poster presentation]. Nephrol Dialy Transplant. 2023;38(Suppl 1):I223–4.
Buatois S, Benkali K, Henrich A, et al. Pharmacokinetic characterization and exposure-response relationship of crovalimab in the COMPOSER and COMMODORE 3 trials of patients with paroxysmal nocturnal hemoglobinuria (PNH) [abstract] [abstract no. Blood. 2022;140(Suppl 1):2918–20.
Röth A, Nishimura JI, Nagy Z, et al. The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood. 2020;135(12):912–20.
Nishimura JI, Usuki K, Ramos J, et al. Crovalimab for treatment of patients with paroxysmal nocturnal haemoglobinuria and complement C5 polymorphism: subanalysis of the phase 1/2 COMPOSER study. Br J Haematol. 2022;198(3):e46–50.
Roth A, Rong FU, He G, et al. Safety of crovalimab versus eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH): pooled esults from the phase III COMMODORE 1, COMMODORE 2, and COMMODORE 3 studies [abstract plus poster presentation]. Blood. 2023;142(Suppl 1):575.
Liu H, Xia L, Weng J, et al. Efficacy and safety of the C5 inhibitor crovalimab in complement inhibitor-naive patients with PNH (COMMODORE 3): a multicenter, phase 3, single-arm study. Am J Hematol. 2023;98(9):1407–14.
Liu H, Xia L, Weng J, et al. Six-month crovalimab extension in the phase 3 commodore 3 study: updated efficacy and safety results in complement inhibitor-naive patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) [abstract no. P785 plus poster]. HemaSphere. 2023;7(Suppl 3): e1287903.
Röth A, He G, Brodsky A, et al. The phase III, randomized COMMODORE 2 trial: results from a multicenter study of crovalimab vs eculizumab in paroxysmal nocturnal hemoglobinuria (PNH) patients naive to complement inhibitors [abstract no. S181 plus oral presentation]. HemaSphere. 2023;7(Suppl 3):187–8.
Panse J, Cermak J, Kyselova O, et al. Patient-reported utcomes (PROs) in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with crovalimab and eculizumab: results from the phase III randomized COMMODORE 2 and COMMODORE 1 trials [abstract and poster presentation]. Blood. 2023;142(Suppl 1):4090.
Kulasekararaj A, Fernandez FAG, Scheinberg P, et al. Patient preferences and treatment satisfaction in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with crovalimab and approved C5 inhibitors in the phase III randomized COMMODORE 1 and COMMODORE 2 trials [abstact]. Blood. 2023;142(Suppl 1):5628.
Lundberg P, de la Iglesia S, Kelly RJ, et al. Biomarker analyses in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with crovalimab and eculizumab: results from the phase III randomized COMMODORE 2 trial [abstract]. Blood. 2023;142(Suppl 1):4088.
Scheinberg P, Cle D, Edwards J, et al. Phase III randomized, multicenter, open-label COMMODORE 1 trial: comparison of crovalimab vs eculizumab in complement inhibitor-experienced patients with paroxysmal nocturnal hemoglobinuria (PNH) [abstract no. S183 plus oral presentation]. HemaSphere. 2023;7(Suppl 3):191–2.
Röth A, Ichikawa S, Ito Y, et al. Crovalimab treatment in patients with paroxysmal nocturnal haemoglobinuria: long-term results from the phase I/II COMPOSER trial. Eur J Haematol. 2023;111(2):300–10.
Peffault de Latour R, Sica S, Ramos J, et al. COMPOSER part 4: an optimized dosing strategy for crovalimab in the treatment of complement inhibitor-naïve or -experienced patients with paroxysmal nocturnal hemoglobinuria [abstract]. Blood. 2020;136(Supplement 1):6–7.
Nishimura JI, Soubret A, Arase N, et al. Mitigating drug-target-drug complexes in patients with paroxysmal nocturnal hemoglobinuria who switch C5 inhibitors. Clin Pharmacol Ther. 2023;113(4):904–15.
Sreckovic S, Brocchieri C, Leon PG, et al. Phase 3 COMMODORE Trials of crovalimab in paroxysmal nocturnal hemoglobinuria (PNH): impact on Kidney Function [abstract no. SA-PO280 plus poster]. J Am Soc Nephrol. 2023;34:800.
Sheerin N, Greenbaum LA, Ito S, et al. Two phase 3 trials evaluating crovalimab in patients with atypical haemolytic uremic syndrome (aHUS): COMMUTE-a and COMMUTE-p [abstract no. INFO19 plus poster]. In: Kidney Week 2022 Annual Meeting of The American Society of Nephrology. 2022.
Callaghan M, Ataga KI, De Franceschi L, et al. Trial in progress: the randomized, double-blind, placebo-controlled phase 2a crosswalk-c trial evaluating the efficacy of crovalimab as adjunct treatment in the prevention of vasoocclusive episodes (VOEs) in patients (pts) with sickle cell disease (SCD) [abstract no. 5612539]. Hemasphere. 2023;7(Suppl 1):26.
Bartolucci P, Ataga KI, Callaghan M, et al. Trial in progress: The randomized, doubleblind, placebo-controlled phase 1b crosswalk-a trial evaluating the safety of crovalimab for the management of acute uncomplicated vaso-occlusive episodes (VOES) in patients (pts) with sickle cell disease (SCD) [abstract no. 5610823]. Hemasphere. 2023;7(Suppl 1):22.
Funding
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Crovalimab: First Approval. Drugs (2024). https://doi.org/10.1007/s40265-024-02032-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02032-5