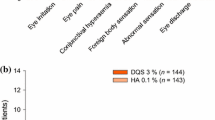
Abstract
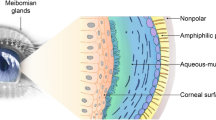
Perfluorohexyloctane ophthalmic solution (Miebo®) is a single-entity, water-, steroid- and preservative-free, first-in-class semifluorinated alkane that is approved in the USA for the treatment of the signs and symptoms of dry eye disease (DED). DED is often linked with meibomian gland dysfunction (MGD), which causes an excessive evaporation of tears. Perfluorohexyloctane ophthalmic solution stabilizes the lipid layer of the tear film and inhibits tear evaporation by forming a monolayer at the air-liquid interface. In the phase III GOBI and MOJAVE trials in adults with DED associated with MGD, one drop of perfluorohexyloctane ophthalmic solution instilled in each eye four times daily over 8 weeks resulted in statistically significant and clinically meaningful improvements in the signs and symptoms of DED compared with hypotonic saline (0.6%). The agent was generally well tolerated, with most ocular adverse events being mild or moderate in severity. The efficacy and tolerability of perfluorohexyloctane ophthalmic solution was sustained for up to 52 weeks in an extension study (KALAHARI). As the first and currently the only prescription treatment approved in the USA directly addressing the pathophysiology of excessive tear evaporation, perfluorohexyloctane ophthalmic solution is a valuable emerging option for the management of DED.
Plain Language Summary
Dry eye disease (DED) is a common eye disorder caused by many factors. In most cases, DED is linked with meibomian gland dysfunction (MGD), which causes an excessive evaporation of tears. Perfluorohexyloctane ophthalmic solution (Miebo®), a single-entity, water-, steroid- and preservative-free, first-in-class semifluorinated alkane, is approved in the USA for the treatment of the signs and symptoms of DED. The agent stabilizes the lipid layer of the tear film and prevents the evaporation of tears by forming a layer on the surface of the tear film. In two phase III clinical trials in adults with MGD-associated DED, one drop of perfluorohexyloctane ophthalmic solution instilled in each eye four times daily over 8 weeks led to significant improvements in the signs and symptoms of DED when compared with hypotonic saline (0.6%). Perfluorohexyloctane ophthalmic solution was generally well tolerated, with most ocular adverse events being mild or moderate in severity. Thus, as the first and currently the only prescription treatment approved in the USA directly addressing excessive tear evaporation, perfluorohexyloctane ophthalmic solution is a valuable emerging option for the management of DED.

Similar content being viewed by others
References
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628.
Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397–418.
Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–65.
Wrobel-Dudzinska D, Osial N, Stepien PW, et al. Prevalence of dry eye symptoms and associated risk factors among university students in Poland. Int J Environ Res Public Health. 2023;20(2):1313.
Barabino S, Benitez-Del-Castillo JM, Fuchsluger T, et al. Dry eye disease treatment: the role of tear substitutes, their future, and an updated classification. Eur Rev Med Pharmacol Sci. 2020;24(17):8642–52.
Sabeti S, Kheirkhah A, Yin J, et al. Management of meibomian gland dysfunction: a review. Surv Ophthalmol. 2020;65(2):205–17.
Mukamal R. Improved dry eye drugs for 2022 and beyond. 2021. https://www.aao.org/eye-health/tips-prevention/new-dry-eye-treatments-ocular-surface-disease. Accessed 27 Feb 2024.
US Food and Drug Administration. MIEBO (perfluorohexyloctane ophthalmic solution) for topical ophthalmic use: US prescribing information. 2024. https://dailymed.nlm.nih.gov/dailymed. Accessed 27 Feb 2024.
Agarwal P, Scherer D, Günther B, et al. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int J Pharm. 2018;538(1–2):119–29.
Tsagogiorgas C, Otto M. Semifluorinated alkanes as new drug carriers-an overview of potential medical and clinical applications. Pharmaceutics. 2023;15(4):1211.
Tauber J, Wirta DL, Sall K, et al. A randomized clinical study (SEECASE) to assess efficacy, safety, and tolerability of NOV03 for treatment of dry eye disease. Cornea. 2021;40(9):1132–40.
Liu X, Riess J, Krafft M. Self-organization of semifluorinated alkanes and related compounds at interfaces: thin films, surface domains and two-dimensional spherulites. Bull Chem Soc Jpn. 2018;91(5):846–57.
Krösser S, Spencer E, Grillenberger R, et al. Ocular and systemic distribution of 14C- perfluorohexyloctane following topical ocular administration to rabbits [abstract no. 2656 plus poster]. Invest Ophthalmol Vis Sci. 2018;59(9):2656.
Schmidl D, Bata AM, Szegedi S, et al. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: a randomized controlled clinical trial. J Ocul Pharmacol Ther. 2020;36(3):154–61.
Steven P, Augustin AJ, Geerling G, et al. Semifluorinated alkane eye drops for treatment of dry eye disease due to meibomian gland disease. J Ocul Pharmacol Ther. 2017;33(9):678–85.
Borchman D, Vittitow J, Kissling R, et al. Spectroscopic characterization of perfluorohexyloctane, an eye drop for dry eye disease [abstract no. 3975-B0292 plus poster]. Invest Ophthalmol Vis Sci. 2023;64(8):3975.
Stolowich N, Vittitow J, Kissling R, et al. Oxygen-carrying capacity of perfluorohexyloctane, a novel eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98(100705):1–5.
Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241–9.
Steven P, Scherer D, Krösser S, et al. Semifluorinated alkane eye drops for treatment of dry eye disease-a prospective, multicenter noninterventional study. J Ocul Pharmacol Ther. 2015;31(8):498–503.
Vittitow J, Kissling R, DeCory H, et al. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98(100704):1–7.
Delicado-Miralles M, Velasco E, Díaz-Tahoces A, et al. Deciphering the action of perfluorohexyloctane eye drops to reduce ocular discomfort and pain. Front Med (Lausanne). 2021. https://doi.org/10.3389/fmed.2021.709712.
Borchman D, Cavet M, DeCory H, et al. Visualization of perfluorohexyloctane, an eye drop for dry eye disease, on the human ocular surface using infrared emissivity [poster]. Annual meeting of the American Academy of Ophthalmology. 2023.
Tauber J, Berdy GJ, Wirta DL, et al. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516–24.
Sheppard JD, Kurata F, Epitropoulos AT, et al. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265–74.
Protzko EE, Segal BA, Korenfeld MS, et al. Long-term safety and efficacy of perfluorohexyloctane ophthalmic solution for the treatment of patients with dry eye disease: the KALAHARI Study. Cornea. 2023. https://doi.org/10.1097/ICO.0000000000003418.
Fahmy A, Evans D, Harthan J, et al. Perfluorohexyloctane (NOV03) for dry eye disease associated with meibomian gland dysfunction: pooled analysis of GOBI and MOJAVE studies [poster]. Annual Meeting of the American Optometric Association. 2023.
Groß D, Kaercher T. Comparison of the clinical efficacy of three different eye drops for the treatment of dry eye. EC Ophthalmol. 2021;12(6):32–44.
Acknowledgments
During the peer review process the manufacturer of perfluorohexyloctane ophthalmic solution was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Amy Zhuang-Yan and Yahiya Y. Syed are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: E. Anastasopoulos, 2nd Department of Ophthalmology, Aristotle University of Thessaloniki, Thessaloniki, Greece; F. Hakim, Department of Ophthalmology, University of Michigan, Ann Arbor, MI, USA; J. Luchs, Florida Vision Institute, West Palm Beach, FL, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuang-Yan, A., Syed, Y.Y. Perfluorohexyloctane Ophthalmic Solution: A Review in Dry Eye Disease. Drugs (2024). https://doi.org/10.1007/s40265-024-02016-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02016-5